Glycemic Control, Basal/Bolus Distribution, BMI and Meal Management in Children and Adolescents with Type 1 Diabetes and Advanced Hybrid Closed Loop
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Subjects
2.2. Outcome Variables
2.3. Statistical Analysis
3. Results
3.1. Population Set
3.2. Device Settings
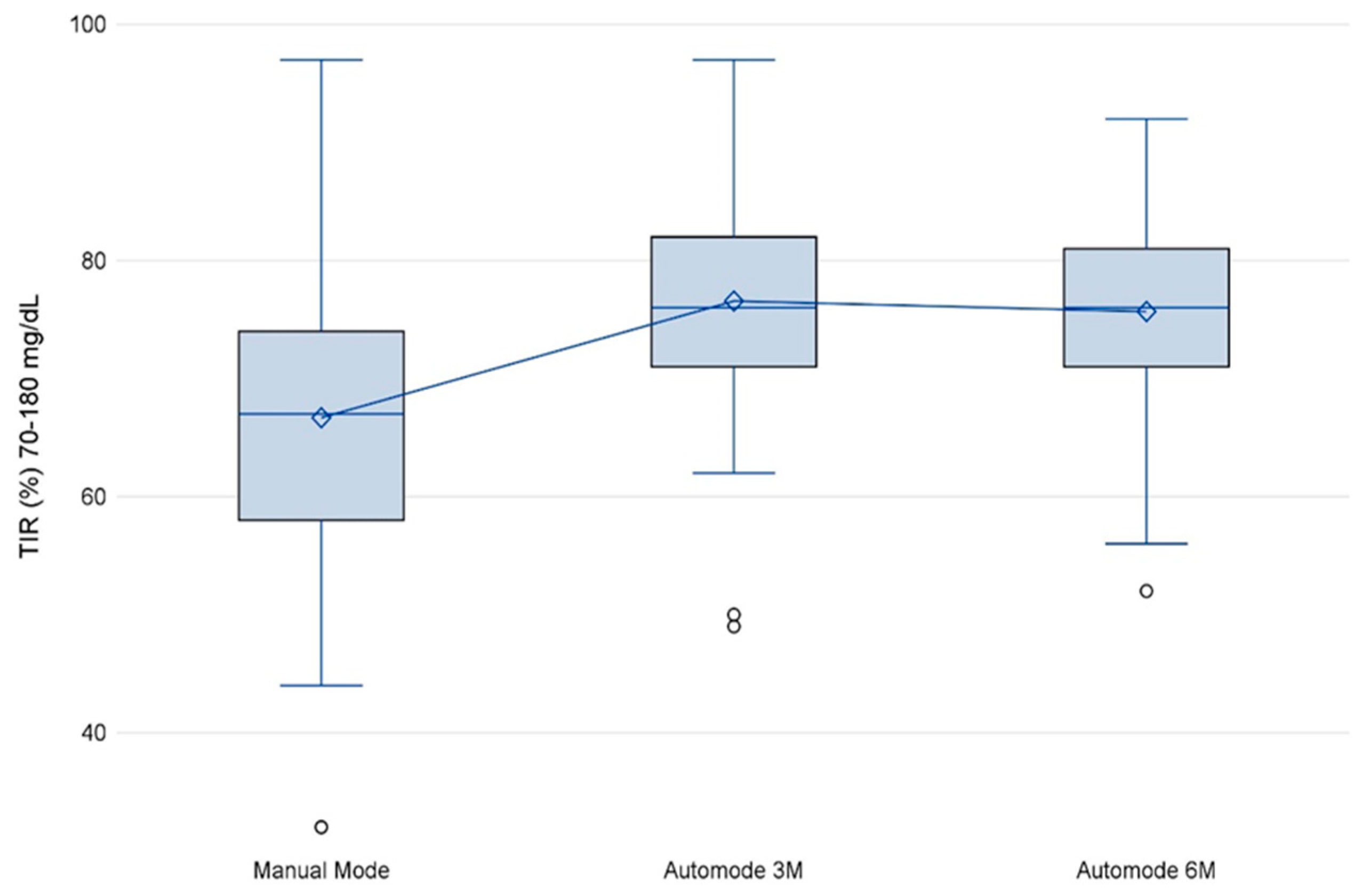
3.3. TIR
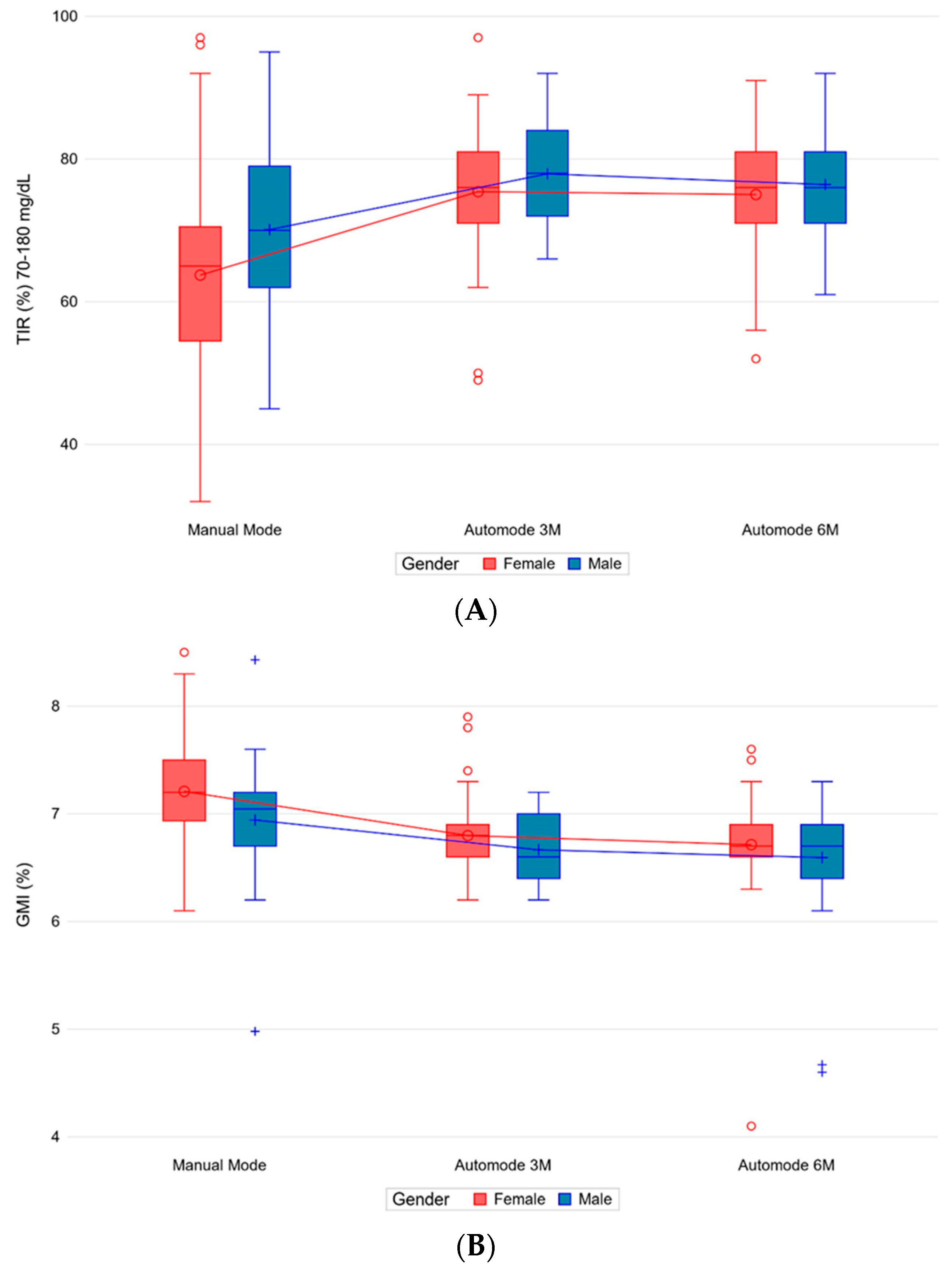
3.4. Glycemic Variability and GMI
3.5. Basal/Bolus Insulin Changes
3.6. HbA1c
3.7. Anthropometric and Nutrition Parameters
3.8. Multivariate Analysis
3.8.1. Gender and Age
3.8.2. Previous Therapy
3.9. Nutrition Parameters from Diaries before and after Use of MiniMedTM 780G
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piccini, B.; Pessina, B.; Casalini, E.; Lenzi, L.; Toni, S. Long-term effectiveness of advanced hybrid closed loop in children and adolescents with type 1 diabetes. Pediatr. Diabetes 2022, 23, 1647–1655. [Google Scholar] [CrossRef]
- Beato-Víbora, P.I.; Gallego-Gamero, F.; Ambrojo-López, A.; Gil-Poch, E.; Martín-Romo, I.; Arroyo-Díez, F.J. Rapid Improvement in Time in Range After the Implementation of an Advanced Hybrid Closed-Loop System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2021, 23, 609–615. [Google Scholar] [CrossRef]
- Arrieta, A.; Battelino, T.; Scaramuzza, A.E.; Da Silva, J.; Castañeda, J.; Cordero, T.L.; John Shin, J.; Cohen, O. Comparison of MiniMed 780G system performance in users aged younger and older than 15 years: Evidence from 12,870 real-world users. Diabetes Obes. Metab. 2022, 24, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, J.; Mathieu, C.; Aanstoot, H.; Arrieta, A.; Da Silva, J.; Shin, J.; Cohen, O. Predictors of time in target glucose range in real-world users of the MiniMed 780G system. Diabetes Obes. Metab. 