Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries—A Population-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Variables
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chen, J.; Viboud, C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: A population-level observational study. Lancet Digit. Health 2020, 2, e201–e208. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Axfors, C.; Ioannidis, J.P. Infection fatality rate of COVID-19 in community-dwelling elderly populations. Eur. J. Epidemiol. 2022, 37, 235–249. [Google Scholar] [CrossRef]
- Massinga Loembe, M.; Tshangela, A.; Salyer, S.J.; Varma, J.K.; Ouma, A.E.O.; Nkengasong, J.N. COVID-19 in Africa: The spread and response. Nat. Med. 2020, 26, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Dyer, O. COVID-19: Excess deaths point to hidden toll in South Africa as cases surge. BMJ 2020, 370, m3038. [Google Scholar] [CrossRef]
- Okonji, E.F.; Okonji, O.C.; Mukumbang, F.C.; Van Wyk, B. Understanding varying COVID-19 mortality rates reported in Africa compared to Europe, Americas and Asia. Trop. Med. Int. Health 2021, 26, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Strang, P.; Furst, P.; Schultz, T. Excess deaths from COVID-19 correlate with age and socio-economic status. A database study in the Stockholm region. Upsala J. Med. Sci. 2020, 125, 297–304. [Google Scholar] [CrossRef]
- Mele, M.; Magazzino, C. Pollution, economic growth, and COVID-19 deaths in India: A machine learning evidence. Environ. Sci. Pollut. Res. Int. 2020, 28, 2669–2677. [Google Scholar] [CrossRef]
- Adams-Prassl, A.; Cloyne, J.; Costa Dias, M.; Parey, M.; Ziliak, J.P. The COVID-19 Economic Crisis. Fisc. Stud. 2020, 41, 489–492. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Chourdakis, M. A critical update on the role of mild and serious vitamin D deficiency prevalence and the COVID-19 epidemic in Europe. Nutrition 2022, 93, 111441. [Google Scholar] [CrossRef] [PubMed]
- Söderström, L.; Rosenblad, A.; Adolfsson, E.T.; Bergkvist, L. Malnutrition is associated with increased mortality in older adults regardless of the cause of death. Br. J. Nutr. 2017, 117, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.; Ovchinnikov, R.S. The relationship between nutrition and infectious diseases: A review. Biomed. Biotechnol. Res. J. 2018, 2, 168–172. [Google Scholar]
- Kearns, M.D.; Alvarez, J.A.; Seidel, N.; Tangpricha, V. Impact of vitamin D on infectious disease. Am. J. Med. Sci. 2015, 349, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.E.D.; Andrade-Silva, M.; Basso, P.J.; Câmara, N.O. Vitamin D and chronic kidney disease: Insights on lipid metabolism of tubular epithelial cell and macrophages in tubulointerstitial fibrosis. Front. Physiol. 2023, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Bzura, B.M. Vitamin D and Influenza-Prevention or Therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: Systematic review and meta-analysis of aggregate data from randomised controlled trials. medRxiv 2020, 9, 276–292. [Google Scholar] [CrossRef]
- Jayawardena, R.; Jeyakumar, D.T.; Francis, T.V.; Misra, A. Impact of the vitamin D deficiency on COVID-19 infection and mortality in Asian countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 757–764. [Google Scholar] [CrossRef]
- Demir, M.; Demir, F.; Aygun, H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J. Med. Virol. 2021, 93, 2992–2999. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 2020, 14, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Basińska-Lewandowska, M.; Lewandowski, K.; Horzelski, W.; Lewiński, A.; Skowrońska-Jóźwiak, E. Frequency of COVID-19 infection as a function of vitamin D levels. Nutrients 2023, 15, 1581. [Google Scholar] [CrossRef] [PubMed]
- Nikniaz, L.; Akbarzadeh, M.A.; Hosseinifard, H.; Hosseini, M. Vitamin D supplementation on mortality rate and clinical outcomes of COVID-19 patients: A systematic review and meta-analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Moghaddam, R.R.; Khorasanchi, Z.; Noor, A.R.; Moghadam, M.S.F.; Esfahani, A.J.; Alyakobi, A.K.M.; Alboresha, M.L.; Sharifan, P.; Bahari, A.; Rezvani, R.; et al. High-dose vitamin D supplementation is related to an improvement in serum alkaline phosphatase in COVID-19 patients; a randomized double-blinded clinical trial. J. Health Popul. Nutr. 2023, 42, 71. [Google Scholar] [CrossRef] [PubMed]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Pugach, I.Z.; Pugach, S. Strong correlation between prevalence of severe vitamin D deficiency and population mortality rate from COVID-19 in Europe. Wien. Klin. Wochenschr. 2021, 133, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Pham, H. Analyzing the relationship between the vitamin D deficiency and COVID-19 mortality rate and modeling the time-delay interactions between body’s immune healthy cells, infected cells, and virus particles with the effect of vitamin D levels. Math. Biosci. Eng. 2022, 19, 8975–9004. [Google Scholar] [CrossRef]
- Hasell, J.; Mathieu, E.; Beltekian, D.; Macdonald, B.; Giattino, C.; Ortiz-Ospina, E.; Roser, M.; Ritchie, H. A cross-country database of COVID-19 testing. Sci. Data 2020, 7, 345. [Google Scholar] [CrossRef]
- Our World in Data. Explore the Global Situation: COVID-19 Data Explorer 2020–2022. Available online: https://ourworldindata.org/coronavirus#explore-the-global-situation (accessed on 15 June 2023).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Herre, B.; Macdonald, B.; Mathieu, E.; Mersmann, S.; et al. Coronavirus Pandemic (COVID-19): Our World Data. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 15 June 2023).
