The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects
Abstract
1. Introduction
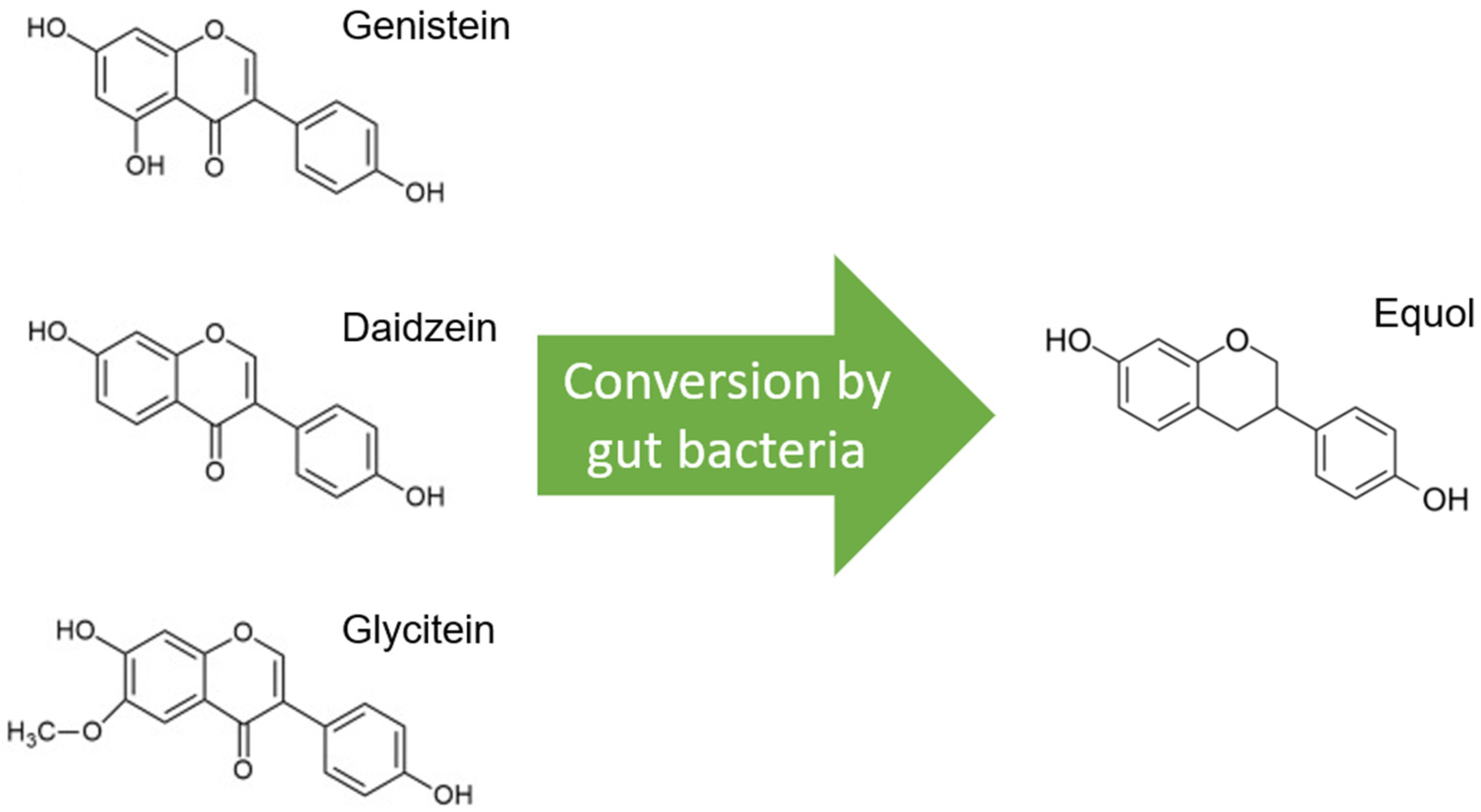
2. Soy—One Word, Different Worlds
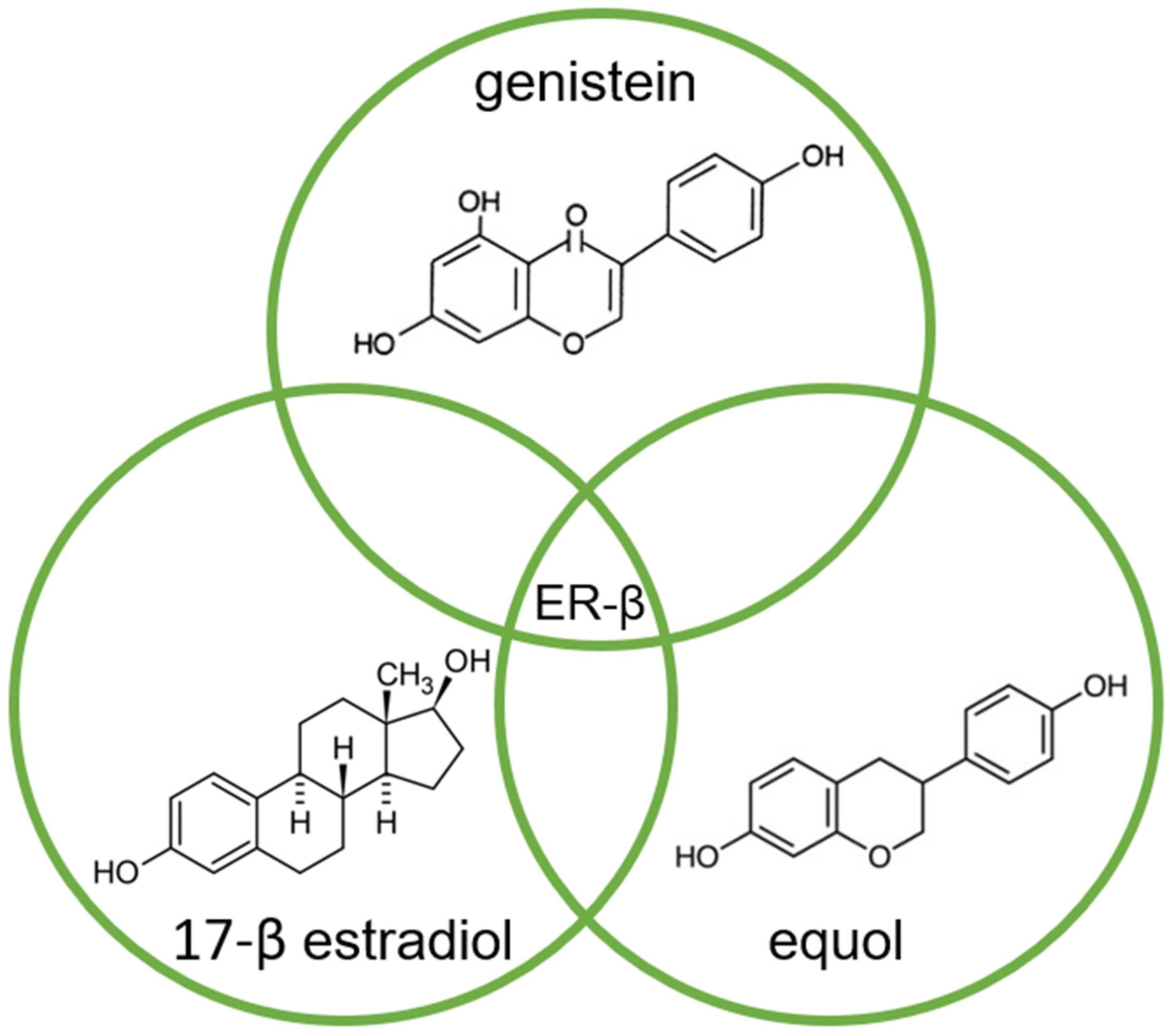
3. The Modification of Androgen- and/or Estrogen-Mediated Carcinogenesis
4. Inhibition of Cancer Cell Growth
5. Effects on Cell Cycle Regulation
6. Angiogenesis
7. Tumor Cell Invasion and Cancer Metastasis
8. Antioxidant Effect
9. Anti-Inflammatory Effect
10. Epigenetics
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marks, L.S.; Kojima, M.; Demarzo, A.; Heber, D.; Bostwick, D.G.; Qian, J.; Dorey, F.J.; Veltri, R.W.; Mohler, J.L.; Partin, A.W. Prostate Cancer in Native Japanese and Japanese-American Men: Effects of Dietary Differences on Prostatic Tissue. Urology 2004, 64, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Ozasa, K.; Nakao, M.; Watanabe, Y.; Hayashi, K.; Miki, T.; Mikami, K.; Mori, M.; Sakauchi, F.; Washio, M.; Ito, Y.; et al. Serum Phytoestrogens and Prostate Cancer Risk in a Nested Case-Control Study among Japanese Men. Cancer Sci. 2004, 95, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, N.; Iwasaki, M.; Inoue, M.; Sasazuki, S.; Tsugane, S. Plasma Isoflavones and Subsequent Risk of Prostate Cancer in a Nested Case-Control Study: The Japan Public Health Center. J. Clin. Oncol. 2008, 26, 5923–5929. [Google Scholar] [CrossRef]
- Perabo, F.G.E.; Von Löw, E.C.; Ellinger, J.; von Rücker, A.; Müller, S.C.; Bastian, P.J. Soy Isoflavone Genistein in Prevention and Treatment of Prostate Cancer. Prostate Cancer Prostatic Dis. 2008, 11, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy Isoflavones and Prostate Cancer: A Review of Molecular Mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Bektic, J.; Guggenberger, R.; Eder, I.E.; Pelzer, A.E.; Berger, A.P.; Bartsch, G.; Klocker, H. Molecular Effects of the Isoflavonoid Genistein in Prostate Cancer. Clin. Prostate Cancer 2005, 4, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Kumar, A.; Sak, K.; Aggarwal, D.; Gupta, D.S.; Kaur, G.; Vashishth, K.; Dhama, K.; Kaur, J.; Saini, A.K.; et al. Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone. Pharmaceuticals 2022, 15, 1418. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding Genistein in Cancer: The “Good” and the “Bad” Effects: A Review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef]
- Trottier, G.; Boström, P.J.; Lawrentschuk, N.; Fleshner, N.E. Nutraceuticals and Prostate Cancer Prevention: A Current Review. Nat. Rev. Urol. 2010, 7, 21–30. [Google Scholar] [CrossRef]
- Barnes, S. The Biochemistry, Chemistry and Physiology of the Isoflavones in Soybeans and Their Food Products. Lymphat. Res. Biol. 2010, 8, 89–98. [Google Scholar] [CrossRef]
- Xiang, T.; Jin, W. Mechanism of Glycitein in the Treatment of Colon Cancer Based on Network Pharmacology and Molecular Docking. Lifestyle Genom. 2023, 16, 1–10. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol—A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Clerici, C.; Lephart, E.D.; Cole, S.J.; Heenan, C.; Castellani, D.; Wolfe, B.E.; Nechemias-Zimmer, L.; Brown, N.M.; Lund, T.D.; et al. S-Equol, a Potent Ligand for Estrogen Receptor β, Is the Exclusive Enantiomeric Form of the Soy Isoflavone Metabolite Produced by Human Intestinal Bacterial Flora1–4. Am. J. Clin. Nutr. 2005, 81, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.H.; Gardner, P.T.; McPhail, D.B.; Morrice, P.C.; Collins, A.R.; Duthie, G.G. Antioxidant Efficacy of Phytoestrogens in Chemical and Biological Model Systems. Arch. Biochem. Biophys. 1998, 360, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Fritz, W.A.; Wang, J.; Eltoum, I.-E.; Lamartiniere, C.A. Dietary Genistein Down-Regulates Androgen and Estrogen Receptor Expression in the Rat Prostate. Mol. Cell Endocrinol. 2002, 186, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Pookot, D.; Noonan, E.J.; Dahiya, R. Genistein Down-Regulates Androgen Receptor by Modulating HDAC6-Hsp90 Chaperone Function. Mol. Cancer Ther. 2008, 7, 3195–3202. [Google Scholar] [CrossRef]
- Sivoňova, M.; Kaplan, P.; Tatarkova, Z.; Lichardusova, L.; Dušenka, R.; Jurečekova, J. Androgen Receptor and Soy Isoflavones in Prostate Cancer (Review). Mol. Clin. Oncol. 2018, 10, 191–204. [Google Scholar] [CrossRef]
- Loutchanwoot, P.; Srivilai, P.; Jarry, H. Lack of Anti-Androgenic Effects of Equol on Reproductive Neuroendocrine Function in the Adult Male Rat. Horm. Behav. 2014, 65, 22–31. [Google Scholar] [CrossRef]