2022, 24, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.N.; Andersen, H.U.; Abrahamson, M.J.; Wolpert, H.A.; Hommel, E.E.; McMullen, W.; Ridderstråle, M. Changes in HbA1c and weight following transition to continuous subcutaneous insulin infusion therapy in adults with type 1 diabetes. J. Diabetes Sci. Technol. 2017, 11, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical targets for continuous glucose monitoring data interpretation: Recommendations from the international consensus on time in range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Bergenstal, R.M.; Nimri, R.; Beck, R.W.; Criego, A.; Laffel, L.; Schatz, D.; Battelino, T.; Danne, T.; Weinzimer, S.A.; Sibayan, J.; et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): A multicentre, randomized, crossover trial. Lancet 2021, 397, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.D.; Lepore, G.; Battelino, T.; Arrieta, A.; Castaneda, J.; Grossman, B.; Shin, J.; Cohen, O. Real-world performance of the MiniMed™ 780G system: First report of outcomes from 4120 users. Diabetes Technol. Ther. 2022, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: Cluster randomized trial (REPOSE). BMJ 2017, 356, j1285. [Google Scholar] [CrossRef]
- Alderisio, A.; Bozzetto, L.; Franco, L.; Riccardi, G.; Rivellese, A.A.; Annuzzi, G. Long-term body weight trajectories and metabolic control in type 1 diabetes patients on insulin pump or multiple daily injections: A 10-year retrospective controlled study. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1110–1117. [Google Scholar] [CrossRef]
- Seget, S.; Jarosz-Chobot, P.; Ochab, A.; Polanska, J.; Rusak, E.; Witoszek, P.; Chobot, A. Body mass index, basal insulin and glycemic control in children with type 1 diabetes treated with the advanced hybrid closed loop system remain stable-1-year prospective, observational, two-center study. Front. Endocrinol. 2022, 13, 1036808. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.; Blackburn, M.; Rankin, D.; Allen, J.; Campbell, F.; Leelarathna, L.; Tauschmann, M.; Thabit, H.; Wilinska, M.E.; Hovorka, R.; et al. The impact of using a closed-loop system on food choices and eating practices among people with Type 1 diabetes: A qualitative study involving adults, teenagers and parents. Diabet. Med. 2019, 36, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.K.; Weinzimer, S.A.; Tamborlane, W.V.; Buckingham, B.A.; Bode, B.W.; Bailey, T.S.; Brazg, R.L.; Ilany, J.; Slover, R.H.; Anderson, S.M.; et al. Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol. Ther. 2017, 19, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Messer, L.H.; Berget, C.; Pyle, L.; Vigers, T.; Cobry, E.; Driscoll, K.A.; Forlenza, G.P. Real-World Use of a New Hybrid Closed Loop Improves Glycemic Control in Youth With Type 1 Diabetes. Diabetes Technol. Ther. Diabetes 2021, 23, 837–843. [Google Scholar] [CrossRef]
- Petrovski, G.; Campbell, J.; Pasha, M.; Day, E.; Hussain, K.; Khalifa, A.; van den Heuvel, T. Simplified Meal Announcement Versus Precise Carbohydrate Counting in Adolescents With Type 1 Diabetes Using the MiniMed 780G Advanced Hybrid Closed Loop System: A Randomized Controlled Trial Comparing Glucose Control. Diabetes Care 2023, 46, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Tornese, G.; Carletti, C.; Giangreco, M.; Nisticò, D.; Faleschini, E.; Barbi, E. Carbohydrate Tolerance Threshold for Unannounced Snacks in Children and Adolescents with Type 1 Diabetes Using an Advanced Hybrid Closed-Loop System. Diabetes Care 2022, 45, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Berget, C.; Messer, L.H.; Vigers, T.; Frohnert, B.I.; Pyle, L.; Wadwa, R.P.; Driscoll, K.-A.; Forlenza, G.P. Six months of hybrid closed loop in the real-world: An evaluation of children and young adults using the 670G system. Pediatr. Diabetes 2020, 21, 310–318. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristics | Summary Statistics | Total (n = 83) |
|---|---|---|
| Age (yrs) | n | 83 (100.0%) |
| Mean ± SD | 13.0 ± 4.5 | |
| Median (IQR) | 12.5 (9.3–15.8) | |