- Singh, S.; Kaur, R.; Singh, R.K. Revisiting the role of vitamin D levels in the prevention of COVID-19 infection and mortality in European countries post infections peak. Aging Clin. Exp. Res. 2020, 32, 1609–1612. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.D.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.A.; Obermayer-Pietsch, B.; Bianchi, M.L.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Frisch, S.; Koertke, H.; Kuhn, J.; Dreier, J.; Obermayer-Pietsch, B.; Wehr, E.; Zittermann, A. Effect of vitamin D supplementation on testosterone levels in men. Horm. Metab. Res. 2011, 43, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.M.; Junker, T.G.; Boelt, S.G.; Cohen, A.S.; Munger, K.L.; Stenager, E.; Ascherio, A.; Boding, L.; Hviid, A. Vitamin D status and severity of COVID-19. Sci. Rep. 2022, 12, 19823. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.; Yue, S.; Xue, W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition 2021, 84, 111106. [Google Scholar] [CrossRef] [PubMed]
- Szeto, B.; Zucker, J.E.; LaSota, E.D.; Rubin, M.R.; Walker, M.D.; Yin, M.T.; Cohen, A. Vitamin D status and COVID-19 clinical outcomes in hospitalized patients. Endocr. Res. 2021, 46, 66–73. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.J.; Lyons, O.C.; Flynn, M.A.; Crowley, R.K.; Twomey, P.J.; Kilbane, M.T. COVID-19 pandemic and vitamin D: Rising trends in status and in daily amounts of vitamin D provided by supplements. BMJ Open 2022, 12, e059477. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Peng, J. Jackknife Empirical likelihood intervals for Spearman’s rho. Am. Actuar. J. 2011, 15, 475–486. [Google Scholar] [CrossRef]
- Vickers, A.J.; Altman, D.G. Statistics notes: Missing outcomes in randomised trials. BMJ 2013, 346, f3438. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 June 2023).
