Carbohydrate Counting, Empowerment and Glycemic Outcomes in Adolescents and Young Adults with Long Duration of Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Questionnaires
2.3. Registry Data
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Advanced Carbohydrate Counting and Glycemic Control
3.3. Advanced Carbohydrate Counting and Empowerment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annan, S.F.; Higgins, L.A.; Jelleryd, E.; Hannon, T.; Rose, S.; Salis, S.; Baptista, J.; Chinchilla, P.; Marcovecchio, M.L. ISPAD Clinical Practice Consensus Guidelines 2022: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1297–1321. [Google Scholar] [CrossRef]
- Schmidt, S.; Schelde, B.; Nørgaard, K. Effects of advanced carbohydrate counting in patients with type 1 diabetes: A systematic review. Diabetes Med. 2014, 31, 886–896. [Google Scholar] [CrossRef]
- Smart, C.E.; Ross, K.; Edge, J.A.; King, B.R.; McElduff, P.; Collins, C.E. Can children with Type 1 diabetes and their caregivers estimate the carbohydrate content of meals and snacks? Diabetes Med. 2010, 27, 348–353. [Google Scholar] [CrossRef]
- Smart, C.E.; Ross, K.; Edge, J.A.; Collins, C.E.; Colyvas, K.; King, B.R. Children and adolescents on intensive insulin therapy maintain postprandial glycaemic control without precise carbohydrate counting. Diabetes Med. 2009, 26, 279–285. [Google Scholar] [CrossRef]
- Olinder, A.L.; DeAbreu, M.; Greene, S.; Haugstvedt, A.; Lange, K.; Majaliwa, E.S.; Pais, V.; Pelicand, J.; Town, M.; Mahmud, F.H. ISPAD Clinical Practice Consensus Guidelines 2022: Diabetes education in children and adolescents. Pediatr. Diabetes 2022, 23, 1229–1242. [Google Scholar] [CrossRef]
- Katarina Eeg-Olofsson, K.Å.; Nåtman, J.; Almskog, I.; Carter, V.H.; Linder, E.; Sjöstedt, C. Nationella Diabetesregistret, Årsrapport 2022. 2022. Available online: https://www.ndr.nu/pdfs/Arsrapport_NDR_2022.pdf (accessed on 5 June 2023).
- Kawamura, T. The importance of carbohydrate counting in the treatment of children with diabetes. Pediatr. Diabetes 2007, 8 (Suppl. 6), 57–62. [Google Scholar] [CrossRef]
