Fermented Stevia Improves Alcohol Poisoning Symptoms Associated with Changes in Mouse Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Medicinal Herbal Extract
2.2. Bacterial Strains and Culture Conditions
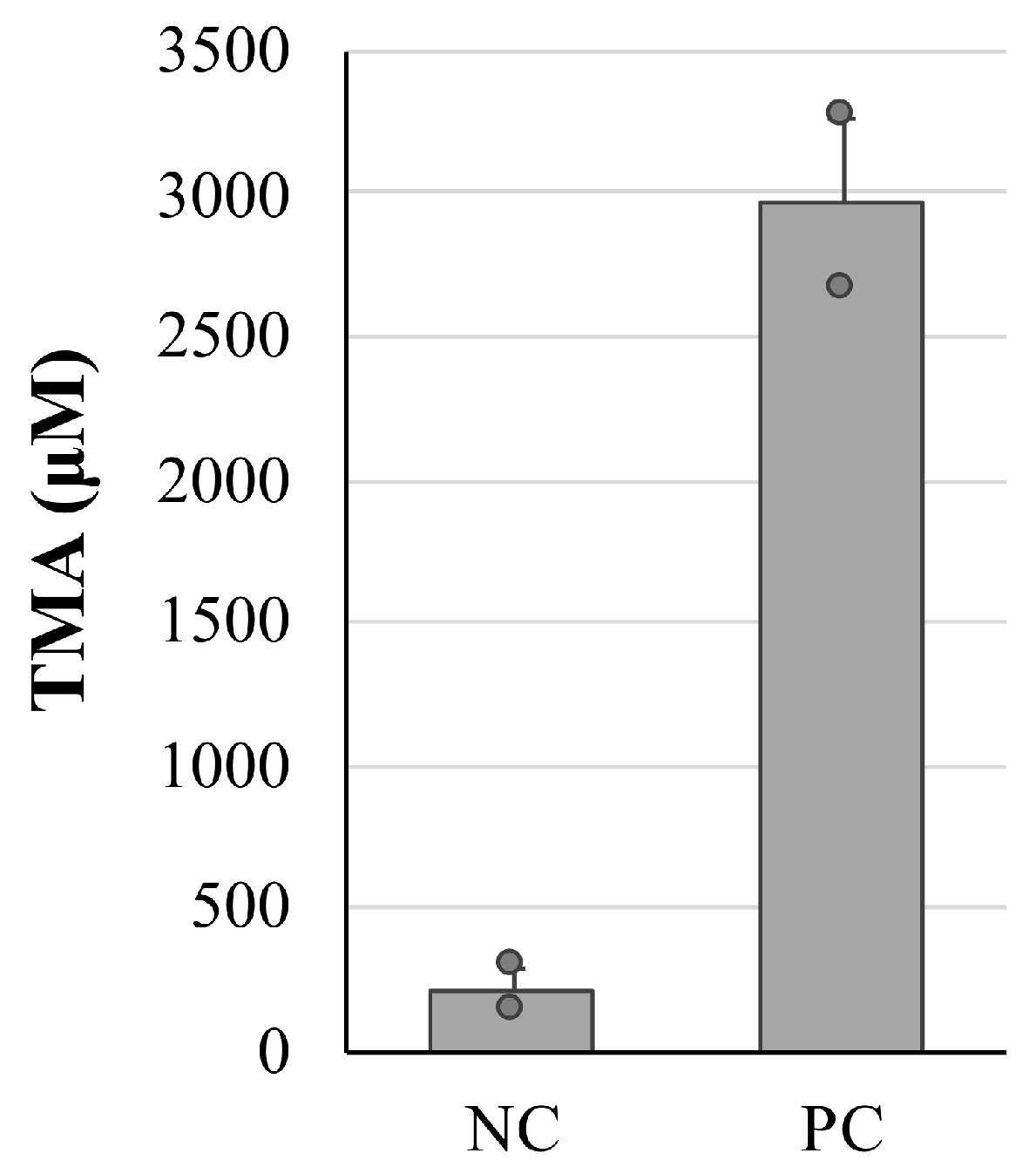
2.3. Establishment of an Alcohol-Poisoning Mouse Model
2.4. In Vitro TMA Production Inhibitory Assay
2.5. Animal Experiments to Evaluate the Impact of the Fermented Stevia Extracts on Alcohol-Poisoning-Model Mice
2.6. Analysis of Cecal Microbiota
3. Results
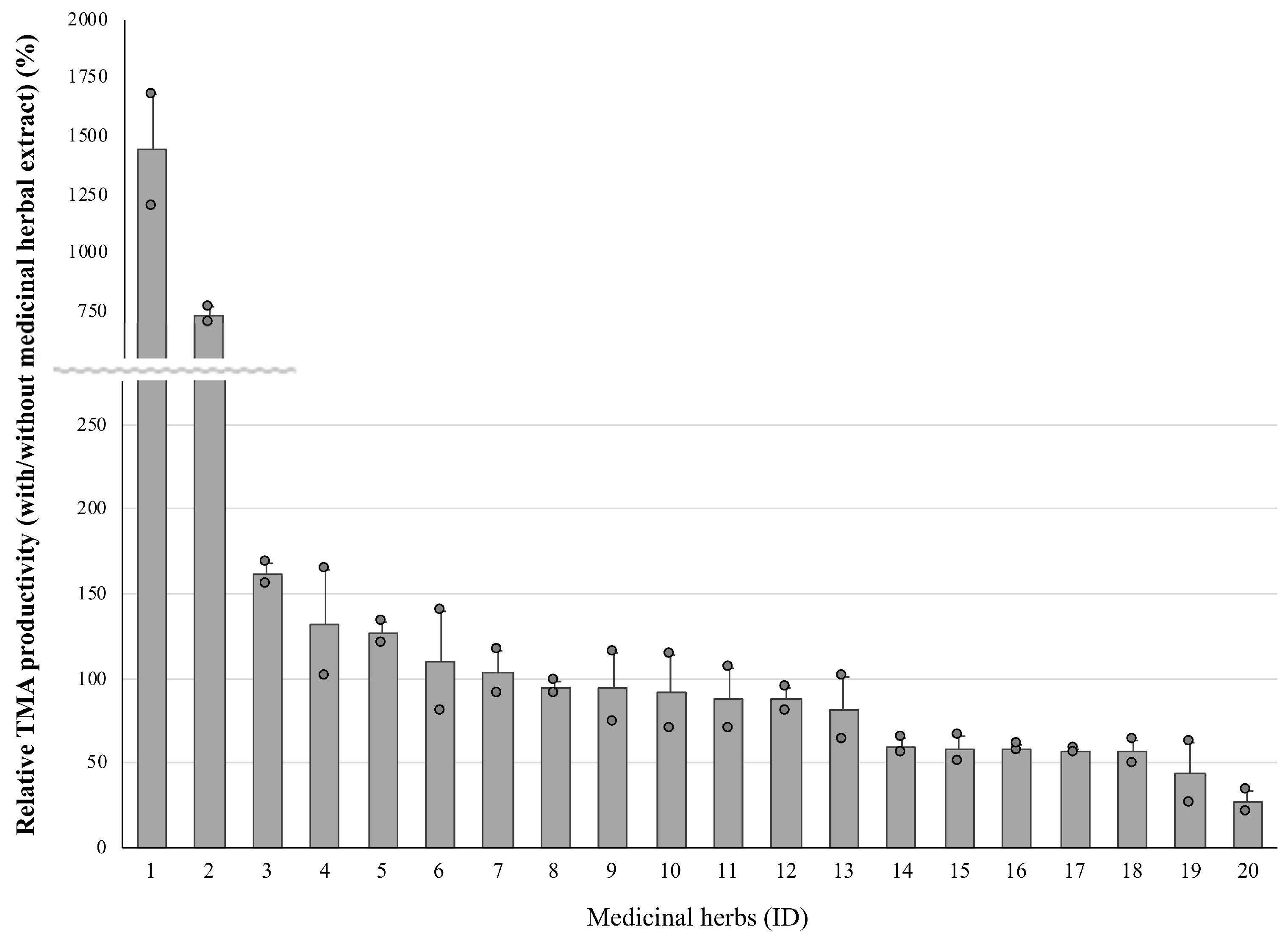
3.1. Effect on the TMA Productivity of the Medicinal Herbal Extract
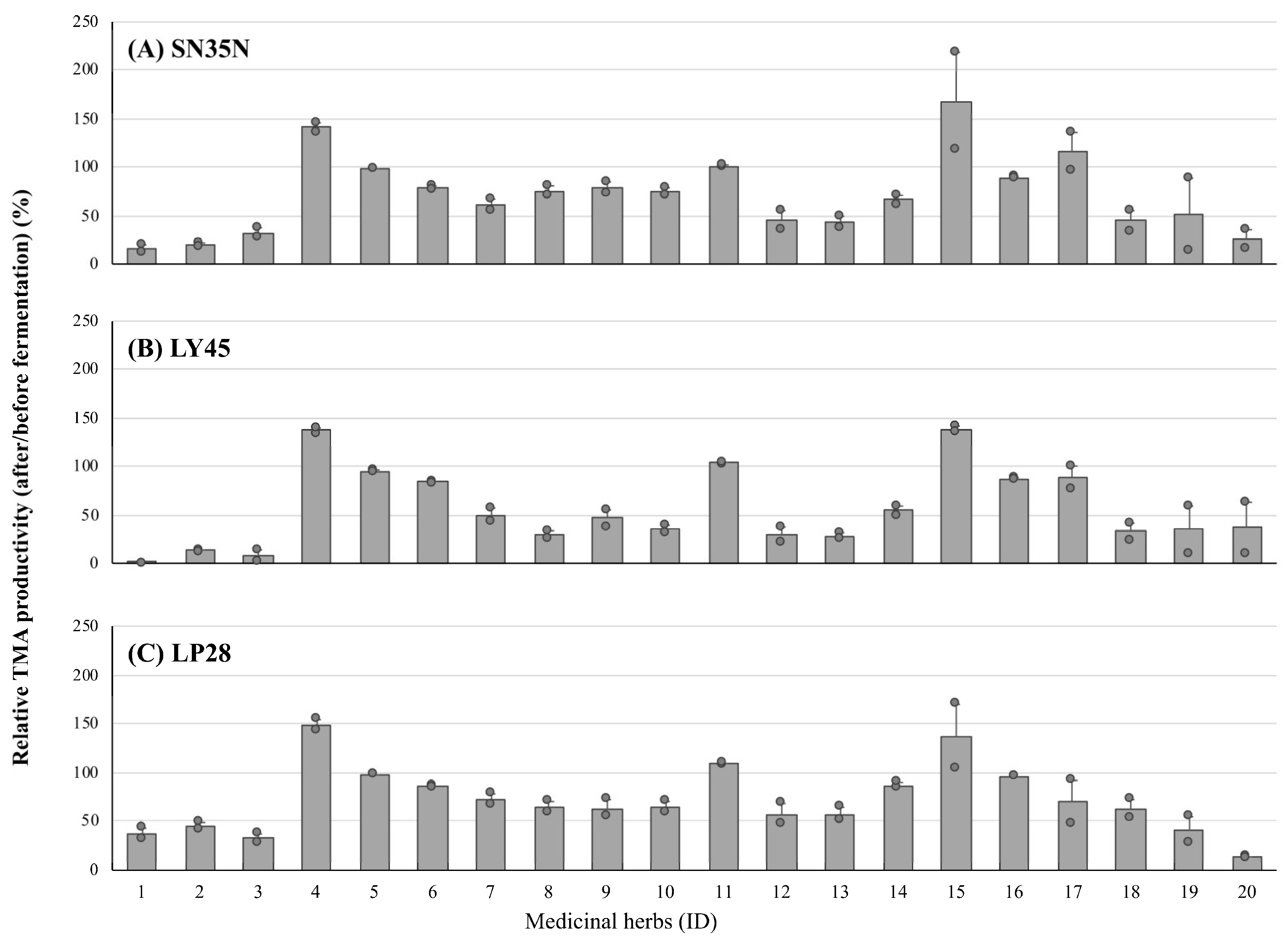
3.2. Inhibitory Activity Alteration of Medicinal Herbal Extract before and after LAB Fermentation
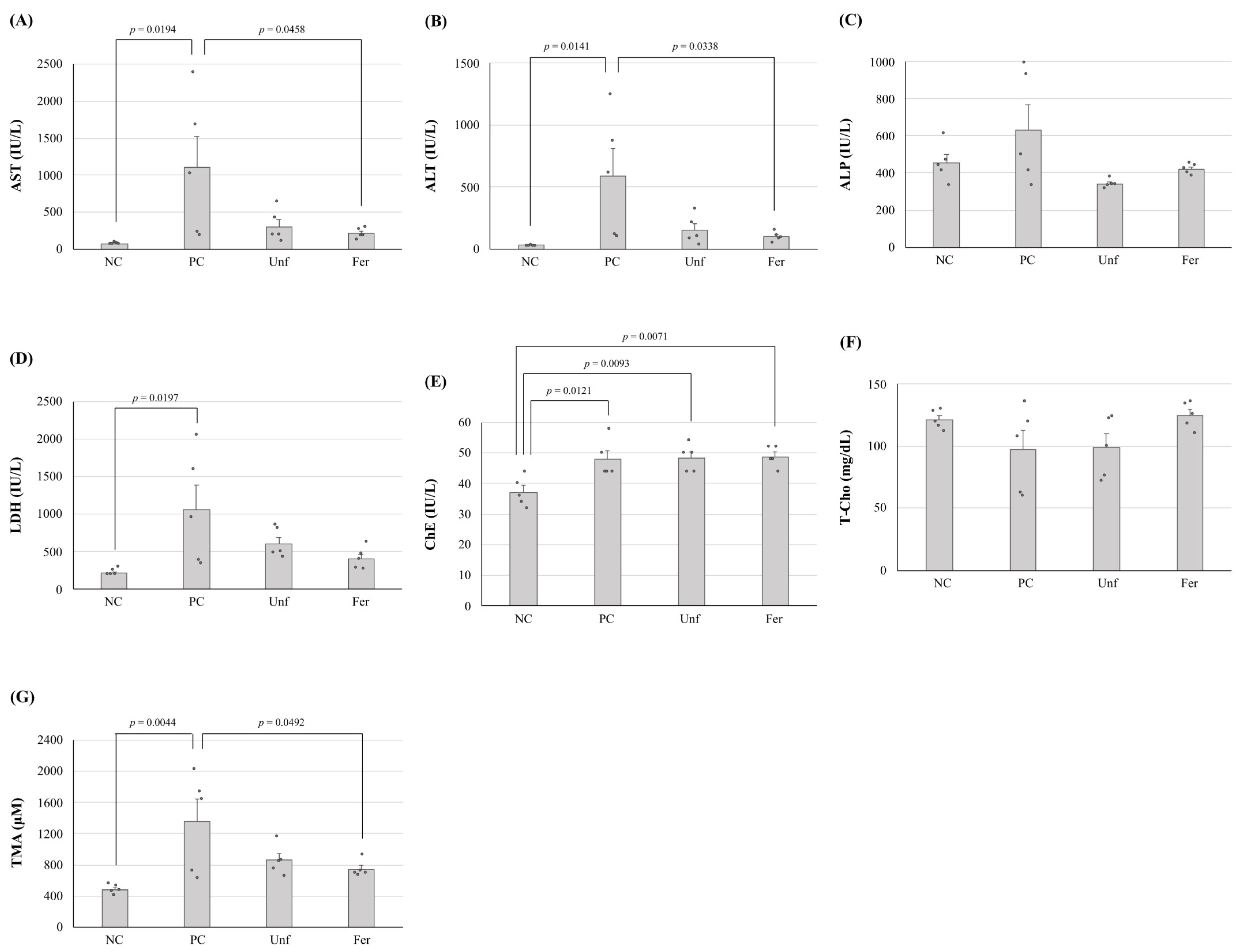
3.3. Improving the Effect of Fermented Stevia Extracts on Alcohol-Poisoning-Model Mice
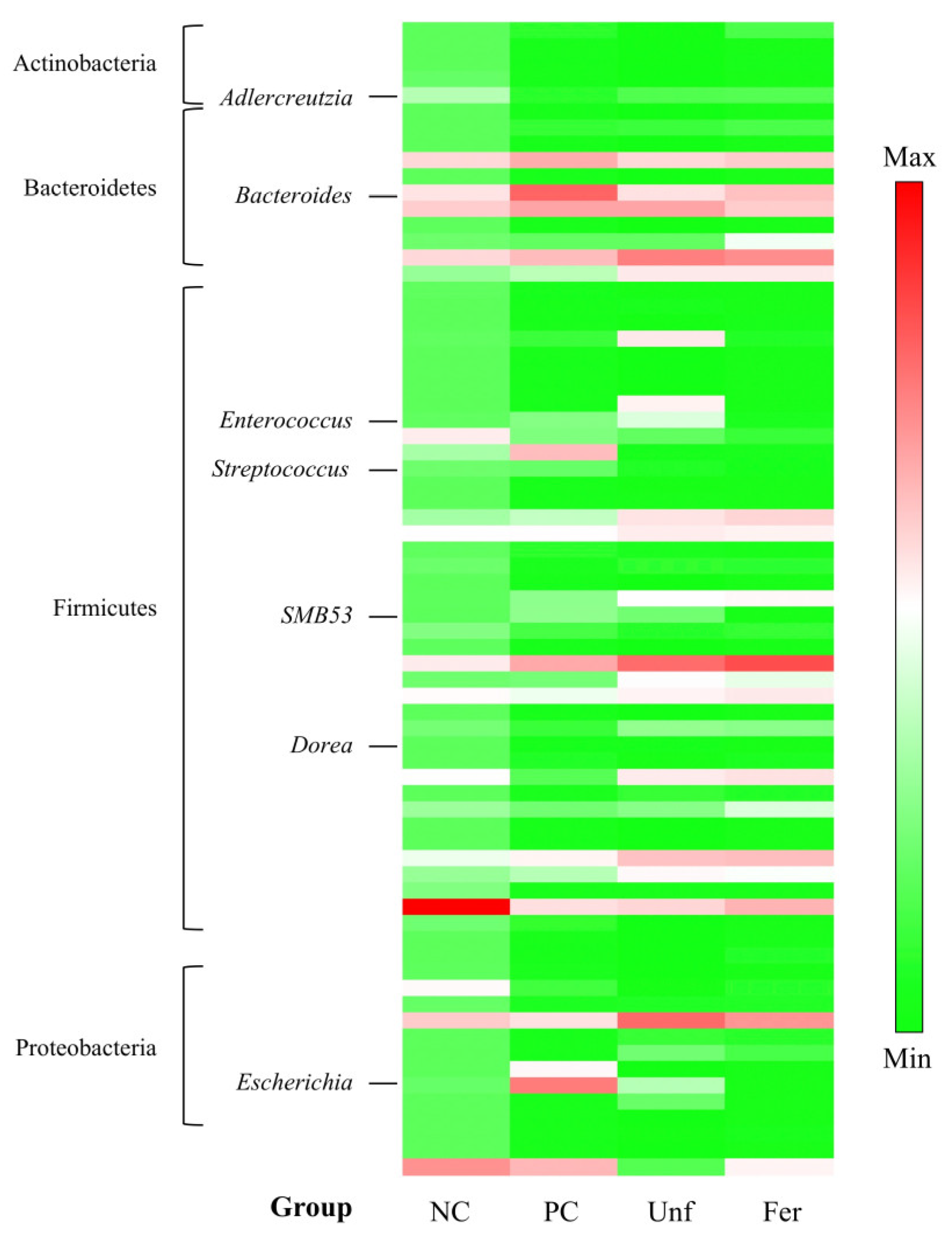
3.4. Alterations of Cecal Microbiota
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Lee, K.H. Research and future trends in the pharmaceutical development of medicinal herbs from Chinese medicine. Public Health Nutr. 2000, 3 (Suppl. 4A), 515–522. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Tyler, V.E. Tyler’s Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies, 4th ed.; The Haworth Herbal Press: New York, NY, USA, 1999. [Google Scholar]
- Lee, N.K.; Paik, H.D. Bioconversion using lactic acid bacteria: Ginsenosides, GABA, and phenolic compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef]
