Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations
Abstract
:1. Introduction
1.1. Cardiovascular Disease
1.2. Atherosclerosis
1.3. Dietary Management of Atherosclerosis
2. Current Status of Knowledge
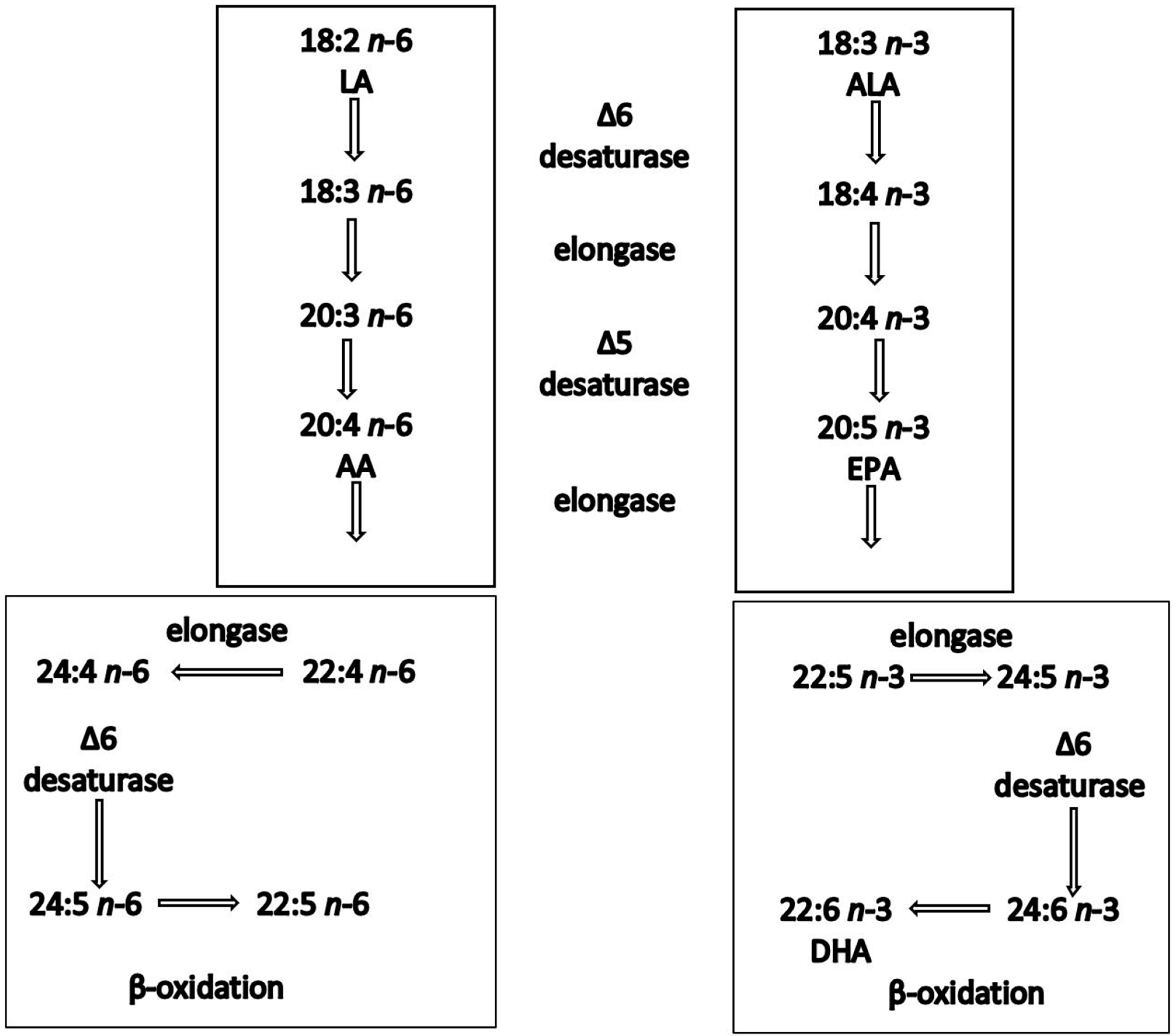
2.1. Biochemistry and Structural Morphology of n-3 PUFAs
2.2. Clinical Evidence on the Effects of n-3 PUFAs on Different CVDs
2.2.1. Effect on Triglycerides
2.2.2. Effect on Low-Density and High-Density Lipoprotein Particle Size
2.2.3. Effect on ‘n-3 Index’
3. Factors Implicated in the Efficacy of PUFAs
3.1. Variability in the Clinical Effects of n-3 PUFAs
3.2. Incorporation of EPA and DHA into the Lipid Pool
3.3. Effects of EPA and DHA on Lipoprotein Metabolism
4. Specific PUFAs as Anti-Inflammatory and Anti-Oxidative Mediators
4.1. Effects of EPA and DHA on Inflammation
4.2. Effects of EPA and DHA on Lipid Oxidation
4.3. Effects of EPA and DHA on Cell Death
5. Epigenetic Determinants of n-3-PUFA Effects
6. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Frostegard, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.J.; Flegal, K.; O’Donnell, C.; Kittner, S.; et al. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151. [Google Scholar] [CrossRef] [PubMed]
- Reimer, K.A.; Jennings, R.B.; Tatum, A.H. Pathobiology of acute myocardial ischemia: Metabolic, functional and ultrastructural studies. Am. J. Cardiol. 1983, 52, 72A–81A. [Google Scholar] [CrossRef] [PubMed]
- Hajouli, S.; Ludhwani, D. Heart Failure and Ejection Fraction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Gasbarrino, K.; Di Iorio, D.; Daskalopoulou, S.S. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur. Heart J. 2022, 43, 460–473. [Google Scholar] [CrossRef]
- Mohd Nor, N.S.; Al-Khateeb, A.M.; Chua, Y.A.; Mohd Kasim, N.A.; Mohd Nawawi, H. Heterozygous familial hypercholesterolaemia in a pair of identical twins: A case report and updated review. BMC Pediatr. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Dichgans, M.; Pulit, S.L.; Rosand, J. Stroke genetics: Discovery, biology, and clinical applications. Lancet Neurol. 2019, 18, 587–599. [Google Scholar] [CrossRef]
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411. [Google Scholar] [CrossRef]
- Kowara, M.; Cudnoch-Jedrzejewska, A. Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. Int. J. Mol. Sci. 2021, 22, 3513. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Amento, E.P.; Ehsani, N.; Palmer, H.; Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. 1991, 11, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Sukhova, G.K.; Kranzhofer, R.; Clark, S.; Libby, P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc. Natl. Acad. Sci. USA 1995, 92, 402–406. [Google Scholar] [CrossRef]
- Lin, J.; Li, H.; Yang, M.; Ren, J.; Huang, Z.; Han, F.; Huang, J.; Ma, J.; Zhang, D.; Zhang, Z.; et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013, 3, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.J.; Libby, P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am. J. Pathol. 1995, 147, 251–266. [Google Scholar]
- Schrijvers, D.M.; De Meyer, G.R.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1256–1261. [Google Scholar] [CrossRef]
- Spannella, F.; Giulietti, F.; Di Pentima, C.; Sarzani, R. Prevalence and Control of Dyslipidemia in Patients Referred for High Blood Pressure: The Disregarded “Double-Trouble” Lipid Profile in Overweight/Obese. Adv. Ther. 2019, 36, 1426–1437. [Google Scholar] [CrossRef]
- Esper, R.J.; Nordaby, R.A. Cardiovascular events, diabetes and guidelines: The virtue of simplicity. Cardiovasc. Diabetol. 2019, 18, 42. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Puttananjaiah, M.K.; Dhale, M.A.; Gaonkar, V.; Keni, S. Statins: 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Appl. Biochem. Biotechnol. 2011, 163, 215–222. [Google Scholar] [CrossRef]
- Li, E.C.; Heran, B.S.; Wright, J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014, 2014, CD009096. [Google Scholar] [CrossRef]
- Misra, S.; Stevermer, J.J. ACE inhibitors and ARBs: One or the other--not both--for high-risk patients. J. Fam. Pract. 2009, 58, 24–27. [Google Scholar]
- Sikand, G.; Severson, T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am. J. Prev. Cardiol. 2020, 4, 100106. [Google Scholar] [CrossRef]