- Oh, H.Y.; Leem, J.; Yoon, S.J.; Yoon, S.; Hong, S.J. Lipid Raft Cholesterol and Genistein Inhibit the Cell Viability of Prostate Cancer Cells via the Partial Contribution of EGFR-Akt/P70S6k Pathway and down-Regulation of Androgen Receptor. Biochem. Biophys. Res. Commun. 2010, 393, 319–324. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Kong, D.; Li, R.; Sarkar, S.H.; Sarkar, F.H. Regulation of Akt/FOXO3a/GSK-3beta/AR Signaling Network by Isoflavone in Prostate Cancer Cells. J. Biol. Chem. 2008, 283, 27707–27716. [Google Scholar] [CrossRef] [PubMed]
- El Touny, L.H.; Banerjee, P.P. Identification of a Biphasic Role for Genistein in the Regulation of Prostate Cancer Growth and Metastasis. Cancer Res. 2009, 69, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Itsumi, M.; Shiota, M.; Takeuchi, A.; Kashiwagi, E.; Inokuchi, J.; Tatsugami, K.; Kajioka, S.; Uchiumi, T.; Naito, S.; Eto, M.; et al. Equol Inhibits Prostate Cancer Growth through Degradation of Androgen Receptor by S-Phase Kinase-Associated Protein 2. Cancer Sci. 2016, 107, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Zhu, T.; Parray, A.; Siddique, H.R.; Yang, W.; Saleem, M.; Bosland, M.C. Differential Effects of Genistein on Prostate Cancer Cells Depend on Mutational Status of the Androgen Receptor. PLoS ONE 2013, 8, e78479. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Agarwal, R. Detrimental Effect of Cancer Preventive Phytochemicals Silymarin, Genistein and Epigallocatechin 3-Gallate on Epigenetic Events in Human Prostate Carcinoma DU145 Cells. Prostate 2001, 46, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dalu, A.; Haskell, J.F.; Coward, L.; Lamartiniere, C.A. Genistein, a Component of Soy, Inhibits the Expression of the EGF and ErbB2/Neu Receptors in the Rat Dorsolateral Prostate. Prostate 1998, 37, 36–43. [Google Scholar] [CrossRef]
- Werner, H.; Le Roith*, D. New Concepts in Regulation and Function of the Insulin-like Growth Factors: Implications for Understanding Normal Growth and Neoplasia. Cell. Mol. Life Sci. 2000, 57, 932–942. [Google Scholar] [CrossRef]
- Takahashi, Y.; Lavigne, J.A.; Hursting, S.D.; Chandramouli, G.V.R.; Perkins, S.N.; Kim, Y.S.; Wang, T.T.Y. Molecular Signatures of Soy-Derived Phytochemicals in Androgen-Responsive Prostate Cancer Cells: A Comparison Study Using DNA Microarray. Mol. Carcinog. 2006, 45, 943–956. [Google Scholar] [CrossRef]
- Rabiau, N.; Kossaï, M.; Braud, M.; Chalabi, N.; Satih, S.; Bignon, Y.-J.; Bernard-Gallon, D.J. Genistein and Daidzein Act on a Panel of Genes Implicated in Cell Cycle and Angiogenesis by Polymerase Chain Reaction Arrays in Human Prostate Cancer Cell Lines. Cancer Epidemiol. 2010, 34, 200–206. [Google Scholar] [CrossRef]
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J.; et al. S-Equol, a Secondary Metabolite of Natural Anticancer Isoflavone Daidzein, Inhibits Prostate Cancer Growth In Vitro and In Vivo, Though Activating the Akt/FOXO3a Pathway. Curr. Cancer Drug Targets 2016, 16, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; DeGroff, V.L.; Clinton, S.K. Tomato and Soy Polyphenols Reduce Insulin-Like Growth Factor-I–Stimulated Rat Prostate Cancer Cell Proliferation and Apoptotic Resistance In Vitro via Inhibition of Intracellular Signaling Pathways Involving Tyrosine Kinase. J. Nutr. 2003, 133, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Liss, M.A.; Schlicht, M.; Kahler, A.; Fitzgerald, R.; Thomassi, T.; Degueme, A.; Hessner, M.; Datta, M.W. Characterization of Soy-Based Changes in Wnt-Frizzled Signaling in Prostate Cancer. Cancer Genom. Proteom. 2010, 7, 245–252. [Google Scholar]
- Lee, J.; Ju, J.; Park, S.; Hong, S.J.; Yoon, S. Inhibition of IGF-1 Signaling by Genistein: Modulation of E-Cadherin Expression and Downregulation of β-Catenin Signaling in Hormone Refractory PC-3 Prostate Cancer Cells. Nutr. Cancer 2012, 64, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Kumi-Diaka, J.; Saddler-Shawnette, S.; Aller, A.; Brown, J. Cancer Cell International Potential Mechanism of Phytochemical-Induced Apoptosis in Human Prostate Adenocarcinoma Cells: Therapeutic Synergy in Genistein and β-Lapachone Combination Treatment. Cancer Cell Int. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Y.; Sarkar, F.H. Gene Expression Profiles of Genistein-Treated PC3 Prostate Cancer Cells. J. Nutr. 2002, 132, 3623–3631. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y. Mechanisms of Cancer Chemoprevention by Soy Isoflavone Genistein. Cancer Metastasis Rev. 2002, 21, 265–280. [Google Scholar] [CrossRef]