- Tsuchihashi, R.; Kodera, M.; Sakamoto, S.; Nakajima, Y.; Yamazaki, T.; Niiho, Y.; Nohara, T.; Kinjo, J. Microbial transformation and bioactivation of isoflavones from Pueraria flowers by human intestinal bacterial strains. J. Nat. Med. 2009, 63, 254–260. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; Vincentini, O.; Silano, M.; Giuliani, G.; Angelis, M.D.; Gobbetti, M. Synthesis of isoflavone aglycones and equol in soy milks fermented by food-related lactic acid bacteria and their effect on human intestinal Caco-2 cells. J. Agric. Food. Chem. 2010, 58, 10338–10346. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, B.; Amin, N.; van Duijn, C. The role of gut microbiota in neuropsychiatric diseases—Creation of an atlas-based on quantified evidence. Front. Cell. Infect. Microbiol. 2022, 12, 831666. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Noda, M.; Maruyama, M.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Improvement of alcohol-poisoning symptoms in mice by the oral administration of live Lactobacillus plantarum SN13T cells. Int. J. Mol. Sci. 2020, 21, 1896. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.C.; Buffa, J.A.; Org, E.; Wang, Z.; Levison, B.S.; Zhu, W.; Wagner, M.A.; Bennett, B.J.; Li, L.; DiDonato, J.A.; et al. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J. Biol. Chem. 2015, 290, 5647–5660. [Google Scholar] [CrossRef]
- Cho, C.E.; Aardema, N.D.J.; Bunnell, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of choline forms and gut microbiota composition on trimethylamine-N-oxide response in healthy men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef]
- Garcia, E.; Shalaurova, I.; Matyus, S.P.; Wolak-Dinsmore, J.; Oskardmay, D.N.; Connelly, M.A. Quantification of choline in serum and plasma using a clinical nuclear magnetic resonance analyzer. Clin. Chim. Acta 2022, 524, 106–112. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef]