- Yagi, S.; Fukuda, D.; Aihara, K.I.; Akaike, M.; Shimabukuro, M.; Sata, M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, R.J.; Worgall, T.S.; Seo, T. n-3 fatty acids and gene expression. Am. J. Clin. Nutr. 2006, 83, 1520S–1525S. [Google Scholar] [CrossRef]
- Deckelbaum, R.J.; Akabas, S.R. n-3 Fatty acids and cardiovascular disease: Navigating toward recommendations. Am. J. Clin. Nutr. 2006, 84, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.J. Education. Is tenure still viable today? J. Prof. Nurs. 1996, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Sokola-Wysoczanska, E.; Wysoczanski, T.; Wagner, J.; Czyz, K.; Bodkowski, R.; Lochynski, S.; Patkowska-Sokola, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 131–134. [Google Scholar]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391. [Google Scholar] [CrossRef]
- Eslick, G.D.; Howe, P.R.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2009, 136, 4–16. [Google Scholar] [CrossRef]
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol. 2009, 32, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.; Wei, L.; et al. Associations of Fish Consumption With Risk of Cardiovascular Disease and Mortality Among Individuals With or Without Vascular Disease From 58 Countries. JAMA Intern. Med. 2021, 181, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef] [PubMed]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef]
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients 2018, 10, 775. [Google Scholar] [CrossRef]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 109–115. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Liu, Y.X.; Yu, J.H.; Sun, J.H.; Ma, W.Q.; Wang, J.J.; Sun, G.J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Kones, R.; Howell, S.; Rumana, U. n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: Principles, Practices, Pitfalls, and Promises—A Contemporary Review. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2017, 26, 497–508. [Google Scholar] [CrossRef]
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch. Cardiovasc. Dis. 2021, 114, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Siegel, D.; Vemuri, M.; Mackey, B.E. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am. J. Clin. Nutr. 2007, 86, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.R.; Edwards, M.C.; Jacobson, T.A. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J. Nutr. 2006, 136, 2844–2848. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Harris, W.S.; Zotor, F.B. n-3 Fatty acids and risk for fatal coronary disease. Proc. Nutr. Soc. 2019, 78, 526–531. [Google Scholar] [CrossRef]
- Milte, C.M.; Coates, A.M.; Buckley, J.D.; Hill, A.M.; Howe, P.R. Dose-dependent effects of docosahexaenoic acid-rich fish oil on erythrocyte docosahexaenoic acid and blood lipid levels. Br. J. Nutr. 2008, 99, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Torrejon, C.; Jung, U.J.; Deckelbaum, R.J. n-3 Fatty acids and cardiovascular disease: Actions and molecular mechanisms. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 319–326. [Google Scholar] [CrossRef]
- BNF. Cardiovascular Disease: Diet, Nutrition and Emerging Risk Factors; Blackwell Publishing: Hoboken, NJ, USA, 2005. [Google Scholar]
- Breslow, J.L. n-3 fatty acids and cardiovascular disease. Am. J. Clin. Nutr. 2006, 83, 1477S–1482S. [Google Scholar] [CrossRef]
- Yusof, H.M.; Miles, E.A.; Calder, P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- SACN. Advice on Fish Consumption: Benefits and Risks; The Stationery Office: London, UK, 2004. [Google Scholar]
- Toth, P.P.; Chapman, M.J.; Parhofer, K.G.; Nelson, J.R. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 17, 100148. [Google Scholar] [CrossRef]
- Geppert, J.; Kraft, V.; Demmelmair, H.; Koletzko, B. Microalgal docosahexaenoic acid decreases plasma triacylglycerol in normolipidaemic vegetarians: A randomised trial. Br. J. Nutr. 2006, 95, 779–786. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691. [Google Scholar] [CrossRef]
- FDA. Letter Responding to Health Claim Petition dated November 3, 2003 (MartekPetition): Omega-3 Fatty Acids and Reduced Risk of Coronary Heart Disease. 2004. Available online: http://www.cfsan.fda.gov/~dms/ds-ltr37.html (accessed on 28 September 2023).