- Kazi, A.; Daniel, K.G.; Smith, D.M.; Kumar, N.B.; Dou, Q.P. Inhibition of the Proteasome Activity, a Novel Mechanism Associated with the Tumor Cell Apoptosis-Inducing Ability of Genistein. Biochem. Pharmacol. 2003, 66, 965–976. [Google Scholar] [CrossRef]
- Li, Y.; Sarkar, F.H. Inhibition of Nuclear Factor KappaB Activation in PC3 Cells by Genistein Is Mediated via Akt Signaling Pathway. Clin. Cancer Res. 2002, 8, 2369–2377. [Google Scholar]
- Davis, J.N.; Kucuk, O.; Sarkar, F.H. Genistein Inhibits NF-KB Activation in Prostate Cancer Cells. Nutr. Cancer 1999, 35, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hagler, J.; Palombella, V.J.; Melandri, F.; Scherer, D.; Ballard, D.; Maniatis, T. Signal-Induced Site-Specific Phosphorylation Targets I Kappa B Alpha to the Ubiquitin-Proteasome Pathway. Genes. Dev. 1995, 9, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Parent, L.; Maniatis, T. Site-Specific Phosphorylation of IκBα by a Novel Ubiquitination-Dependent Protein Kinase Activity. Cell 1996, 84, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Tepper, C.G.; Vinall, R.L.; Wee, C.B.; Xue, L.; Shi, X.-B.; Burich, R.; Mack, P.C.; de Vere White, R.W. GCP-Mediated Growth Inhibition and Apoptosis of Prostate Cancer Cells via Androgen Receptor-Dependent and -Independent Mechanisms. Prostate 2007, 67, 521–535. [Google Scholar] [CrossRef] [PubMed]
- OUCHI, H.; ISHIGURO, H.; IKEDA, N.; HORI, M.; KUBOTA, Y.; UEMURA, H. Genistein Induces Cell Growth Inhibition in Prostate Cancer through the Suppression of Telomerase Activity. Int. J. Urol. 2005, 12, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Z.; Hill, D.L.; Chen, X.; Wang, H.; Zhang, R. Genistein, a Dietary Isoflavone, Down-Regulates the MDM2 Oncogene at Both Transcriptional and Posttranslational Levels. Cancer Res. 2005, 65, 8200–8208. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-C.; Klein, R.D.; Wei, Q.; Guan, Y.; Contois, J.H.; Wang, T.T.Y.; Chang, S.; Hursting, S.D. Low-Dose Genistein Induces Cyclin-Dependent Kinase Inhibitors and G1 Cell-Cycle Arrest in Human Prostate Cancer Cells. Mol. Carcinog. 2000, 29, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Singh, B.; Bhuiyan, M.; Sarkar, F.H. Genistein-induced Upregulation of P21WAF1, Downregulation of Cyclin B, and Induction of Apoptosis in Prostate Cancer Cells. Nutr. Cancer 1998, 32, 123–131. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, W.H.; Park, K.-Y.; Zhang, L. P53-Independent Induction of P21 (WAF1/CIP1), Reduction of Cyclin B1 and G2/M Arrest by the Isoflavone Genistein in Human Prostate Carcinoma Cells. Jpn. J. Cancer Res. 2000, 91, 164–173. [Google Scholar] [CrossRef]
- Handayani, R.; Rice, L.; Cui, Y.; Medrano, T.A.; Samedi, V.G.; Baker, H.V.; Szabo, N.J.; Shiverick, K.T. Soy Isoflavones Alter Expression of Genes Associated with Cancer Progression, Including Interleukin-8, in Androgen-Independent PC-3 Human Prostate Cancer Cells. J. Nutr. 2006, 136, 75–82. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Hoot, D.R.; Clinton, S.K. Suppression of VEGF-Mediated Autocrine and Paracrine Interactions between Prostate Cancer Cells and Vascular Endothelial Cells by Soy Isoflavones. J. Nutr. Biochem. 2007, 18, 408–417. [Google Scholar] [CrossRef]
- Ambra, R.; Rimbach, G.; de Pascual Teresa, S.; Fuchs, D.; Wenzel, U.; Daniel, H.; Virgili, F. Genistein Affects the Expression of Genes Involved in Blood Pressure Regulation and Angiogenesis in Primary Human Endothelial Cells. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Xu, L.; Liu, Y.; Deb, D.K.; Platanias, L.C.; Bergan, R.C. Genistein Inhibits P38 Map Kinase Activation, Matrix Metalloproteinase Type 2, and Cell Invasion in Human Prostate Epithelial Cells. Cancer Res. 2005, 65, 3470–3478. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Ma, D.; Shi, Y.; Liu, C.; Wang, P. (±)Equol Inhibits Invasion in Prostate Cancer DU145 Cells Possibly via down-Regulation of Matrix Metalloproteinase-9, Matrix Metalloproteinase-2 and Urokinase-Type Plasminogen Activator by Antioxidant Activity. J. Clin. Biochem. Nutr. 2012, 51, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, Y.; Ma, D.; Li, G.; Wang, P. [Anti-Invasion Effects of R- and S-Enantiomers of Equol on Prostate Cancer PC3, DU145 Cells]. Wei Sheng Yan Jiu 2011, 40, 423–425, 430. [Google Scholar] [PubMed]