- Phimister, E.G.; Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar]
- Okamoto, T.; Sugimoto, S.; Noda, M.; Yokooji, T.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Interleukin-8 release inhibitors generated by fermentation of Artemisia princeps Pampanini herb extract with Lactobacillus plantarum SN13T. Front. Microbiol. 2020, 11, 1159. [Google Scholar] [CrossRef]
- Noda, M.; Danshiitsoodol, N.; Inoue, Y.; Okamoto, T.; Sultana, N.; Sugiyama, M. Antibiotic susceptibility of plant-derived lactic acid bacteria conferring health benefits to human. J. Antibiot. 2019, 72, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Panthavee, W.; Noda, M.; Danshiitsoodol, N.; Kumagai, T.; Sugiyama, M. Characterization of exopolysaccharides produced by thermophilic lactic acid bacteria isolated from tropical fruits of Thailand. Biol. Pharm. Bull. 2017, 40, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Miyauchi, R.; Danshiitsoodol, N.; Matoba, Y.; Kumagai, T.; Sugiyama, M. Expression of genes involved in bacteriocin production and self-resistance in Lactobacillus brevis 174A is mediated by two regulatory proteins. Appl. Environ. Microbiol. 2018, 84, e02707-17. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Kanno, K.; Danshiitsoodol, N.; Higashikawa, F.; Sugiyama, M. Plant-derived Lactobacillus paracasei IJH-SONE68 improves chronic allergy status: A randomized, double-blind, placebo-controlled clinical trial. Nutrients 2021, 13, 4022. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Sugihara, N.; Sugimoto, Y.; Hayashi, I.; Sugimoto, S.; Danshiitsoodol, N.; Sugiyama, M. Lactobacillus reuteri BM53-1 produces a compound that inhibits sticky glucan synthesis by Streptococcus mutans. Microorganisms 2021, 9, 1390. [Google Scholar] [CrossRef]