- Chary, A.; Tohidi, M.; Hedayati, M. Association of LDL-cholesterol subfractions with cardiovascular disorders: A systematic review. BMC Cardiovasc. Disord. 2023, 23, 533. [Google Scholar] [CrossRef]
- Buckley, R.; Shewring, B.; Turner, R.; Yaqoob, P.; Minihane, A.M. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br. J. Nutr. 2004, 92, 477–483. [Google Scholar] [CrossRef]
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q. Cholesterol and Lipoprotein Metabolism and Atherosclerosis: Recent Advances In reverse Cholesterol Transport. Ann. Hepatol. 2017, 16, s27–s42. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Siegel, D.; Vemuri, M.; Chung, G.H.; Mackey, B.E. Docosahexaenoic acid supplementation decreases remnant-like particle-cholesterol and increases the (n-3) index in hypertriglyceridemic men. J. Nutr. 2008, 138, 30–35. [Google Scholar] [CrossRef]
- Harris, W.S. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol. Res. 2007, 55, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Ostermann, A.I.; Stork, L.; Kutzner, L.; Kohrs, H.; Greupner, T.; Hahn, A.; Schebb, N.H. Effects of docosahexaenoic acid supplementation on PUFA levels in red blood cells and plasma. Prostaglandins Leukot. Essent. Fat. Acids 2016, 115, 12–23. [Google Scholar] [CrossRef]
- Walker, C.G.; West, A.L.; Browning, L.M.; Madden, J.; Gambell, J.M.; Jebb, S.A.; Calder, P.C. The Pattern of Fatty Acids Displaced by EPA and DHA Following 12 Months Supplementation Varies between Blood Cell and Plasma Fractions. Nutrients 2015, 7, 6281–6293. [Google Scholar] [CrossRef]
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Group, A.S.C.; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.E.; Gaine, S.P.; Michos, E.D. Eicosapentaenoic acid vs. docosahexaenoic acid for the prevention of cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2023, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Metherel, A.H.; Irfan, M.; Klingel, S.L.; Mutch, D.M.; Bazinet, R.P. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: A randomized control trial. Am. J. Clin. Nutr. 2019, 110, 823–831. [Google Scholar] [CrossRef]
- Singer, P.; Berger, I.; Wirth, M.; Godicke, W.; Jaeger, W.; Voigt, S. Slow desaturation and elongation of linoleic and alpha-linolenic acids as a rationale of eicosapentaenoic acid-rich diet to lower blood pressure and serum lipids in normal, hypertensive and hyperlipemic subjects. Prostaglandins Leukot. Med. 1986, 24, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, P.V.; Kaufman, D.; Bagdade, J.D. Incorporation of dietary n-3 fatty acids into molecular species of phosphatidyl choline and cholesteryl ester in normal human plasma. Am. J. Clin. Nutr. 1993, 58, 360–368. [Google Scholar] [CrossRef]
- Sanders, T.A.; Sullivan, D.R.; Reeve, J.; Thompson, G.R. Triglyceride-lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis 1985, 5, 459–465. [Google Scholar] [CrossRef]
- Madsen, L.; Rustan, A.C.; Vaagenes, H.; Berge, K.; Dyroy, E.; Berge, R.K. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 1999, 34, 951–963. [Google Scholar] [CrossRef]
- Nordoy, A.; Barstad, L.; Connor, W.E.; Hatcher, L. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am. J. Clin. Nutr. 1991, 53, 1185–1190. [Google Scholar] [CrossRef]
- Krokan, H.E.; Bjerve, K.S.; Mork, E. The enteral bioavailability of eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl esters as from glyceryl esters in spite of lower hydrolytic rates by pancreatic lipase in vitro. Biochim. Biophys. Acta 1993, 1168, 59–67. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef]
- Hedengran, A.; Szecsi, P.B.; Dyerberg, J.; Harris, W.S.; Stender, S. n-3 PUFA esterified to glycerol or as ethyl esters reduce non-fasting plasma triacylglycerol in subjects with hypertriglyceridemia: A randomized trial. Lipids 2015, 50, 165–175. [Google Scholar] [CrossRef]
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 2011, 65, 247–254. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Neubronner, J.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in statin-treated dyslipidemic subjects: Results from a six month randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 381–386. [Google Scholar] [CrossRef]