| Min–Max | 3.3–22.3 | |
| Gender (male) | %, n/Pts | 45.8% (38/83) |
| Age at diagnosis (yrs) | n | 83 (100.0%) |
| Mean ± SD | 6.0 ± 3.8 | |
| Median (IQR) | 5.7 (2.3–8.5) | |
| Min–Max | 0.9–16.6 | |
| DM duration (yrs) | n | 83 (100.0%) |
| Mean ± SD | 7.0 ± 4.5 | |
| Median (IQR) | 6.6 (2.8–9.4) | |
| Min–Max | 0.3–19.2 | |
| Previous therapy | ||
| MDI | %, n/Pts | 37.3% (31/83) |
| CSII | %, n/Pts | 62.7% (52/83) |
| Manual mode duration (days) | n | 83 (100.0%) |
| Mean ± SD | 32.6 ± 38.6 | |
| Median (IQR) | 20.0 (13.0–32.0) | |
| Min–Max | 3.0–232.0 |
| Summary Statistics | Manual Mode (n = 83) | Auto-Mode 3M (n = 83) | Auto-Mode 6M (n = 83) | Mean Change 95% CI p-Value | ||
|---|---|---|---|---|---|---|
| Auto-Mode 3M (vs. Manual Mode) | Auto-Mode 6M (vs. Manual Mode) | |||||
| SG CV (%) | n | 82 (98.8%) | 83 (100.0%) | 81 (97.6%) | −0.9 (−2.4–0.5) 0.204 | −0.2 (−1.4–1.0) 0.754 |
| Mean ± SD | 34.2 ± 6.2 | 33.3 ± 4.5 | 34.1 ± 5.0 | |||
| Median (IQR) | 32.7 (31.2–38.1) | 33.0 (30.3–36.2) | 33.9 (30.5–37.0) | |||
| Min–Max | 21.3–66.7 | 22.0–44.4 | 24.1–50.0 | |||
| GMI (%) | n | 82 (98.8%) | 83 (100.0%) | 81 (97.6%) | −0.3 (−0.5–0.2) <0.001 | −0.4 (−0.5–0.3) <0.001 |
| Mean ± SD | 7.1 ± 0.5 | 6.7 ± 0.3 | 6.7 ± 0.5 | |||
| Median (IQR) | 7.1 (6.9–7.4) | 6.7 (6.5–7.0) | 6.7 (6.5–6.9) | |||
| Min–Max | 5.0–8.5 | 6.2–7.9 | 4.1–7.6 | |||
| Summary Statistics | Manual Mode (n = 83) | Auto-Mode 3M (n = 83) | Auto-Mode 6M (n = 83) | Mean Change 95% CI p-Value | ||
|---|---|---|---|---|---|---|
| Auto-Mode 3M (vs. Manual Mode) | Auto-Mode 6M (vs. Manual Mode) | |||||
| Number of meals/day | n | 82 (98.8%) | 83 (100.0%) | 80 (96.4%) | 0.6 (0.2–1.0) 0.002 | 0.7 (0.4–1.0) <0.001 |
| Mean ± SD | 4.4 ± 1.2 | 5.0 ± 1.5 | 5.1 ± 1.7 | |||
| Median (IQR) | 4.3 (3.7–5.0) | 4.8 (4.0–5.6) | 4.9 (4.0–5.9) | |||
| Min–Max | 1.3–7.5 | 2.4–8.7 | 1.8–10.7 | |||
| Weight (Kg) | n | 83 (100.0%) | 83 (100.0%) | 81 (97.6%) | 0.7 (−0.0–1.4) 0.052 | 1.7 (1.2–2.2) <0.001 |