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Kümmel, L.S.; Krumbein, H.; Fragkou, P.C.; Hünerbein, B.L.; Reiter, R.; Papathanasiou, K.A.; Thölken, C.; Weiss, S.T.; Renz, H.; Skevaki, C. Vitamin D supplementation for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Front. Immunol. 2022, 13, 1023903. [Google Scholar] [CrossRef]
- Mariani, J.; Antonietti, L.; Tajer, C.; Ferder, L.; Inserra, F.; Cunto, M.S.; Brosio, D.; Ross, F.; Zylberman, M.; López, D.E.; et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 2022, 17, e0267918. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Dantas Damascena, A.; Galvao Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 62, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Varikasuvu, S.R.; Thangappazham, B.; Vykunta, A.; Duggina, P.; Manne, M.; Raj, H.; Aloori, S. COVID-19 andvitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti Infect. Ther. 2022, 20, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Radujkovic, A.; Merle, U. Reply to: Vitamin D Insufficiency May Account for Almost Nine of Ten COVID-19 Deaths: Time to Act. Comment on: Vitamin D Deficiency and Outcome of COVID-19 Patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R. Vitamin D in the prevention or treatment of COVID-19. Proc. Nutr. Soc. 2023, 82, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M. COVID-19 and the endocrine system: Exploring the unexplored. J. Endocrinol. Investig. 2020, 43, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Sulli, A.; Gotelli, E.; Casabella, A.; Paolino, S.; Pizzorni, C.; Alessandri, E.; Grosso, M.; Ferone, D.; Smith, V.; Cutolo, M. Vitamin D and lung outcomes in elderly COVID-19 patients. Nutrients 2021, 13, 717. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubée, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: The GERIA-COVID quasi-experimental study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Mandal, A.K.; Baktash, V.; Hosack, T.; Missouris, C.G. Vitamin D status and COVID-19 in older adults. Aging Clin. Exp. Res. 2020, 32, 2425–2426. [Google Scholar] [CrossRef]
- Lim, Z.J.; Subramaniam, A.; Reddy, M.P.; Blecher, G.; Kadam, U.; Afroz, A.; Billah, B.; Ashwin, S.; Kubicki, M.; Bilotta, F.; et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. a meta-analysis. Am. J. Respir. Crit. Care Med. 2021, 203, 54–66. [Google Scholar] [CrossRef]
- Zittermann, A.; Pilz, S.; Hoffmann, H.; März, W. Vitamin D and airway infections: A European perspective. Eur. J. Med. Res. 2016, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Kisters, K. Influence of drugs on vitamin D and calcium metabolism. Derm. Endocrinol. 2012, 4, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Buttriss, J.; Lanham-New, S.A. Is a vitamin D fortification strategy needed? Nutr. Bull. 2020, 45, 115. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, T.; Itkonen, S.T.; Lundqvist, A.; Erkkola, M.; Koskela, T.; Lakkala, K.; Dowling, K.G.; Hull, G.L.; Kröger, H.; Karppinen, J.; et al. The positive impact of general vitamin D food fortification policy on vitamin D status in a representative adult Finnish population: Evidence from an 11-y follow-up based on standardized 25-hydroxyvitamin D data. Am. J. Clin. Nutr. 2017, 105, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Vitamin D 2022. Available online: https://www.nice.org.uk/guidance/ng187 (accessed on 15 June 2023).
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 9, 322–350. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open. 2020, 3, e2019722. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanidou, E.; Vlachakis, D.; Tsarouhas, K.; Panidis, D.; Tsitsimpikou, C.; Darviri, C.; Kouretas, D.; Bacopoulou, F. The prognostic role of micronutrient status and supplements in COVID-19 outcomes: A systematic review. Food Chem. Toxicol. 2022, 162, 112901. [Google Scholar] [CrossRef] [PubMed]
- Gerken, J.; Zapata, D.; Kuivinen, D.; Zapata, I. Comorbidities, sociodemographic factors, and determinants of health on COVID-19 fatalities in the United States. Front. Public Health 2022, 10, 993662. [Google Scholar] [CrossRef]
- Fang, F.; Kasperzyk, J.L.; Shui, I.; Hendrickson, W.; Hollis, B.W.; Fall, K.; Ma, J.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; et al. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS ONE 2011, 6, e18625. [Google Scholar] [CrossRef]
- National Health Service Laboratory. Direct Public Vitamin D Testing from our NHS Laboratory in West Bromwich 2022. Available online: https://www.vitamindtest.org.uk/ (accessed on 15 June 2023).
- Karahan, S.; Katkat, F. Impact of Serum 25(OH) Vitamin D Level on Mortality in Patients with COVID-19 in Turkey. J. Nutr. Health Aging 2021, 25, 189–196. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Ebrahimi, M.; Pazoki, M.; Kafan, S.; Tabriz, H.M.; Hadadi, A.; Montazeri, M.; Nasiri, M.; Shirvani, A.; et al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS ONE 2020, 15, e0239799. [Google Scholar] [CrossRef]
- Tylavsky, F.A.; Cheng, S.; Lyytikäinen, A.; Viljakainen, H.; Lamberg-Allardt, C. Strategies to improve vitamin D status in northern European children: Exploring the merits of vitamin D fortification and supplementation. J. Nutr. 2006, 136, 1130–1134. [Google Scholar] [CrossRef]
- Public Health England. COVID-19: Understanding the Impact on BAME Communities 2020. Available online: https://www.gov.uk/government/publications/covid-19-understanding-the-impact-on-bame-communities (accessed on 15 June 2023).