- Gabani, M.; Shapiro, M.D.; Toth, P.P. The Role of Triglyceride-rich Lipoproteins and Their Remnants in Atherosclerotic Cardiovascular Disease. Eur. Cardiol. 2023, 18, e56. [Google Scholar] [CrossRef] [PubMed]
- Rustan, A.C.; Nossen, J.O.; Christiansen, E.N.; Drevon, C.A. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J. Lipid Res. 1988, 29, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Fisher, E.A. n-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J. Clin. Investig. 1993, 91, 1380–1389. [Google Scholar] [CrossRef]
- Bordin, P.; Bodamer, O.A.; Venkatesan, S.; Gray, R.M.; Bannister, P.A.; Halliday, D. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur. J. Clin. Nutr. 1998, 52, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Zhang, Y.; Hua, T.; Liu, X.L.; Liu, T.; Yuan, R.Y.; Li, G.P.; Zhu, Y.; Zhang, X. Omega-3 polyunsaturated fatty acid supplementation improves lipid metabolism and endothelial function by providing a beneficial eicosanoid-pattern in patients with acute myocardial infarction: A randomized, controlled trial. Clin. Nutr. 2021, 40, 445–459. [Google Scholar] [CrossRef]
- Rustan, A.C.; Hustvedt, B.E.; Drevon, C.A. Dietary supplementation of very long-chain n-3 fatty acids decreases whole body lipid utilization in the rat. J. Lipid Res. 1993, 34, 1299–1309. [Google Scholar] [CrossRef]
- Lorente-Cebrian, S.; Bustos, M.; Marti, A.; Fernandez-Galilea, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid inhibits tumour necrosis factor-alpha-induced lipolysis in murine cultured adipocytes. J. Nutr. Biochem. 2012, 23, 218–227. [Google Scholar] [CrossRef]
- Park, Y.; Harris, W.S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J. Lipid Res. 2003, 44, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, G.; Zhou, Q.; Chu, X.; Su, M.; Wei, Y.; Li, L.; Zhang, Z. Effects of eicosapentaenoic acid and docosahexaenoic acid versus alpha-linolenic acid supplementation on cardiometabolic risk factors: A meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 1919–1932. [Google Scholar] [CrossRef]
- Borja-Magno, A.; Guevara-Cruz, M.; Flores-Lopez, A.; Carrillo-Dominguez, S.; Granados, J.; Arias, C.; Perry, M.; Sears, B.; Bourges, H.; Gomez, F.E. Differential effects of high dose omega-3 fatty acids on metabolism and inflammation in patients with obesity: Eicosapentaenoic and docosahexaenoic acid supplementation. Front. Nutr. 2023, 10, 1156995. [Google Scholar] [CrossRef]
- Hande, L.N.; Kjellmo, C.; Pettersen, K.; Ljunggren, S.; Karlsson, H.; Cederbrant, K.; Marcusson-Stahl, M.; Hovland, A.; Lappegard, K.T. Effect of n-3 Polyunsaturated Fatty Acids on Lipid Composition in Familial Hypercholesterolemia: A Randomized Crossover Trial. Biomedicines 2022, 10, 1809. [Google Scholar] [CrossRef] [PubMed]
- Pena-de-la-Sancha, P.; Munoz-Garcia, A.; Espinola-Zavaleta, N.; Bautista-Perez, R.; Mejia, A.M.; Luna-Luna, M.; Lopez-Olmos, V.; Rodriguez-Perez, J.M.; Fragoso, J.M.; Carreon-Torres, E.; et al. Eicosapentaenoic and Docosahexaenoic Acid Supplementation Increases HDL Content in n-3 Fatty Acids and Improves Endothelial Function in Hypertriglyceridemic Patients. Int. J. Mol. Sci. 2023, 24, 5390. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Harris, W.S.; Vanden Heuvel, J.P.; Wagner, P.R.; West, S.G. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am. J. Clin. Nutr. 2011, 93, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lepine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Allaire, J.; Vors, C.; Harris, W.S.; Jackson, K.H.; Tchernof, A.; Couture, P.; Lamarche, B. Comparing the serum TAG response to high-dose supplementation of either DHA or EPA among individuals with increased cardiovascular risk: The ComparED study. Br. J. Nutr. 2019, 121, 1223–1234. [Google Scholar] [CrossRef]
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009, 139, 861–868. [Google Scholar] [CrossRef]
- Grimsgaard, S.; Bonaa, K.H.; Hansen, J.B.; Nordoy, A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am. J. Clin. Nutr. 1997, 66, 649–659. [Google Scholar] [CrossRef]