| Mean ± SD | 43.2 ± 18.2 | 43.9 ± 18.0 | 45.1 ± 18.2 | |||
| Median (IQR) | 37.5 (30.0–60.0) | 38.5 (31.1–60.0) | 40.7 (31.3–60.0) | |||
| Min–Max | 14.9–84.0 | 14.7–84.9 | 15.1–88.5 | |||
| BMI (kg/m²) | n | 83 (100.0%) | 82 (98.8%) | 81 (97.6%) | 0.0 (−0.2–0.3) 0.775 | 0.1 (−0.1–0.3) 0.205 |
| Mean ± SD | 19.0 ± 3.2 | 19.0 ± 3.1 | 19.2 ± 3.2 | |||
| Median (IQR) | 18.3 (16.5–21.3) | 18.2 (16.6–20.7) | 18.9 (16.5–21.2) | |||
| Min–Max | 14.3–27.0 | 13.7–27.2 | 14.1–28.7 | |||
| BMI z-score | n | 83 (100.0%) | 82 (98.8%) | 80 (96.4%) | −0.1 (−0.2–0.0) 0.154 | −0.1 (−0.2–0.0) 0.050 |
| Mean ± SD | 0.2 ± 0.9 | 0.1 ± 0.9 | 0.1 ± 0.9 | |||
| Median (IQR) | 0.2 (−0.3–0.9) | 0.2 (−0.4–0.8) | 0.2 (−0.3–0.8) | |||
| Min–Max | −2.1–1.9 | −2.1–1.8 | −2.1–1.9 | |||
| TDD (units) | n | 82 (98.8%) | 83 (100.0%) | 81 (97.6%) | 2.4 (0.6–4.3) 0.010 | 4.3 (2.9–5.6) <0.001 |
| Mean ± SD | 31.0 ± 16.5 | 33.3 ± 17.8 | 35.4 ± 17.7 | |||
| Median (IQR) | 27.9 (18.3–38.4) | 30.6 (20.6–42.9) | 32.6 (22.8–43.1) | |||
| Min–Max | 4.8–78.8 | 5.7–80.5 | 6.0–85.1 | |||
| TDD/Kg (units) | n | 83 (100.0%) | 83 (100.0%) | 81 (97.6%) | 0.1 (0.0–0.1) 0.008 | 0.1 (0.0–0.1) <0.001 |
| Mean ± SD | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.2 | |||
| Median (IQR) | 0.7 (0.6–0.9) | 0.7 (0.6–1.0) | 0.8 (0.7–0.9) | |||
| Min–Max | 0.0–1.2 | 0.2–1.4 | 0.1–1.4 | |||
| CHO (gr) | n | 82 (98.8%) | 83 (100.0%) | 81 (97.6%) | 6.3 (−13–25.6) 0.522 | 12.7 (−2.2–27.7) 0.094 |
| Mean ± SD | 197.3 ± 81.7 | 203.5 ± 86.7 | 210.7 ± 104.0 | |||
| Median (IQR) | 182.0 (149.0–229.0) | 184.0 (154.0–236.0) | 191.0 (144.0–257.0) | |||
| Min–Max | 64.0–605.0 | 81.0–509.0 | 31.0–814.0 | |||
| CHO/Kg (gr) | n | 83 (100.0%) | 83 (100.0%) | 81 (97.6%) | 0.1 (−0.4–0.5) 0.810 | 0.1 (−0.3–0.4) 0.666 |
| Mean ± SD | 5.1 ± 2.1 | 5.1 ± 2.1 | 5.2 ± 2.6 | |||
| Median (IQR) | 5.1 (3.4–6.7) | 5.2 (3.4–6.4) | 5.0 (3.5–6.3) | |||
| Min–Max | 0.0–11.0 | 1.6–14.8 | 0.6–21.1 | |||