- Bell, K.J.; Barclay, A.W.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Efficacy of carbohydrate counting in type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014, 2, 133–140. [Google Scholar] [CrossRef]
- Tascini, G.; Berioli, M.G.; Cerquiglini, L.; Santi, E.; Mancini, G.; Rogari, F.; Toni, G.; Esposito, S. Carbohydrate Counting in Children and Adolescents with Type 1 Diabetes. Nutrients 2018, 10, 109. [Google Scholar] [CrossRef]
- Cheng, L.J.; Wang, W.; Lim, S.T.; Wu, V.X. Factors associated with glycaemic control in patients with diabetes mellitus: A systematic literature review. J. Clin. Nurs. 2019, 28, 1433–1450. [Google Scholar] [CrossRef] [PubMed]
- Small, N.; Bower, P.; Chew-Graham, C.A.; Whalley, D.; Protheroe, J. Patient empowerment in long-term conditions: Development and preliminary testing of a new measure. BMC Health Serv. Res. 2013, 13, 263. [Google Scholar] [CrossRef]
- Mora, M.A.; Luyckx, K.; Sparud-Lundin, C.; Peeters, M.; van Staa, A.; Sattoe, J.; Bratt, E.-L.; Moons, P. Patient empowerment in young persons with chronic conditions: Psychometric properties of the Gothenburg Young Persons Empowerment Scale (GYPES). PLoS ONE 2018, 13, e0201007. [Google Scholar]
- Fridholm, O. The relationship of target glucose intervals: A Swedish retrospective study. In Proceedings of the 47th International Society for Pediatric and Adolescent Diabetes (ISPAD), Virtual, 13–15 October 2021. [Google Scholar]
- Baretić, M.; Pavić, E.; Rabađija, N.; Uroić, V.; Koletić, C.; Renar, P. Type 1 diabetes from adolescence to adulthood: Is there a permanent need for nutrition education and re-education? Minerva Endocrinol. 2018, 43, 27–33. [Google Scholar] [CrossRef]
- Ullah, A.; Graue, M.; Haugstvedt, A. Adolescent’s Experiences with Diabetes Self-Management and the Use of Carbohydrate Counting in Their Everyday Life with Type 1 Diabetes. Patient Prefer. Adherence 2022, 16, 887–896. [Google Scholar] [CrossRef]
- Braun, M.; Tomasik, B.; Wrona, E.; Fendler, W.; Jarosz-Chobot, P.; Szadkowska, A.; Zmysłowska, A.; Wilson, J.; Mlynarski, W. The Stricter the Better? The Relationship between Targeted HbA1c Values and Metabolic Control of Pediatric Type 1 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 5490258. [Google Scholar] [CrossRef]