- Nestel, P.; Shige, H.; Pomeroy, S.; Cehun, M.; Abbey, M.; Raederstorff, D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am. J. Clin. Nutr. 2002, 76, 326–330. [Google Scholar] [CrossRef]
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am. J. Clin. Nutr. 2002, 76, 1007–1015. [Google Scholar] [CrossRef]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef]
- Klingel, S.L.; Metherel, A.H.; Irfan, M.; Rajna, A.; Chabowski, A.; Bazinet, R.P.; Mutch, D.M. EPA and DHA have divergent effects on serum triglycerides and lipogenesis, but similar effects on lipoprotein lipase activity: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Rustan, A.C.; Christiansen, E.N.; Drevon, C.A. Serum lipids, hepatic glycerolipid metabolism and peroxisomal fatty acid oxidation in rats fed omega-3 and omega-6 fatty acids. Biochem. J. 1992, 283 Pt 2, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update1. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Xu, J.; Nakamura, M.T.; Cho, H.P.; Clarke, S.D. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids. A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J. Biol. Chem. 1999, 274, 23577–23583. [Google Scholar] [CrossRef]
- Xu, J.; Teran-Garcia, M.; Park, J.H.; Nakamura, M.T.; Clarke, S.D. Polyunsaturated fatty acids suppress hepatic sterol regulatory element-binding protein-1 expression by accelerating transcript decay. J. Biol. Chem. 2001, 276, 9800–9807. [Google Scholar] [CrossRef]
- Song, S.; Attia, R.R.; Connaughton, S.; Niesen, M.I.; Ness, G.C.; Elam, M.B.; Hori, R.T.; Cook, G.A.; Park, E.A. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol. Cell Endocrinol. 2010, 325, 54–63. [Google Scholar] [CrossRef]
- Rudkowska, I.; Caron-Dorval, D.; Verreault, M.; Couture, P.; Deshaies, Y.; Barbier, O.; Vohl, M.C. PPARalpha L162V polymorphism alters the potential of n-3 fatty acids to increase lipoprotein lipase activity. Mol. Nutr. Food Res. 2010, 54, 543–550. [Google Scholar] [CrossRef]
- Gani, O.A.; Sylte, I. Molecular recognition of docosahexaenoic acid by peroxisome proliferator-activated receptors and retinoid-X receptor alpha. J. Mol. Graph. Model. 2008, 27, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Berge, R.K.; Madsen, L.; Vaagenes, H.; Tronstad, K.J.; Gottlicher, M.; Rustan, A.C. In contrast with docosahexaenoic acid, eicosapentaenoic acid and hypolipidaemic derivatives decrease hepatic synthesis and secretion of triacylglycerol by decreased diacylglycerol acyltransferase activity and stimulation of fatty acid oxidation. Biochem. J. 1999, 343 Pt 1, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.; Hexeberg, S.; Skorve, J.; Lundquist, M.; Berge, R.K. Docosahexaenoic acid shows no triglyceride-lowering effects but increases the peroxisomal fatty acid oxidation in liver of rats. J. Lipid Res. 1993, 34, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Froyland, L.; Madsen, L.; Vaagenes, H.; Totland, G.K.; Auwerx, J.; Kryvi, H.; Staels, B.; Berge, R.K. Mitochondrion is the principal target for nutritional and pharmacological control of triglyceride metabolism. J. Lipid Res. 1997, 38, 1851–1858. [Google Scholar] [CrossRef]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 387–393. [Google Scholar] [CrossRef]
- Li, K.; Huang, T.; Zheng, J.; Wu, K.; Li, D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: A meta-analysis. PLoS ONE 2014, 9, e88103. [Google Scholar] [CrossRef]
- Farimani, A.R.; Hariri, M.; Azimi-Nezhad, M.; Borji, A.; Zarei, S.; Hooshmand, E. The effect of n-3 PUFAs on circulating adiponectin and leptin in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2018, 55, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Allaire, J.; Mejia, S.B.; Khan, T.A.; Sievenpiper, J.L.; Lamarche, B. Comparing the Effects of Docosahexaenoic and Eicosapentaenoic Acids on Inflammation Markers Using Pairwise and Network Meta-Analyses of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 128–140. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Shearer, G.C.; Harris, W.S.; Pedersen, T.L.; Newman, J.W. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 2010, 51, 2074–2081. [Google Scholar] [CrossRef]
- Elajami, T.K.; Colas, R.A.; Dalli, J.; Chiang, N.; Serhan, C.N.; Welty, F.K. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016, 30, 2792–2801. [Google Scholar] [CrossRef]