- Noda, M.; Danshiitsoodol, N.; Sakaguchi, T.; Kanno, K.; Sugiyama, M. Exopolysaccharide produced by plant-derived Lactobacillus plantarum SN35N exhibits antiviral activity. Biol. Pharm. Bull. 2021, 44, 1886–1890. [Google Scholar] [CrossRef]
- Dyer, W.J. Amines in fish muscle: I. colorimetric determination of trimethylamine as the picrate salt. J. Fish. Res. Bd. Canada 1945, 6, 351–358. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Okauchi, T. On the determination of TMA and TMAO: A modification of the Dyer method. Bull. Jpn. Soc. Sci. Fish 1957, 23, 269–272. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Conn, J.W.; Rovner, D.R.; Cohen, E.L. Licorice-induced pseudoaldosteronism, hypertension, hypokalemia, aldosteronopenia, and suppressed plasma renin activity. JAMA 1968, 205, 492–496. [Google Scholar] [CrossRef]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Corbin, K.D. Choline. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., MacDonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012. [Google Scholar]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Arboleya, S.; Allison, J.; Kaliszewska, A.; Higarza, S.G.; Gueimonde, M.; Arias, J.L. The relationship between choline bioavailability from diet, intestinal microbiota composition, and its modulation of human diseases. Nutrients 2020, 12, 2340. [Google Scholar] [CrossRef] [PubMed]