- Lakshman, M.; Xu, L.; Ananthanarayanan, V.; Cooper, J.; Takimoto, C.H.; Helenowski, I.; Pelling, J.C.; Bergan, R.C. Dietary Genistein Inhibits Metastasis of Human Prostate Cancer in Mice. Cancer Res. 2008, 68, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bergan, R.C. Genistein Inhibits Matrix Metalloproteinase Type 2 Activation and Prostate Cancer Cell Invasion by Blocking the Transforming Growth Factor β-Mediated Activation of Mitogen-Activated Protein Kinase-Activated Protein Kinase 2-27-KDa Heat Shock Protein Pathway. Mol. Pharmacol. 2006, 70, 869–877. [Google Scholar] [CrossRef]
- Bellou, S.; Karali, E.; Bagli, E.; Al-Maharik, N.; Morbidelli, L.; Ziche, M.; Adlercreutz, H.; Murphy, C.; Fotsis, T. The Isoflavone Metabolite 6-Methoxyequol Inhibits Angiogenesis and Suppresses Tumor Growth. Mol. Cancer 2012, 11, 35. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Rogozin, E.A.; Cho, Y.-Y.; Heo, Y.-S.; Bode, A.M.; Lee, H.J.; Dong, Z. Equol, a Metabolite of the Soybean Isoflavone Daidzein, Inhibits Neoplastic Cell Transformation by Targeting the MEK/ERK/P90RSK/Activator Protein-1 Pathway. J. Biol. Chem. 2007, 282, 32856–32866. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.B.; Peehl, D.M.; Feldman, D. Calcitriol and Genistein Actions to Inhibit the Prostaglandin Pathway: Potential Combination Therapy to Treat Prostate Cancer. J. Nutr. 2007, 137, 205S–210S. [Google Scholar] [CrossRef] [PubMed]
- ZHANG, L.; LI, L.; WU, D.; FAN, J.; LI, X.; WU, K.; WANG, X.; HE, D. A Novel Anti-Cancer Effect of Genistein: Reversal of Epithelial Mesenchymal Transition in Prostate Cancer Cells 1. Acta Pharmacol. Sin. 2008, 29, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gu, Y.; Hui, K.; Huang, J.; Xu, S.; Wu, S.; Li, L.; Fan, J.; Wang, X.; Hsieh, J.-T.; et al. AKR1C3, a Crucial Androgenic Enzyme in Prostate Cancer, Promotes Epithelial-Mesenchymal Transition and Metastasis through Activating ERK Signaling. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 472.e11–472.e20. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yan, J.; Li, Y.; Cheng, J.; Zheng, L.; Fu, T.; Zhu, Y. Inhibition of Castration-Resistant Prostate Cancer Growth by Genistein through Suppression of AKR1C3. Food Nutr. Res. 2023, 67. [Google Scholar] [CrossRef] [PubMed]
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.A.; Greenberg, N.M.; Elgavish, A. Dietary Genistein Improves Survival and Reduces Expression of Osteopontin in the Prostate of Transgenic Mice with Prostatic Adenocarcinoma (TRAMP). J. Nutr. 2005, 135, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Foti, P.; Erba, D.; Riso, P.; Spadafranca, A.; Criscuoli, F.; Testolin, G. Comparison between Daidzein and Genistein Antioxidant Activity in Primary and Cancer Lymphocytes. Arch. Biochem. Biophys. 2005, 433, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Koike, H.; Matsui, H.; Ono, Y.; Hasumi, M.; Nakazato, H.; Okugi, H.; Sekine, Y.; Oki, K.; Ito, K.; et al. Genistein, a Soy Isoflavone, Induces Glutathione Peroxidase in the Human Prostate Cancer Cell Lines LNCaP and PC-3. Int. J. Cancer 2002, 99, 846–852. [Google Scholar] [CrossRef]
- Raschke, M.; Rowland, I.R.; Magee, P.J.; Pool-Zobel, B.L. Genistein Protects Prostate Cells against Hydrogen Peroxide-Induced DNA Damage and Induces Expression of Genes Involved in the Defence against Oxidative Stress. Carcinogenesis 2006, 27, 2322–2330. [Google Scholar] [CrossRef]
- Alfa, H.H.; Arroo, R.R.J. Over 3 Decades of Research on Dietary Flavonoid Antioxidants and Cancer Prevention: What Have We Achieved? Phytochem. Rev. 2019, 18, 989–1004. [Google Scholar] [CrossRef]
- Park, C.E.; Yun, H.; Lee, E.-B.; Min, B.-I.; Bae, H.; Choe, W.; Kang, I.; Kim, S.-S.; Ha, J. The Antioxidant Effects of Genistein Are Associated with AMP-Activated Protein Kinase Activation and PTEN Induction in Prostate Cancer Cells. J. Med. Food 2010, 13, 815–820. [Google Scholar] [CrossRef]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.-B.; Lee, K.; Park, S.-K.; Kim, H.M. Equol Inhibits Nitric Oxide Production and Inducible Nitric Oxide Synthase Gene Expression through Down-Regulating the Activation of Akt. Int. Immunopharmacol. 2007, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.-B.; Lee, K.; Kang, M.R.; Moon, E.-Y.; Jeon, Y.J.; Park, S.-K.; Kim, H.M. Estrogen Receptor-Independent Inhibition of Tumor Necrosis Factor-α Gene Expression by Phytoestrogen Equol Is Mediated by Blocking Nuclear Factor-ΚB Activation in Mouse Macrophages. Biochem. Pharmacol. 