- Polus, A.; Zapala, B.; Razny, U.; Gielicz, A.; Kiec-Wilk, B.; Malczewska-Malec, M.; Sanak, M.; Childs, C.E.; Calder, P.C.; Dembinska-Kiec, A. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim. Biophys. Acta 2016, 1861, 1746–1755. [Google Scholar] [CrossRef]
- Schwarz, D.; Kisselev, P.; Chernogolov, A.; Schunck, W.H.; Roots, I. Human CYP1A1 variants lead to differential eicosapentaenoic acid metabolite patterns. Biochem. Biophys. Res. Commun. 2005, 336, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.; Markovic, M.; Blossey, K.; Wallukat, G.; Fischer, R.; Dechend, R.; Konkel, A.; von Schacky, C.; Luft, F.C.; Muller, D.N.; et al. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of omega-3 fatty acids. J. Biol. Chem. 2010, 285, 32720–32733. [Google Scholar] [CrossRef]
- Oster, R.T.; Tishinsky, J.M.; Yuan, Z.; Robinson, L.E. Docosahexaenoic acid increases cellular adiponectin mRNA and secreted adiponectin protein, as well as PPARgamma mRNA, in 3T3-L1 adipocytes. Appl. Physiol. Nutr. Metab. 2010, 35, 783–789. [Google Scholar] [CrossRef]
- Tishinsky, J.M.; Ma, D.W.; Robinson, L.E. Eicosapentaenoic acid and rosiglitazone increase adiponectin in an additive and PPARgamma-dependent manner in human adipocytes. Obesity 2011, 19, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wei, H.; Luo, H.; Jiang, S.; Peng, J. EPA inhibits the inhibitor of kappaBalpha (IkappaBalpha)/NF-kappaB/muscle RING finger 1 pathway in C2C12 myotubes in a PPARgamma-dependent manner. Br. J. Nutr. 2011, 105, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Mazzotta, S.; Vega-Holm, M.; Iglesias-Guerra, F.; Vega-Perez, J.M.; Aiello, F.; Brizzi, A. GPR120/FFAR4 Pharmacology: Focus on Agonists in Type 2 Diabetes Mellitus Drug Discovery. J. Med. Chem. 2021, 64, 4312–4332. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sheu, W.H.; Chiang, A.N. Docosahexaenoic acid and eicosapentaenoic acid suppress adhesion molecule expression in human aortic endothelial cells via differential mechanisms. Mol. Nutr. Food Res. 2015, 59, 751–762. [Google Scholar] [CrossRef]
- Mobraten, K.; Haug, T.M.; Kleiveland, C.R.; Lea, T. Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health Dis. 2013, 12, 101. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.P.; Park, S.J.; Kang, S.; Huang, J.; Lee, J.M.; Sato, K.; Chung, H.Y.; Okajima, F.; Im, D.S. Omega-3 fatty acids induce Ca(2+) mobilization responses in human colon epithelial cell lines endogenously expressing FFA4. Acta Pharmacol. Sin. 2015, 36, 813–820. [Google Scholar] [CrossRef]
- Watson, S.J.; Brown, A.J.; Holliday, N.D. Differential signaling by splice variants of the human free fatty acid receptor GPR120. Mol. Pharmacol. 2012, 81, 631–642. [Google Scholar] [CrossRef]
- Wang, C.P.; Lee, C.C.; Wu, D.Y.; Chen, S.Y.; Lee, T.M. Differential effects of EPA and DHA on PPARgamma-mediated sympathetic innervation in infarcted rat hearts by GPR120-dependent and -independent mechanisms. J. Nutr. Biochem. 2022, 103, 108950. [Google Scholar] [CrossRef]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013, 2013, 152786. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Sherratt, S.C.; Jacob, R.F. Eicosapentaenoic Acid Inhibits Oxidation of ApoB-containing Lipoprotein Particles of Different Size In Vitro When Administered Alone or in Combination With Atorvastatin Active Metabolite Compared With Other Triglyceride-lowering Agents. J. Cardiovasc. Pharmacol. 2016, 68, 33–40. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim. Biophys. Acta 2015, 1848, 502–509. [Google Scholar] [CrossRef]
- Sakai, C.; Ishida, M.; Ohba, H.; Yamashita, H.; Uchida, H.; Yoshizumi, M.; Ishida, T. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS ONE 2017, 12, e0187934. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Morvaridzadeh, M.; Maroufizadeh, S.; Akbari, A.; Yavari, M.; Amirinejad, A.; Maleki-Hajiagha, A.; Sepidarkish, M. Omega-3 fatty acids supplementation and oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 149, 104462. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Juliano, R.A.; Mason, R.P. Eicosapentaenoic acid (EPA) has optimal chain length and degree of unsaturation to inhibit oxidation of small dense LDL and membrane cholesterol domains as compared to related fatty acids in vitro. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183254. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Mason, R.P. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem. Biophys. Res. Commun. 2018, 496, 335–338. [Google Scholar] [CrossRef]