- Romano, K.A.; Vivas, E.I.; Amador-Noguez, D.; Rey, F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio 2015, 6, e02481. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Bao, Q.; Di, S.; Zhao, Y.; Zhao, S.; Zhang, H.; Lian, F.; Tong, X. The interaction between the gut microbiota and herbal medicines. Biomed. Pharmacother. 2019, 118, 109252. [Google Scholar] [CrossRef]
- Hattori, M. Metabolism of crude drug components by intestinal flora. Bifidus 1991, 5, 27–40. [Google Scholar]
- Hattori, M. Intestinal bacteria play a significant role in the medicinal effects of kampo medicines. J. Intest. Microbiol. 2012, 26, 159–169. [Google Scholar]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Martinson, J.N.V.; Walk, S.T. Escherichia coli residency in the gut of healthy human adults. EcoSal Plus 2020, 9, ESP-003. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The many faces of Enterococcus spp.—Commensal, probiotic and opportunistic pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Marteau, P.; Seksik, P.; Lepage, P.; Dore, J. Cellular and physiological effects of probiotics and prebiotics. Mini-Rev. Med. Chem. 2012, 4, 889–896. [Google Scholar] [CrossRef]
- Bringel, F. Carbamoylphosphate and natural auxotrophies in lactic acid bacteria. Lait 1998, 78, 31–37. [Google Scholar] [CrossRef]
- Li, L.; Chen, B.; Zhu, R.; Li, R.; Tian, Y.; Liu, C.; Jia, Q.; Wang, L.; Tang, J.; Zhao, D.; et al. . Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging 2019, 11, 9348–9368. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Miura, T.; Hirakata, S.; Hosoyama, A.; Sugino, S.; Umeno, A.; Murotomi, K.; Yoshida, Y.; Koike, T. Comparative analysis of the intestinal flora in type 2 diabetes and nondiabetic mice. Exp. Anim. 2017, 66, 405–416. [Google Scholar] [CrossRef]