2005, 71, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, Y.; Shima, I.; Stearns, M.E. IL-10/IL-10 Receptor Signaling Regulates TIMP-1 Expression. Cancer Biol. Ther. 2002, 1, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Bladé, C.; Arola, L.; Salvadó, J. Isoflavone Effect on Gene Expression Profile and Biomarkers of Inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Adjakly, M.; Bosviel, R.; Rabiau, N.; Boiteux, J.-P.; Bignon, Y.-J.; Guy, L.; Bernard-Gallon, D. DNA Methylation and Soy Phytoestrogens: Quantitative Study in DU-145 and PC-3 Human Prostate Cancer Cell Lines. Epigenomics 2011, 3, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.-P.; Fontana, L.; Bignon, Y.-J.; Guy, L.; et al. Soy Phytoestrogens Modify DNA Methylation of GSTP1, RASSF1A, EPH2 and BRCA1 Promoter in Prostate Cancer Cells. In Vivo 2010, 24, 393–400. [Google Scholar] [PubMed]
- Fang, M.Z.; Chen, D.; Sun, Y.; Jin, Z.; Christman, J.K.; Yang, C.S. Reversal of Hypermethylation and Reactivation of P16INK4a, RARβ, and MGMT Genes by Genistein and Other Isoflavones from Soy. Clin. Cancer Res. 2005, 11, 7033–7041. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein Reverses Hypermethylation and Induces Active Histone Modifications in Tumor Suppressor Gene B-Cell Translocation Gene 3 in Prostate Cancer. Cancer 2010, 116, 66–76. [Google Scholar] [CrossRef]
- Rabiau, N.; Trraf, H.-K.; Adjakly, M.; Bosviel, R.; Guy, L.; Fontana, L.; Bignon, Y.-J.; Bernard-Gallon, D.J. MiRNAs Differentially Expressed in Prostate Cancer Cell Lines after Soy Treatment. In Vivo 2011, 25, 917–921. [Google Scholar]
- Li, Y.; Kong, D.; Ahmad, A.; Bao, B.; Dyson, G.; Sarkar, F.H. Epigenetic Deregulation of MiR-29a and MiR-1256 by Isoflavone Contributes to the Inhibition of Prostate Cancer Cell Growth and Invasion. Epigenetics 2012, 7, 940–949. [Google Scholar] [CrossRef]
- Murata, M.; Marugame, Y.; Yamada, S.; Lin, I.; Yamashita, S.; Fujimura, Y.; Tachibana, H. Circulating MiRNA Profiles in Mice Plasma Following Flavonoid Intake. Mol. Biol. Rep. 2022, 49, 10399–10407. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Lin, I.; Oka, C.; Kumazoe, M.; Komatsu, S.; Murata, M.; Kamachi, S.; Tachibana, H. Soy Isoflavone Metabolite Equol Inhibits Cancer Cell Proliferation in a PAP Associated Domain Containing 5-Dependent and an Estrogen Receptor-Independent Manner. J. Nutr. Biochem. 2022, 100, 108910. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H. Estrogen Receptor Signaling in Prostate Cancer: Implications for Carcinogenesis and Tumor Progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Bektic, J.; Berger, A.P.; Pfeil, K.; Dobler, G.; Bartsch, G.; Klocker, H. Androgen Receptor Regulation by Physiological Concentrations of the Isoflavonoid Genistein in Androgen-Dependent LNCaP Cells Is Mediated by Estrogen Receptor β. Eur. Urol. 2004, 45, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Lavigne, J.A.; Hursting, S.D.; Chandramouli, G.V.R.; Perkins, S.N.; Barrett, J.C.; Wang, T.T.Y. Using DNA Microarray Analyses to Elucidate the Effects of Genistein in Androgen-Responsive Prostate Cancer Cells: Identification of Novel Targets. Mol. Carcinog. 2004, 41, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.T.; Young, C.Y.-F.; Bilhartz, D.L.; Andrews, P.E.; Thompson, N.F.; Tindall, D.J.; Prescott, J.L. Hormonal Regulation of Prostate-Specific Antigen (PSA) Glycoprotein in the Human Prostatic Adenocarcinoma Cell Line, LNCaP. Prostate 1992, 21, 63–73. [Google Scholar] [CrossRef]
- Davis, J.N.; Muqim, N.; Bhuiyan, M.; Kucuk, O.; Pienta, K.J.; Sarkar, F.H. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int. J. Oncol. 2000, 16, 1091–1098. [Google Scholar] [CrossRef]
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.E.; Greenberg, N.M.; Elgavish, A. Genistein in the Diet Reduces the Incidence of Poorly Differentiated Prostatic Adenocarcinoma in Transgenic Mice (TRAMP). Cancer Res. 2001, 61, 6777–6782. [Google Scholar]
- Pollard, M.; Wolter, W. Prevention of Spontaneous Prostate-Related Cancer in Lobund-Wistar Rats by a Soy Protein Isolate/Isoflavone Diet. Prostate 2000, 45, 101–105. [Google Scholar] [CrossRef]
- Peterson, G.; Barnes, S. Genistein and Biochanin A Inhibit the Growth of Human Prostate Cancer Cells but Not Epidermal Growth Factor Receptor Tyrosine Autophosphorylation. Prostate 1993, 22, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.D.; Munson, D.J.; Haldy, M.E.; Setchell, K.D.R.; Lephart, E.D.; Handa, R.J. Equol Is a Novel Anti-Androgen That Inhibits Prostate Growth and Hormone Feedback. Biol. Reprod. 2004, 70, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Sionit, L.; Partido, C.; Li, L.; Tan, X.; Youngkin, T.; Nachtsheim, D.; Hoffman, R.M. Genistein Inhibits the Growth of Human-Patient BPH and Prostate Cancer in Histoculture. Prostate 1998, 34, 75–79. [Google Scholar] [CrossRef]