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020, 93, 508–511. [Google Scholar] [CrossRef]
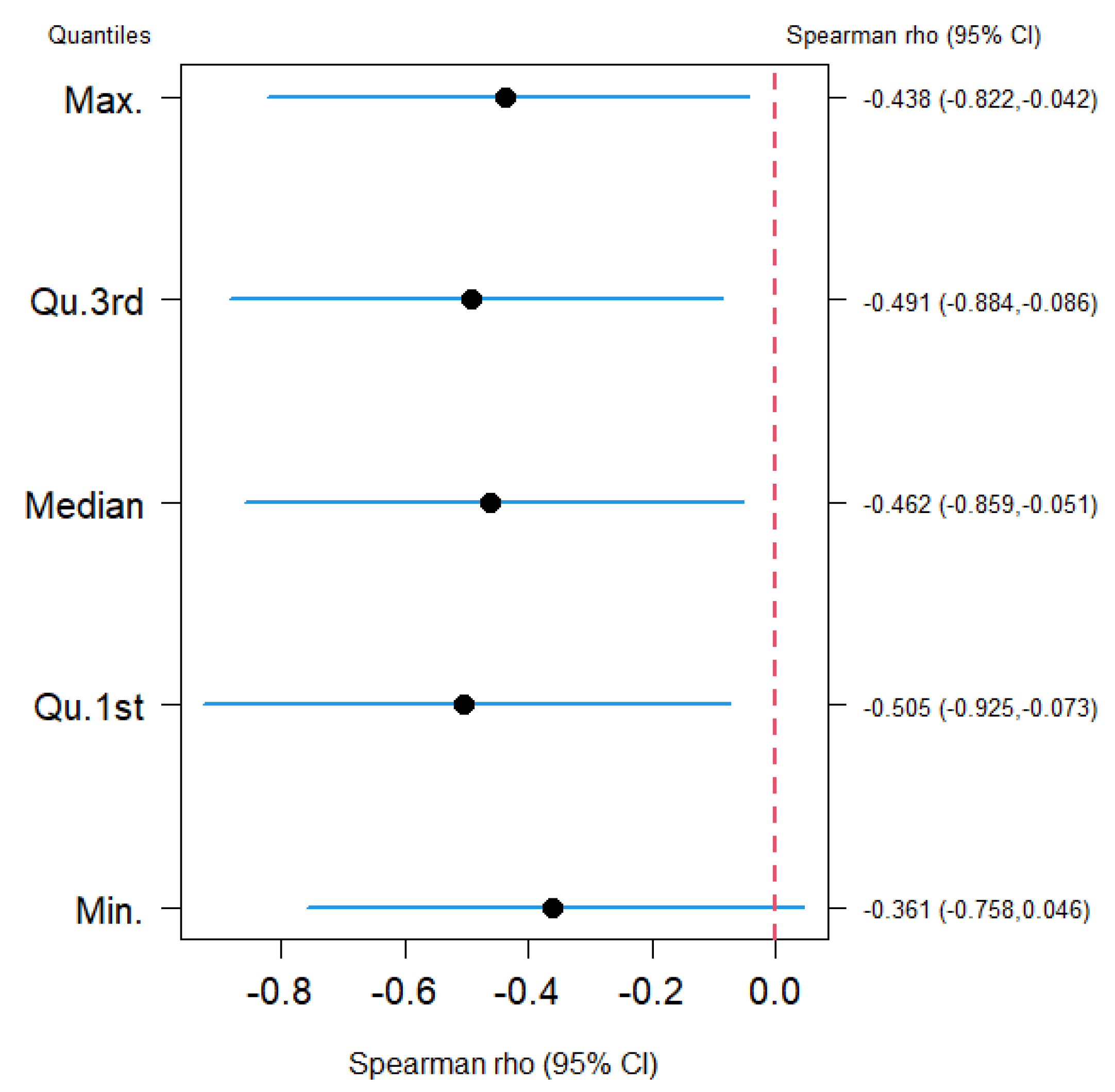
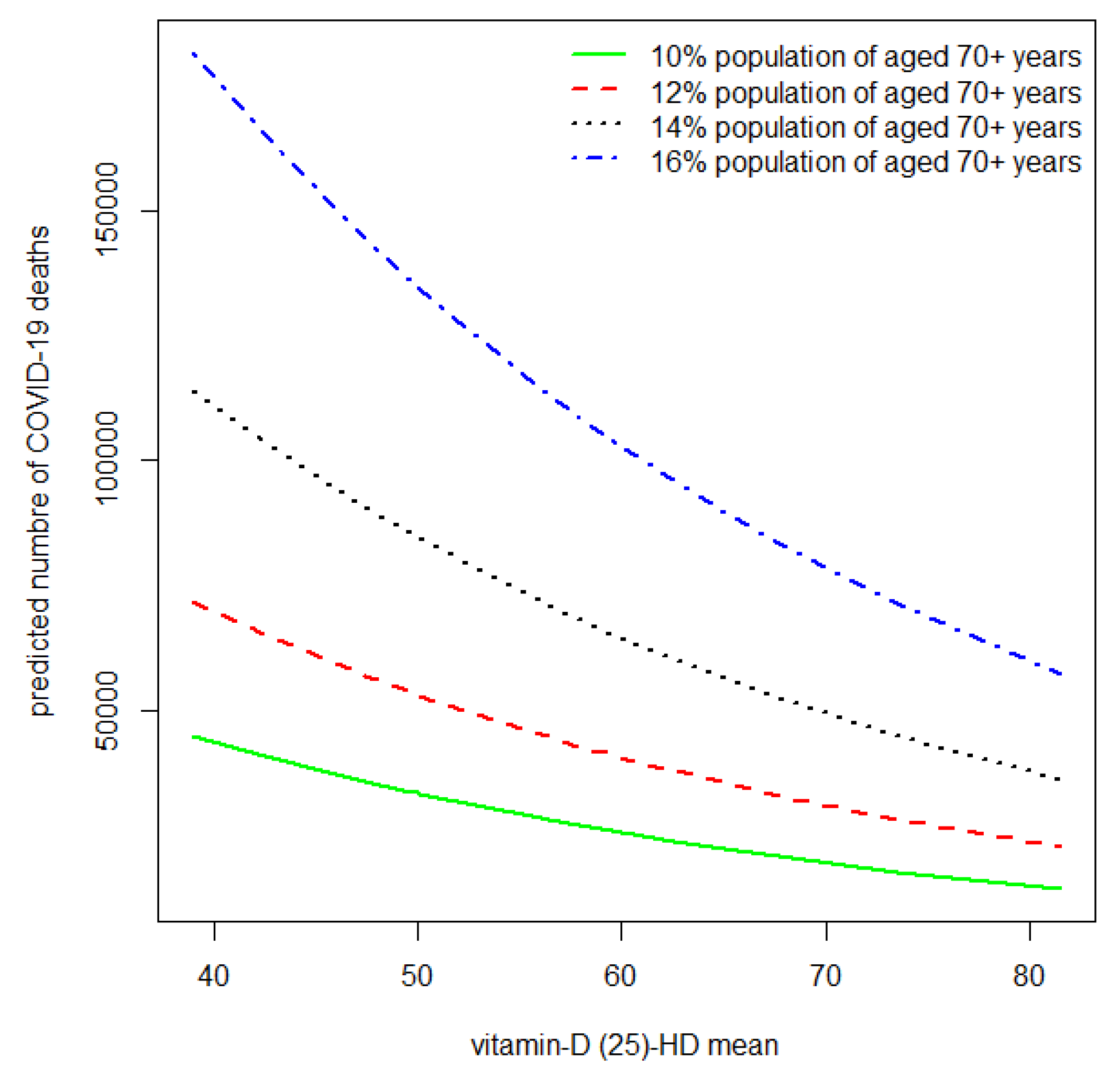

| Country/ Location | Min. | Qu.1st | Median | Qu.3rd | Max. | Population | Age 70+ Population | 25(OH) Vit D (nmol/L) | 25(OH) Vit D (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|
| Portugal | 1 | 6490 | 18,109 | 24,805 | 26824 | 10,270,857 | 14.924 | 39.00 | 15.63 |
| Spain | 1 | 53,606 | 89,004 | 114,946 | 121,416 | 47,558,632 | 13.799 | 42.50 | 17.03 |
| Switzerland | 2 | 7085 | 10,800 | 13,540 | 14,020 | 8,740,471 | 12.644 | 46.00 | 18.43 |
| United Kingdom | 3 | 89,820 | 166,690 | 206,036 | 226,977 | 67,508,936 | 12.527 | 47.40 | 18.99 |
| Belgium | 1 | 19,341 | 25,984 | 32,449 | 34,360 | 11,655,923 | 12.849 | 49.30 | 19.75 |
| Italy | 29 | 71,359 | 131,724 | 174,300 | 190,625 | 59,037,472 | 16.24 | 50.00 | 20.03 |
| Germany | 1 | 45,885 | 97,611 | 150,593 | 174,545 | 83,369,840 | 15.957 | 50.10 | 20.07 |
| Austria | 1 | 6979 | 13,658 | 20,513 | 22,518 | 8,939,617 | 13.748 | 56.00 | 22.44 |
| Ireland | 1 | 2228 | 5492 | 7927 | 8998 | 5,023,108 | 8.678 | 56.40 | 22.60 |
| Greece | 1 | 4507 | 15,519 | 32,077 | 37,052 | 10,384,972 | 14.524 | 57.95 | 23.22 |
| The Netherlands | 1 | 10,891 | 18,277 | 22,564 | 22,992 | 17,564,020 | 11.881 | 59.50 | 23.84 |