| Summary Statistics | Pre-780G (n = 83) | Post-780G (n = 83) | p-Value | |
|---|---|---|---|---|
| Kcal | n | 8 (9.6%) | 8 (9.6%) | 0.461 |
| Mean ± SD | 1622.3 ± 464.4 | 1538.4 ± 371.9 | ||
| Median (IQR) | 1688.8 (1513.5–1871.0) | 1638.0 (1419.5–1686.8) | ||
| Min–Max | 637.0–2195.0 | 771.0–2048.0 | ||
| P (gr) | n | 8 (9.6%) | 8 (9.6%) | 0.195 |
| Mean ± SD | 62.5 ± 18.1 | 55.0 ± 21.6 | ||
| Median (IQR) | 62.8 (56.2–71.6) | 52.9 (43.0–59.9) | ||
| Min–Max | 27.8–90.9 | 27.4–101.2 | ||
| P (%) | n | 8 (9.6%) | 8 (9.6%) | 0.195 |
| Mean ± SD | 15.7 ± 2.4 | 14.2 ± 3.5 | ||
| Median (IQR) | 14.9 (14.5–17.0) | 13.6 (12.1–16.6) | ||
| Min–Max | 12.1–20.3 | 9.5–19.8 | ||
| L (gr) | n | 8 (9.6%) | 8 (9.6%) | 0.641 |
| Mean ± SD | 63.6 ± 22.9 | 59.3 ± 19.8 | ||
| Median (IQR) | 70.4 (49.1–75.3) | 63.5 (47.8–71.0) | ||
| Min–Max | 24.6–94.5 | 25.5–84.9 | ||
| L (%) | n | 8 (9.6%) | 8 (9.6%) | 0.844 |
| Mean ± SD | 35.0 ± 6.1 | 34.2 ± 6.2 | ||
| Median (IQR) | 36.7 (32.0–39.0) | 34.0 (31.2–36.8) | ||
| Min–Max | 22.6–41.5 | 23.9–45.4 | ||
| SFA (gr) | n | 8 (9.6%) | 8 (9.6%) | 0.109 |
| Mean ± SD | 17.8 ± 4.5 | 15.4 ± 5.3 | ||
| Median (IQR) | 17.4 (15.8–20.8) | 16.6 (14.5–18.4) | ||
| Min–Max | 9.7–24.6 | 3.3–20.8 | ||
| SFA (%) | n | 8 (9.6%) | 8 (9.6%) | 0.195 |
| Mean ± SD | 10.3 ± 2.2 | 8.7 ± 2.1 | ||
| Median (IQR) | 9.5 (8.7–12.2) | 9.1 (8.4–9.8) | ||
| Min–Max | 7.5–13.7 | 3.9–10.9 | ||
| CHO (gr) | n | 8 (9.6%) | 8 (9.6%) | 1.000 |
| Mean ± SD | 208.8 ± 71.7 | 208.3 ± 46.3 | ||
| Median (IQR) | 208.8 (196.8–259.2) | 207.6 (191.8–246.1) | ||
| Min–Max | 53.0–288.2 | 114.8–260.7 | ||
| CHO (%) | n | 8 (9.6%) | 8 (9.6%) | 0.148 |
| Mean ± SD | 47.1 ± 8.4 | 51.4 ± 5.4 | ||
| Median (IQR) | 46.1 (43.5–54.8) | 53.1 (47.5–55.7) | ||
| Min–Max | 31.2–56.5 | 41.4–56.8 | ||
| Simple CHO (gr) | n | 8 (9.6%) | 8 (9.6%) | 0.383 |
| Mean ± SD | 48.9 ± 25.5 | 42.4 ± 11.6 | ||
| Median (IQR) | 44.5 (28.4–69.3) | 39.7 (32.1–53.2) | ||