- Hempstock; Kavanagh; George. Growth Inhibition of Prostate Cell Lines in Vitro by Phyto-Oestrogens. BJU Int. 1998, 82, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Shenouda, N.S.; Zhou, C.; Browning, J.D.; Ansell, P.J.; Sakla, M.S.; Lubahn, D.B.; MacDonald, R.S. Phytoestrogens in Common Herbs Regulate Prostate Cancer Cell Growth in Vitro. Nutr. Cancer 2004, 49, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Onozawa, M.; Fukuda, K.; Ohtani, M.; Akaza, H.; Sugimura, T.; Wakabayashi, K. Effects of Soybean Isoflavones on Cell Growth and Apoptosis of the Human Prostatic Cancer Cell Line LNCaP. Jpn. J. Clin. Oncol. 1998, 28, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef]
- Graff, J.R.; Konicek, B.W.; McNulty, A.M.; Wang, Z.; Houck, K.; Allen, S.; Paul, J.D.; Hbaiu, A.; Goode, R.G.; Sandusky, G.E.; et al. Increased AKT Activity Contributes to Prostate Cancer Progression by Dramatically Accelerating Prostate Tumor Growth and Diminishing P27Kip1 Expression. J. Biol. Chem. 2000, 275, 24500–24505. [Google Scholar] [CrossRef]
- Uzgare, A.R.; Isaacs, J.T. Enhanced Redundancy in Akt and Mitogen-Activated Protein Kinase-Induced Survival of Malignant versus Normal Prostate Epithelial Cells. Cancer Res. 2004, 64, 6190–6199. [Google Scholar] [CrossRef]
- Renehan, A.G.; Zwahlen, M.; Minder, C.; O’Dwyer, S.T.; Shalet, S.M.; Egger, M. Insulin-like Growth Factor (IGF)-I, IGF Binding Protein-3, and Cancer Risk: Systematic Review and Meta-Regression Analysis. Lancet 2004, 363, 1346–1353. [Google Scholar] [CrossRef]
- Ozkan, E.E. Plasma and Tissue Insulin-like Growth Factor-I Receptor (IGF-IR) as a Prognostic Marker for Prostate Cancer and Anti-IGF-IR Agents as Novel Therapeutic Strategy for Refractory Cases: A Review. Mol. Cell Endocrinol. 2011, 344, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gennigens, C.; Menetrier-Caux, C.; Droz, J.P. Insulin-Like Growth Factor (IGF) Family and Prostate Cancer. Crit. Rev. Oncol. Hematol. 2006, 58, 124–145. [Google Scholar] [CrossRef] [PubMed]
- Burgering, B.M.T. A Brief Introduction to FOXOlogy. Oncogene 2008, 27, 2258–2262. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Srivastava, R.K.; Shankar, S. Inhibition of PI3K/AKT and MAPK/ERK Pathways Causes Activation of FOXO Transcription Factor, Leading to Cell Cycle Arrest and Apoptosis in Pancreatic Cancer. J. Mol. Signal 2010, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Traenckner, E.B.; Pahl, H.L.; Henkel, T.; Schmidt, K.N.; Wilk, S.; Baeuerle, P.A. Phosphorylation of Human I Kappa B-Alpha on Serines 32 and 36 Controls I Kappa B-Alpha Proteolysis and NF-Kappa B Activation in Response to Diverse Stimuli. EMBO J. 1995, 14, 2876–2883. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.P.; Shapiro, V.S.; Stokoe, D.; Weiss, A. Induction of NF-ΚB by the Akt/PKB Kinase. Curr. Biol. 1999, 9, 601-S1. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.; Milner, J. Diet, Autophagy, and Cancer: A Review. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-MTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Mol. Cell Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Agarwal, R. Cell Signaling and Regulators of Cell Cycle as Molecular Targets for Prostate Cancer Prevention by Dietary Agents. Biochem. Pharmacol. 2000, 60, 1051–1059. [Google Scholar] [CrossRef]
- Oki, T.; Sowa, Y.; Hirose, T.; Takagaki, N.; Horinaka, M.; Nakanishi, R.; Yasuda, C.; Yoshida, T.; Kanazawa, M.; Satomi, Y.; et al. Genistein Induces Gadd45 Gene and G2/M Cell Cycle Arrest in the DU145 Human Prostate Cancer Cell Line. FEBS Lett. 2004, 577, 55–59. [Google Scholar] [CrossRef]
- Wang, B.F.; Wang, J.S.; Lu, J.F.; Kao, T.H.; Chen, B.H. Antiproliferation Effect and Mechanism of Prostate Cancer Cell Lines as Affected by Isoflavones from Soybean Cake. J. Agric. Food Chem. 2009, 57, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the Angiogenic Switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Diamond, E.J.; Mamkine, B.; Stone, N.N.; Stock, R.G. Serum Interleukin-8 Is Elevated in Men with Prostate Cancer and Bone Metastases. Technol. Cancer Res. Treat. 2004, 3, 411. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Troncoso, P.; Johnston, D.; Bucana, C.D.; Dinney, C.; Dong, Z.; Fidler, I.J.; Pettaway, C.A. Expression of Interleukin-8 Gene in Radical Prostatectomy Specimens Is Associated with Advanced Pathologic Stage. Prostate 2005, 64, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Slaton, J.W.; Eve, B.Y.; Kim, S.J.; Perrotte, P.; Balbay, M.D.; Yano, S.; Bar-Eli, M.; Radinsky, R.; Pettaway, C.A.; et al. Interleukin 8 Expression Regulates Tumorigenicity and Metastases in Androgen-Independent Prostate Cancer. Clin. Cancer Res. 2000, 6, 2104–2119. [Google Scholar] [PubMed]