| France | 2 | 62,051 | 115,014 | 149,846 | 163,787 | 67,813,000 | 13.079 | 60.00 | 24.04 |
| Hungary | 1 | 8951 | 30,492 | 47,083 | 48,790 | 9,967,304 | 11.976 | 60.60 | 24.28 |
| Czechia | 1 | 11,242 | 30,707 | 40,807 | 42,806 | 10,493,990 | 11.580 | 62.50 | 25.04 |
| Denmark | 1 | 1140 | 2696 | 6833 | 8736 | 5,882,259 | 12.325 | 65.00 | 26.04 |
| Norway | 1 | 421 | 929 | 3958 | 5556 | 5,434,324 | 10.813 | 65.00 | 26.04 |
| Finland | 1 | 574 | 1199 | 5782 | 9798 | 5,540,745 | 13.264 | 67.70 | 27.12 |
| Sweden | 1 | 9229 | 14,999 | 19,796 | 24,391 | 10,549,349 | 13.433 | 73.50 | 29.45 |
| Slovakia | 1 | 1732 | 12,886 | 20,322 | 21,167 | 5,643,455 | 9.167 | 81.50 | 32.65 |
| Multivariate | ||
|---|---|---|
| Variable | RR (95% CI) | Robust z-Value (p-Value) |
| 25(OH) vitamin D ≤ 50 nmol/L | 1.000 (Reference Group) | |
| 25(OH) vitamin D > 50 nmol/L | 0.794 (0.662, 0.953) | −2.478 (0.013) |
| Age 70+ years in population | 0.981 (0.926, 1.038) | −0.676 (0.499) |
| Year 2020 | 1.000 (Reference Group) | |
| Year 2021 | 0.878 (0.720, 1.072) | −1.274 (0.203) |
| Year 2022 | 0.540 (0.428, 0.681) | −5.214 (<0.001) |
| Year 2023 | 0.196 (0.154, 0.249) | −13.204 (<0.001) |
| Multivariate | ||
|---|---|---|
| Variable | RR (95% CI) | Robust z-Value (p-Value) |
| 25(OH) vitamin D ≤ 50 nmol/L | 1.000 (Reference Group) | |