- Cerreto, M.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Bariatric surgery and liver disease: General considerations and role of the gut-liver axis. Nutrients 2021, 13, 2649. [Google Scholar] [CrossRef]
- Guo, W.; Kim, S.H.; Wu, D.; Li, L.; Ortega, E.F.; Thomas, M.; Meydani, S.N.; Meydani, M. Dietary fruit and vegetable supplementation suppresses diet-induced atherosclerosis in LDL receptor knockout mice. J. Nutr. 2021, 151, 902–910. [Google Scholar] [CrossRef]
- Cassano, M.; Offner, S.; Planet, E.; Piersigilli, A.; Jang, S.M.; Henry, H.; Geuking, M.B.; Mooser, C.; McCoy, K.D.; Macpherson, A.J.; et al. Polyphenic trait promotes liver cancer in a model of epigenetic instability in mice. Hepatology 2017, 66, 235–251. [Google Scholar] [CrossRef]
- Zeybel, M.; Arif, M.; Li, X.; Altay, O.; Yang, H.; Shi, M.; Akyildiz, M.; Saglam, B.; Gonenli, M.G.; Yigit, B.; et al. Multiomics analysis reveals the impact of microbiota on host metabolism in hepatic steatosis. Adv. Sci. 2022, 9, e2104373. [Google Scholar] [CrossRef]
- Loomba, R.; Seguritan, V.; Li, W.; Long, T.; Klitgord, N.; Bhatt, A.; Dulai, P.S.; Caussy, C.; Bettencourt, R.; Highlander, S.K.; et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017, 25, 1054–1062. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.B.; Jun, D.W.; Kang, B.K.; Lim, J.H.; Lim, S.; Chung, M.J. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci. Rep. 2019, 9, 5688. [Google Scholar] [CrossRef] [PubMed]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Hansen, T.; Pedersen, O.; Astrup, A.; Ehrlich, S.D.; Larsen, L.H. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes 2015, 5, e159. [Google Scholar] [CrossRef]