- Yu, X.; Zhu, J.; Mi, M.; Chen, W.; Pan, Q.; Wei, M. Anti-Angiogenic Genistein Inhibits VEGF-Induced Endothelial Cell Activation by Decreasing PTK Activity and MAPK Activation. Med. Oncol. 2012, 29, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Pu, H.; Kyprianou, N. Targeting TGF-β in Prostate Cancer: Therapeutic Possibilities during Tumor Progression. Expert. Opin. Ther. Targets 2009, 13, 227–234. [Google Scholar] [CrossRef]
- Klein, C.A. The Metastasis Cascade. Science (1979) 2008, 321, 1785–1787. [Google Scholar] [CrossRef]
- Kumi-Diaka, J.K.; Hassanhi, M.; Merchant, K.; Horman, V. Influence of Genistein Isoflavone on Matrix Metalloproteinase-2 Expression in Prostate Cancer Cells. J. Med. Food 2006, 9, 491–497. [Google Scholar] [CrossRef]
- Li, Y.; Kucuk, O.; Hussain, M.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Antitumor and Antimetastatic Activities of Docetaxel Are Enhanced by Genistein through Regulation of Osteoprotegerin/Receptor Activator of Nuclear Factor-ΚB (RANK)/RANK Ligand/MMP-9 Signaling in Prostate Cancer. Cancer Res. 2006, 66, 4816–4825. [Google Scholar] [CrossRef]
- Liu, Y.; Kyle, E.; Lieberman, R.; Crowell, J.; Kelloff, G.; Bergan, R.C. Focal Adhesion Kinase (FAK) Phosphorylation Is Not Required for Genistein-Induced FAK-β-1-Integrin Complex Formation. Clin. Exp. Metastasis 2000, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Figel, S.; H Gelman, I. Focal Adhesion Kinase Controls Prostate Cancer Progression Via Intrinsic Kinase and Scaffolding Functions. Anticancer Agents Med. Chem. 2011, 11, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, S.; Bergan, R.C. MAPKAPK2 and HSP27 Are Downstream Effectors of P38 MAP Kinase-Mediated Matrix Metalloproteinase Type 2 Activation and Cell Invasion in Human Prostate Cancer. Oncogene 2006, 25, 2987–2998. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.F. The Role of Prostaglandin Synthesis in Prostate Cancer. BJU Int. 2000, 85, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Gupta, S.; Mukhtar, H. Cyclooxygenase-2 and Prostate Carcinogenesis. Cancer Lett. 2003, 191, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Koki, A.T.; Masferrer, J.L. Celecoxib: A Specific COX-2 Inhibitor with Anticancer Properties. Cancer Control 2002, 9, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Aparicio Gallego, G.; Díaz Prado, S.; Jiménez Fonseca, P.; García Campelo, R.; Cassinello Espinosa, J.; Antón Aparicio, L.M. Cyclooxygenase-2 (COX-2): A Molecular Target in Prostate Cancer. Clin. Transl. Oncol. 2007, 9, 694–702. [Google Scholar] [CrossRef]
- Fosslien, E. Review: Molecular Pathology of Cyclooxygenase-2 in Cancer-Induced Angiogenesis. Ann. Clin. Lab. Sci. 2001, 31, 325–348. [Google Scholar]
- Swami, S.; Krishnan, A.V.; Moreno, J.; Bhattacharyya, R.S.; Gardner, C.; Brooks, J.D.; Peehl, D.M.; Feldman, D. Inhibition of Prostaglandin Synthesis and Actions by Genistein in Human Prostate Cancer Cells and by Soy Isoflavones in Prostate Cancer Patients. Int. J. Cancer 2009, 124, 2050–2059. [Google Scholar] [CrossRef]
- Thalmann, G.N.; Sikes, R.A.; Devoll, R.E.; Kiefer, J.A.; Markwalder, R.; Klima, I.; Farach-Carson, C.M.; Studer, U.E.; Chung, L.W. Osteopontin: Possible Role in Prostate Cancer Progression. Clin. Cancer Res. 1999, 5, 2271–2277. [Google Scholar]
- Jain, S.; Chakraborty, G.; Kundu, G.C. The Crucial Role of Cyclooxygenase-2 in Osteopontin-Induced Protein Kinase C α/c-Src/IκB Kinase α/β–Dependent Prostate Tumor Progression and Angiogenesis. Cancer Res. 2006, 66, 6638–6648. [Google Scholar] [CrossRef]