- Wu, T.; Geigerman, C.; Lee, Y.S.; Wander, R.C. Enrichment of LDL with EPA and DHA decreased oxidized LDL-induced apoptosis in U937 cells. Lipids 2002, 37, 789–796. [Google Scholar] [CrossRef]
- Mebarek, S.; Ermak, N.; Benzaria, A.; Vicca, S.; Dubois, M.; Nemoz, G.; Laville, M.; Lacour, B.; Vericel, E.; Lagarde, M.; et al. Effects of increasing docosahexaenoic acid intake in human healthy volunteers on lymphocyte activation and monocyte apoptosis. Br. J. Nutr. 2009, 101, 852–858. [Google Scholar] [CrossRef]
- Muralidhar, B.; Carpenter, K.L.; Muller, K.; Skepper, J.N.; Arends, M.J. Potency of arachidonic acid in polyunsaturated fatty acid-induced death of human monocyte-macrophages: Implications for atherosclerosis. Prostaglandins Leukot. Essent. Fat. Acids 2004, 71, 251–262. [Google Scholar] [CrossRef]
- Novinbahador, T.; Nourazarian, A.; Asgharzadeh, M.; Rahbarghazi, R.; Avci, C.B.; Bagca, B.G.; Ozates, N.P.; Karbasforoush, S.; Khaki-Khatibi, F. Docosahexaenoic acid attenuates the detrimental effect of palmitic acid on human endothelial cells by modulating genes from the atherosclerosis signaling pathway. J. Cell Biochem. 2018, 119, 9752–9763. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Ahn, S.H.; Kim, D.S.; Lee, T.; Jeong, J.H. Maresin 1 attenuates pro-inflammatory reactions and ER stress in HUVECs via PPARalpha-mediated pathway. Mol. Cell Biochem. 2018, 448, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Liang, C.P.; Thorp, E.B.; Han, S.; Jehle, A.W.; Saraswathi, V.; Pridgen, B.; Kanter, J.E.; Li, R.; et al. Defective phagocytosis of apoptotic cells by macrophages in atherosclerotic lesions of ob/ob mice and reversal by a fish oil diet. Circ. Res. 2009, 105, 1072–1082. [Google Scholar] [CrossRef]
- Chang, H.Y.; Lee, H.N.; Kim, W.; Surh, Y.J. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci. 2015, 120, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Z.; Marinello, M.; Decker, C.; Sansbury, B.E.; Sadhu, S.; Gerlach, B.D.; Bossardi Ramos, R.; Adam, A.P.; Spite, M.; Fredman, G. Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1062–1075. [Google Scholar] [CrossRef]
- Gerlach, B.D.; Marinello, M.; Heinz, J.; Rymut, N.; Sansbury, B.E.; Riley, C.O.; Sadhu, S.; Hosseini, Z.; Kojima, Y.; Tang, D.D.; et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020, 27, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Polymorphisms, de novo lipogenesis, and plasma triglyceride response following fish oil supplementation. J. Lipid Res. 2013, 54, 2866–2873. [Google Scholar] [CrossRef]
- Ouellette, C.; Cormier, H.; Rudkowska, I.; Guenard, F.; Lemieux, S.; Couture, P.; Vohl, M.C. Polymorphisms in genes involved in the triglyceride synthesis pathway and marine omega-3 polyunsaturated fatty acid supplementation modulate plasma triglyceride levels. J. Nutr. Nutr. 2013, 6, 268–280. [Google Scholar] [CrossRef]
- Tremblay, B.L.; Cormier, H.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Association between polymorphisms in phospholipase A2 genes and the plasma triglyceride response to an n-3 PUFA supplementation: A clinical trial. Lipids Health Dis. 2015, 14, 12. [Google Scholar] [CrossRef]
- Bouchard-Mercier, A.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Polymorphisms in genes involved in fatty acid beta-oxidation interact with dietary fat intakes to modulate the plasma TG response to a fish oil supplementation. Nutrients 2014, 6, 1145–1163. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.; Joshi, A.; Joshi, S. Differential regulation of hepatic transcription factors in the Wistar rat offspring born to dams fed folic acid, vitamin B12 deficient diets and supplemented with omega-3 fatty acids. PLoS ONE 2014, 9, e90209. [Google Scholar] [CrossRef]