- Lee, G.; You, H.J.; Bajaj, J.S.; Joo, S.K.; Yu, J.; Park, S.; Kang, H.; Park, J.H.; Kim, J.H.; Lee, D.H.; et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat. Commun. 2020, 11, 4982. [Google Scholar] [CrossRef] [PubMed]

| Species | Strain | Cultivation Temperature | Notes | References |
|---|---|---|---|---|
| Enterococcus avium | G-15 | 37 °C | γ-Aminobutyric acid (GABA) production | [22] |
| Enterococcus mundtii | 15-1A | 37 °C | Bacteriocin production | [22] |
| Pediococcus pentosaceus | LP28 | 28 °C | Exopolysaccharide (EPS) production, Anti-obesity | [22] |
| LY45 | 45 °C | Thermophilic, EPS production | [23] | |
| Lactobacillus amylovorus | PY45 | 45 °C | Thermophilic, EPS production | [23] |
| Lactobacillus brevis | 174A | 28 °C | Bacteriocin production | [24] |
| Lactobacillus paracasei | IJH-SONE68 | 28 °C | EPS production, Anti-inflammation | [22,25] |
| Lactobacillus reuteri | BM53-1 | 28 °C | Anti-biofilm | [26] |
| Lactobacillus plantarum | SN13T | 28 °C | Anti-constipation, Improving liver function | [15,22] |
| SN35N | 28 °C | EPS production, Anti-virus infection | [22,27] |
| Phylum | Restore 3 | Class | Restore | Order | Restore | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fer | Unf | Fer | Unf | Fer | Unf | ||||
| Increased 1 | Gammaproteobacteria | † | Enterobacteriales | ||||||
| Bacilli | Bacillales | ||||||||
| Decreased 2 | Actinobacteria | † | † | Erysipelotrichi | Erysipelotrichales | ||||
| Verrucomicrobiae | Burkholderiales | ||||||||
| Betaproteobacteria | Coriobacteriales | † | † | ||||||
| Coriobacteriia | |||||||||
| Deltaproteobacteria | † | † | |||||||
| Family | Restore | Genus | Restore | ||||||
| Fer | Unf | Fer | Unf | ||||||
| Increased 1 | Enterobacteriaceae | † | Escherichia | † | |||||