| Min–Max | 17.1–89.9 | 29.7–59.7 | ||
| Simple CHO (%) | n | 8 (9.6%) | 8 (9.6%) | 0.742 |
| Mean ± SD | 11.3 ± 4.6 | 10.8 ± 3.5 | ||
| Median (IQR) | 10.8 (8.0–14.4) | 10.2 (8.3–11.9) | ||
| Min–Max | 4.9–19.3 | 7.3–18.2 | ||
| Fiber (gr) | n | 8 (9.6%) | 8 (9.6%) | 0.195 |
| Mean ± SD | 17.7 ± 6.2 | 15.2 ± 3.6 | ||
| Median (IQR) | 18.5 (14.5–21.5) | 15.0 (13.0–17.4) | ||
| Min–Max | 6.3–26.9 | 9.5–21.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccini, B.; Felicioni, M.; Pessina, B.; Bertini, M.; Casalini, E.; Ceccotti, C.; Farina, S.; Ferrari, M.; Lenzi, L.; Monzali, F.; et al. Glycemic Control, Basal/Bolus Distribution, BMI and Meal Management in Children and Adolescents with Type 1 Diabetes and Advanced Hybrid Closed Loop. Nutrients 2023, 15, 4875. https://doi.org/10.3390/nu15234875
Piccini B, Felicioni M, Pessina B, Bertini M, Casalini E, Ceccotti C, Farina S, Ferrari M, Lenzi L, Monzali F, et al. Glycemic Control, Basal/Bolus Distribution, BMI and Meal Management in Children and Adolescents with Type 1 Diabetes and Advanced Hybrid Closed Loop. Nutrients. 2023; 15(23):4875. https://doi.org/10.3390/nu15234875
Chicago/Turabian StylePiccini, Barbara, Matteo Felicioni, Benedetta Pessina, Mattia Bertini, Emilio Casalini, Chiara Ceccotti, Silvia Farina, Marta Ferrari, Lorenzo Lenzi, Francesca Monzali, and et al. 2023. "Glycemic Control, Basal/Bolus Distribution, BMI and Meal Management in Children and Adolescents with Type 1 Diabetes and Advanced Hybrid Closed Loop" Nutrients 15, no. 23: 4875. https://doi.org/10.3390/nu15234875
APA StylePiccini, B., Felicioni, M., Pessina, B., Bertini, M., Casalini, E., Ceccotti, C., Farina, S., Ferrari, M., Lenzi, L., Monzali, F., & Toni, S. (2023). Glycemic Control, Basal/Bolus Distribution, BMI and Meal Management in Children and Adolescents with Type 1 Diabetes and Advanced Hybrid Closed Loop. Nutrients, 15(23), 4875. https://doi.org/10.3390/nu15234875