- Aslibekyan, S.; Wiener, H.W.; Havel, P.J.; Stanhope, K.L.; O’Brien, D.M.; Hopkins, S.E.; Absher, D.M.; Tiwari, H.K.; Boyer, B.B. DNA methylation patterns are associated with n-3 fatty acid intake in Yup’ik people. J. Nutr. 2014, 144, 425–430. [Google Scholar] [CrossRef]
- de la Rocha, C.; Perez-Mojica, J.E.; Leon, S.Z.; Cervantes-Paz, B.; Tristan-Flores, F.E.; Rodriguez-Rios, D.; Molina-Torres, J.; Ramirez-Chavez, E.; Alvarado-Caudillo, Y.; Carmona, F.J.; et al. Associations between whole peripheral blood fatty acids and DNA methylation in humans. Sci. Rep. 2016, 6, 25867. [Google Scholar] [CrossRef] [PubMed]
- Hoile, S.P.; Clarke-Harris, R.; Huang, R.C.; Calder, P.C.; Mori, T.A.; Beilin, L.J.; Lillycrop, K.A.; Burdge, G.C. Supplementation with n-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS ONE 2014, 9, e109896. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, C.L.; Milagro, F.I.; Curi, R.; Martinez, J.A. DNA methylation pattern in overweight women under an energy-restricted diet supplemented with fish oil. Biomed. Res. Int. 2014, 2014, 675021. [Google Scholar] [CrossRef]
- Lee, H.S.; Barraza-Villarreal, A.; Biessy, C.; Duarte-Salles, T.; Sly, P.D.; Ramakrishnan, U.; Rivera, J.; Herceg, Z.; Romieu, I. Dietary supplementation with polyunsaturated fatty acid during pregnancy modulates DNA methylation at IGF2/H19 imprinted genes and growth of infants. Physiol. Genom. 2014, 46, 851–857. [Google Scholar] [CrossRef]
- Hoyo, C.; Fortner, K.; Murtha, A.P.; Schildkraut, J.M.; Soubry, A.; Demark-Wahnefried, W.; Jirtle, R.L.; Kurtzberg, J.; Forman, M.R.; Overcash, F.; et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control. 2012, 23, 635–645. [Google Scholar] [CrossRef]
- Perkins, E.; Murphy, S.K.; Murtha, A.P.; Schildkraut, J.; Jirtle, R.L.; Demark-Wahnefried, W.; Forman, M.R.; Kurtzberg, J.; Overcash, F.; Huang, Z.; et al. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J. Pediatr. 2012, 161, 31–39. [Google Scholar] [CrossRef]
- van Dijk, S.J.; Zhou, J.; Peters, T.J.; Buckley, M.; Sutcliffe, B.; Oytam, Y.; Gibson, R.A.; McPhee, A.; Yelland, L.N.; Makrides, M.; et al. Effect of prenatal DHA supplementation on the infant epigenome: Results from a randomized controlled trial. Clin. Epigenetics 2016, 8, 114. [Google Scholar] [CrossRef]
- Lind, M.V.; Martino, D.; Harslof, L.B.; Kyjovska, Z.O.; Kristensen, M.; Lauritzen, L. Genome-wide identification of mononuclear cell DNA methylation sites potentially affected by fish oil supplementation in young infants: A pilot study. Prostaglandins Leukot. Essent. Fat. Acids 2015, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of gene expression. Nutr. Rev. 2004, 62, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.S.; Miller, M.; Tighe, A.P.; Davidson, M.H.; Schaefer, E.J. Omega-3 fatty acids and coronary heart disease risk: Clinical and mechanistic perspectives. Atherosclerosis 2008, 197, 12–24. [Google Scholar] [CrossRef]
- Rundblad, A.; Larsen, S.V.; Myhrstad, M.C.; Ottestad, I.; Thoresen, M.; Holven, K.B.; Ulven, S.M. Differences in peripheral blood mononuclear cell gene expression and triglyceride composition in lipoprotein subclasses in plasma triglyceride responders and non-responders to omega-3 supplementation. Genes. Nutr. 2019, 14, 10. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaeloudes, C.; Christodoulides, S.; Christodoulou, P.; Kyriakou, T.-C.; Patrikios, I.; Stephanou, A. Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations. Nutrients 2023, 15, 4830. https://doi.org/10.3390/nu15224830
Michaeloudes C, Christodoulides S, Christodoulou P, Kyriakou T-C, Patrikios I, Stephanou A. Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations. Nutrients. 2023; 15(22):4830. https://doi.org/10.3390/nu15224830
Chicago/Turabian StyleMichaeloudes, Charalambos, Stephanos Christodoulides, Panayiota Christodoulou, Theodora-Christina Kyriakou, Ioannis Patrikios, and Anastasis Stephanou. 2023. "Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations" Nutrients 15, no. 22: 4830. https://doi.org/10.3390/nu15224830
APA StyleMichaeloudes, C., Christodoulides, S., Christodoulou, P., Kyriakou, T.-C., Patrikios, I., & Stephanou, A. (2023). Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease—Possible Causes and Future Considerations. Nutrients, 15(22), 4830. https://doi.org/10.3390/nu15224830