| Enterococcaceae | † | Bacteroides | † | † | |||||
| Streptococcaceae | Enterococcus | † | |||||||
| Clostridiaceae | Lactococcus | ||||||||
| Staphylococcaceae | Clostridium | ||||||||
| Rikenellaceae | Staphylococcus | ||||||||
| Bacteroidaceae | † | † | SMB53 | † | |||||
| Mogibacteriaceae | † | Streptococcus | † | ||||||
| Dorea | † | ||||||||
| Decreased 2 | Erysipelotrichaceae | Allobaculum | |||||||
| Alcaligenaceae | Sutterella | ||||||||
| Lactobacillaceae | Lactobacillus | ||||||||
| Desulfovibrionaceae | † | † | Adlercreutzia | † | † | ||||
| Coriobacteriaceae | † | † | Anaerofustis | ||||||
| Christensenellaceae | † | † | Prevotella | ||||||
| Eubacteriaceae | |||||||||
| Prevotellaceae | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Noda, M.; Danshiitsoodol, N.; Sugiyama, M. Fermented Stevia Improves Alcohol Poisoning Symptoms Associated with Changes in Mouse Gut Microbiota. Nutrients 2023, 15, 3708. https://doi.org/10.3390/nu15173708
Ma Q, Noda M, Danshiitsoodol N, Sugiyama M. Fermented Stevia Improves Alcohol Poisoning Symptoms Associated with Changes in Mouse Gut Microbiota. Nutrients. 2023; 15(17):3708. https://doi.org/10.3390/nu15173708
Chicago/Turabian StyleMa, Qingmiao, Masafumi Noda, Narandalai Danshiitsoodol, and Masanori Sugiyama. 2023. "Fermented Stevia Improves Alcohol Poisoning Symptoms Associated with Changes in Mouse Gut Microbiota" Nutrients 15, no. 17: 3708. https://doi.org/10.3390/nu15173708
APA StyleMa, Q., Noda, M., Danshiitsoodol, N., & Sugiyama, M. (2023). Fermented Stevia Improves Alcohol Poisoning Symptoms Associated with Changes in Mouse Gut Microbiota. Nutrients, 15(17), 3708. https://doi.org/10.3390/nu15173708