| 25(OH) vitamin D > 50 nmol/L | 0.780 (0.653, 0.931) | −2.750 (0.006) |
| age 70+ | 0.977 (0.924, 1.032) | −0.840 (0.401) |
| Year 2020 | 1.000 (Reference Group) | |
| Year 2021 | 0.878 (0.715, 1.078) | −1.241 (0.215) |
| Year 2022 | 0.525 (0.418, 0.659) | −5.545 (<0.001) |
| Year 2023 | 0.204 (0.154, 0.269) | −11.188 (<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.S.; Juber, N.F.; Al-Naseri, H.; Heumann, C.; Ali, R.; Oliver, T. Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries—A Population-Based Study. Nutrients 2023, 15, 4818. https://doi.org/10.3390/nu15224818
Ahmad AS, Juber NF, Al-Naseri H, Heumann C, Ali R, Oliver T. Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries—A Population-Based Study. Nutrients. 2023; 15(22):4818. https://doi.org/10.3390/nu15224818
Chicago/Turabian StyleAhmad, Amar S., Nirmin F. Juber, Heba Al-Naseri, Christian Heumann, Raghib Ali, and Tim Oliver. 2023. "Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries—A Population-Based Study" Nutrients 15, no. 22: 4818. https://doi.org/10.3390/nu15224818
APA StyleAhmad, A. S., Juber, N. F., Al-Naseri, H., Heumann, C., Ali, R., & Oliver, T. (2023). Association between Average Vitamin D Levels and COVID-19 Mortality in 19 European Countries—A Population-Based Study. Nutrients, 15(22), 4818. https://doi.org/10.3390/nu15224818