- Bouchal, J.; Santer, F.R.; Höschele, P.P.S.; Tomastikova, E.; Neuwirt, H.; Culig, Z. Transcriptional Coactivators P300 and CBP Stimulate Estrogen Receptor-Beta Signaling and Regulate Cellular Events in Prostate Cancer. Prostate 2011, 71, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Miękus, K.; Madeja, Z. Genistein Inhibits the Contact-Stimulated Migration of Prostate Cancer Cells. Cell Mol. Biol. Lett. 2007, 12, 348–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, J.-R.; Yu, L.; Zhong, Y.; Nassr, R.L.; Franke, A.A.; Gaston, S.M.; Blackburn, G.L. Inhibition of Orthotopic Growth and Metastasis of Androgen-Sensitive Human Prostate Tumors in Mice by Bioactive Soybean Components. Prostate 2002, 53, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.E.; Cook, L.M.; Patel, B.B.; Wang, J.; Eltoum, I.A.; Arabshahi, A.; Shirai, T.; Lamartiniere, C.A. Genistein and Resveratrol, Alone and in Combination, Suppress Prostate Cancer in SV-40 Tag Rats. Prostate 2009, 69, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Joseph, I.B.J.K.; Isaacs, J.T. Macrophage Role in the Anti-Prostate Cancer Response to One Class of Antiangiogenic Agents. JNCI J. Natl. Cancer Inst. 1998, 90, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Hidaka, H.; Majid, S.; Saini, S.; Arora, S.; Deng, G.; Shahryari, V.; Chang, I.; et al. Genistein Up-Regulates Tumor Suppressor MicroRNA-574-3p in Prostate Cancer. PLoS ONE 2013, 8, e58929. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A.; et al. Genistein as a Regulator of Signaling Pathways and MicroRNAs in Different Types of Cancers. Cancer Cell Int. 2021, 21, 388. [Google Scholar] [CrossRef]
| Effect | Mechanism | G | D | E | References |
|---|---|---|---|---|---|
| Receptor-mediated carcinogenesis | Downregulation AR | x | [17,18,19,20] | ||
| x | [7,21,22,23] | ||||
| x | [24] | ||||
| Inhibition of cancer cell growth | ↓TK | x | [25] | ||
| ↓EGFR or ErbB-1 | x | [26] | |||
| ↓ErbB-2 or HER2 | x | [27] | |||
| ↓IGF1 (PI3K/AKT and RAS/MAPK pathway) | x | [28,29,30] | |||
| ↓phosphorylation Src, AKT, GSK-3β, FOXO3a and p70S6k | x | [21,22,23] | |||
| ↓phosphorylation FOXO, AKT | x | x | [29,31] | ||
| ↓IRS-1 | x | [32] | |||
| ↓Wnt/β-catenin | x | [33,34,35] | |||
| ↑caspase3 | x | [36] | |||
| ↓survivin, ↓PAR-2, ↑elafin | x | [37] | |||
| ↑Bax, ↓Bcl-2 | x | [38] | |||
| ↓proteasome | x | [39] | |||
| ↓NF-Kβ | x | [37,40,41] | |||
| ↓phosphorylation IKB | x | [42,43] | |||
| ↓mTOR | x | [44] | |||
| ↓TRT, ↓c-Myc, ↓MDM2 | x | [45,46] | |||
| Cell cycle regulation | G1 arrest | x | [30,47,48,49] | ||
| G2/M arrest | x | x | x | [31] | |
| ↓cyclin B1 | x | [48] | |||
| x | x | [31] | |||
| ↑p21WAF1 | x | [48] | |||
| ↓adaptor protein Shc, ↓ERK1/2 | x | [26] | |||
| ↓CDK1 | x | [31] | |||
| ↓CDK4 | x | [48] | |||
| ↑p21, ↑p27 | x | [39,48] | |||
| x | [31] | ||||
| ↑FasL, ↑Bim | x | [31] | |||
| Angiogenesis | ↓VEGF,↓ HIF-1α | x | x | [50,51] | |
| ↓VEGFR1, ↓VEGFR2 | x | [52] | |||
| ↓ECGF1, ↓FGF1, ↓IGF1, ↓ FGFR3, ↓CXC, ↓ IL-1β, ↓IL-6, ↓IL-8, ↓ Ligand 10, ↓PECAM1 | x | x | [30,50,53] | ||
| ↓TGF-β, ↓MMP-2, ↓ MAPK p38 | x | [54] | |||
| Anti-metastatic | ↓urokinase-type plasminogen activator, ↓MMP-2, ↓MMP-9 | x | x | x | [55,56] |
| ↓MAPKAPK2, ↓ HSP27, ↓FAK | x | [54,57,58] | |||
| ↓VEGF, ↓FGF2, ↓MEK (or MAPK)1/2, ↓ERK1/2 | x | [7,59,60] | |||
| ↓PG, ↓ COX-1, ↓ COX-2 | x | [61] | |||
| ↓EMT | x | [62] | |||
| ↓AKR1C3 | x | [63,64] | |||
| ↓OPN | x | [23,65] | |||
| Antioxidant | ↑SOD, ↑catalase, ↑glutathione peroxidase | x | x | x | [66,67,68,69] |
| ↑AMPK, ↑PTEN, ↓NO, ↓NOS | x | [70] | |||
| ↓NO, ↓AKT, ↓NF-κB, ↓ TNF-α, ↓iNOS | x | [71,72] | |||
| Anti-inflammatory | ↓TAM, ↓ TNF-α, ↓GM-CSF | x | x | x | [69] |
| ↓IL-10 | x | [73] | |||
| ↓PG-E2 | x | x | [74] | ||
| Epigenetics | ↓methylation (e.g., BRCA1) | x | x | [75] | |
| ↓methylation (BTG3, RASSF1A) | x | [76] | |||
| ↓methyl binding domain proteins | x | [77] | |||
| ↓DNA methyl transferase enzymes | x | [78] | |||
| ↓miRNA | x | x | [79] | ||
| ↓miR-29a, miR-1256, TRIM68, PGK-1 | x | [80] | |||
| miRNA | x | [81] | |||
| snoRNAs | x | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Eecken, H.; Joniau, S.; Berghen, C.; Rans, K.; De Meerleer, G. The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects. Nutrients 2023, 15, 4856. https://doi.org/10.3390/nu15234856
Van der Eecken H, Joniau S, Berghen C, Rans K, De Meerleer G. The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects. Nutrients. 2023; 15(23):4856. https://doi.org/10.3390/nu15234856
Chicago/Turabian StyleVan der Eecken, Hans, Steven Joniau, Charlien Berghen, Kato Rans, and Gert De Meerleer. 2023. "The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects" Nutrients 15, no. 23: 4856. https://doi.org/10.3390/nu15234856
APA StyleVan der Eecken, H., Joniau, S., Berghen, C., Rans, K., & De Meerleer, G. (2023). The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects. Nutrients, 15(23), 4856. https://doi.org/10.3390/nu15234856