- Hanberger, L.; Samuelsson, U.; Bertero, C.; Ludvigsson, J. The influence of structure, process, and policy on HbA(1c) levels in treatment of children and adolescents with type 1 diabetes. Diabetes Res. Clin. Pract. 2012, 96, 331–338. [Google Scholar] [CrossRef]
- Cherubini, V.; Marino, M.; Marigliano, M.; Maffeis, C.; Zanfardino, A.; Rabbone, I.; Giorda, S.; Schiaffini, R.; Lorubbio, A.; Rollato, S.; et al. Rethinking Carbohydrate Intake and Time in Range in Children and Adolescents with Type 1 Diabetes. Nutrients 2021, 13, 3869. [Google Scholar] [CrossRef]
| Fixed Insulin Dose from Onset n = 53 | ACC from Onset n = 58 | p-Value | ||
|---|---|---|---|---|
| Age (mean) | 18.81 ± 4.76 | 17.93 ± 4.86 | 0.338 | |
| Age at onset | 8.4 ± 4.65 | 9.4 ± 4.82 | 0.247 | |
| Diabetes duration (SD) | 11.25 (0.61) | 9.34 (0.55) | <0.001 * | |
| Male/Female | 25/28 | 29/29 | 0.766 | |
| Weight ** (n = 95) | Normal weight | 31 (69%) | 37 (74%) | 0.681 |
| Overweight | 13 (29%) | 11 (22%) | ||
| Obese | 1 (2%) | 2 (4%) | ||
| HbA1c mean last year (mmol/mol) (SD) (n = 110) | 55.7 (12.8) | 55.0 (10.8) | 0.754 | |
| HbA1c mean last year (% DCCT) (SD) (n = 110) | 7.25 (0.6) | 7.18 (0.6) | ||
| Pen/Pump | 16/37 | 20/38 | 0.629 | |
| ACC strategy | 41 (77%) | 47 (81%) | 0.065 | |
| Mixed strategy | 3 (6%) | 8 (14%) | ||
| Other strategy (no ACC) | 9 (17%) | 3 (5%) | ||
| ACC Strategy (n = 88) | Mixed Strategy (n = 11) | Other Strategy (n = 12) | p-Value | ||
|---|---|---|---|---|---|
| Age | Mean | 17.6 | 19.4 | 22.7 | 0.001 * |
| CI | 16.6, 18.6 | 16.3, 22.4 | 20.3, 25.2 | ||
| Age at onset | Mean | 8.3 | 10.3 | 12.7 | 0.006 * |
| CI | 7.3, 9.3 | 7.1, 13.5 | 10.2, 15.1 | ||
| Diabetes duration | Mean | 10.1 | 9.6 | 11.0 | 0.027 * |
| CI | 9.8,10.4 | 9.1, 10.6 | 10.3, 11.7 | ||
| Pen / Pump | n | 21/67 | 7/4 | 8/4 | <0.001 * |
| % | 23.9/76.1 | 63.6/36.4 | 66.7/33.3 | ||
| Hba1c last year IFCC (mmol/mol) (n = 110) | Mean | 54.6 | 60.4 | 56.5 | 0.281 |
| CI | 52.9, 57.0 | 50.7, 70.2 | 50.8, 62.1 | ||
| HbA1c last year DCCT (%) (n = 110) | Mean | 7.15 | 7.7 | 7.3 | |
| CI | 7.0, 9.0 | 6.8, 8.6 | 6.8, 7.8 | ||
| Mean blood glucose (mmol/L) (n = 92) | Mean | 9.0 | 10.3 | 8.4 | 0.009 * |
| CI | 8.7, 9.3 | 8.5, 12.2 | 7.3, 9.6 | ||
| Time in range (TIR) last year % (n = 88) | Mean | 60.7 | 48.9 | 63.2 | 0.085 |
| CI | 57.2, 64.1 | 32.7, 65.1 | 48.6, 77.8 | ||
| Standard deviation (SD) (n = 82) | Mean | 3.4 | 4.1 | 3.3 | 0.078 |
| CI | 3.2, 3.6 | 3.2, 4.9 | 2.7, 3.9 |
| Empowerment | ACC Strategy (n = 88) | Mixed Strategy (n = 11) | Other Strategy (n = 12) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Median | Min Max | Median | Min Max | Median | Min Max | ||
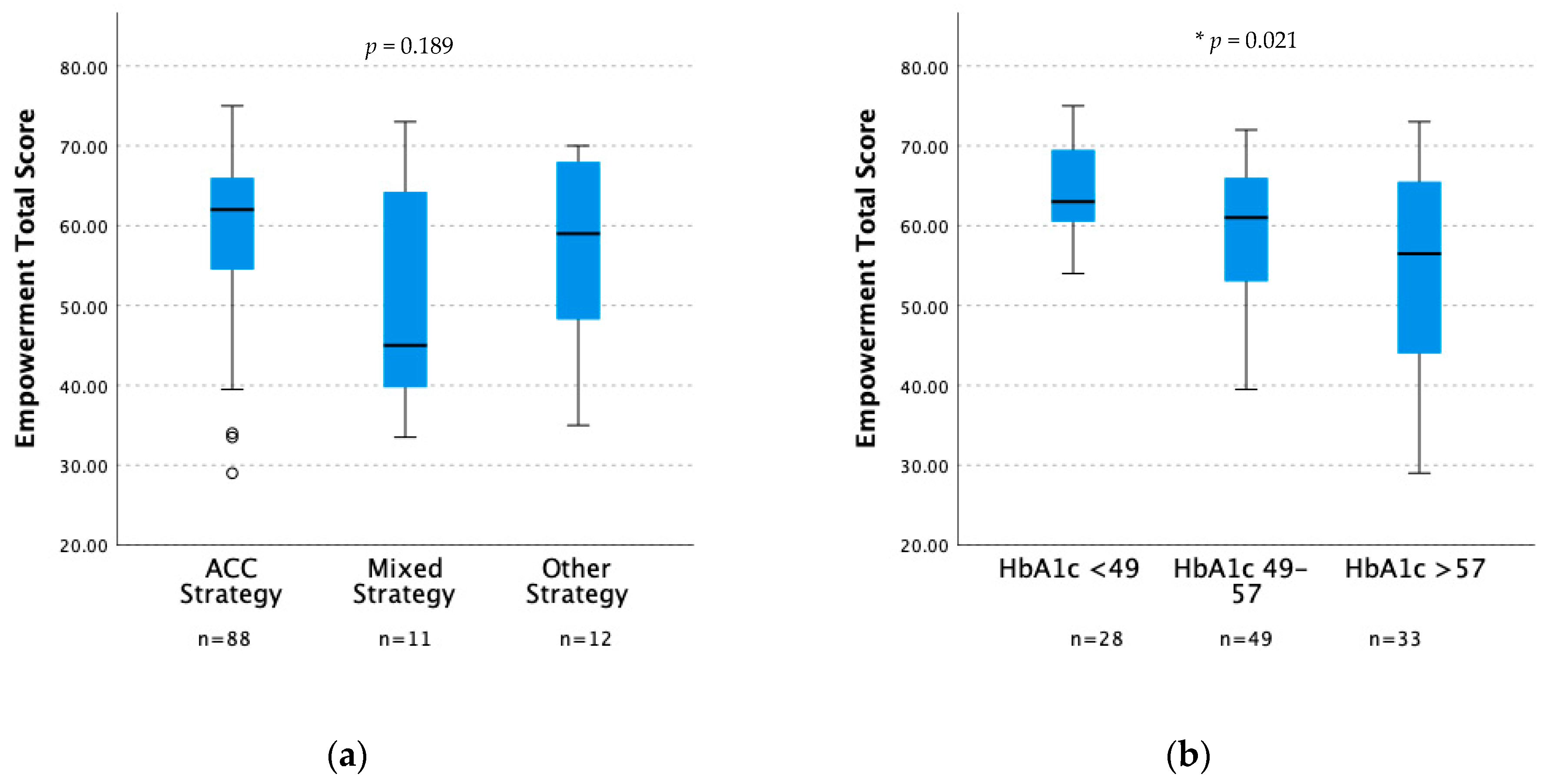
| Total score | 62 | 29, 75 | 45 | 33.5, 73 | 59 | 35, 70 | 0.189 |
| Knowledge | 14 | 5, 15 | 11 | 7, 15 | 15 | 7, 15 | 0.104 |
| Personal control | 13.5 | 6, 15 | 12 | 6, 15 | 14.2 | 6, 15 | 0.408 |
| Identity | 12 | 4, 15 | 10 | 6, 14 | 11 | 5, 13 | 0.186 |
| Shared decision making | 12 | 4, 15 | 12 | 6, 15 | 12 | 6, 15 | 0.663 |
| Enabling others | 11.5 | 3, 15 | 8 | 3, 15 | 9 | 3, 15 | 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelleryd, E.; Brorsson, A.L.; Smart, C.E.; Käck, U.; Lindholm Olinder, A. Carbohydrate Counting, Empowerment and Glycemic Outcomes in Adolescents and Young Adults with Long Duration of Type 1 Diabetes. Nutrients 2023, 15, 4825. https://doi.org/10.3390/nu15224825
Jelleryd E, Brorsson AL, Smart CE, Käck U, Lindholm Olinder A. Carbohydrate Counting, Empowerment and Glycemic Outcomes in Adolescents and Young Adults with Long Duration of Type 1 Diabetes. Nutrients. 2023; 15(22):4825. https://doi.org/10.3390/nu15224825
Chicago/Turabian StyleJelleryd, Elisabeth, Anna Lena Brorsson, Carmel E. Smart, Ulrika Käck, and Anna Lindholm Olinder. 2023. "Carbohydrate Counting, Empowerment and Glycemic Outcomes in Adolescents and Young Adults with Long Duration of Type 1 Diabetes" Nutrients 15, no. 22: 4825. https://doi.org/10.3390/nu15224825
APA StyleJelleryd, E., Brorsson, A. L., Smart, C. E., Käck, U., & Lindholm Olinder, A. (2023). Carbohydrate Counting, Empowerment and Glycemic Outcomes in Adolescents and Young Adults with Long Duration of Type 1 Diabetes. Nutrients, 15(22), 4825. https://doi.org/10.3390/nu15224825





