Aqueous Extract of Brassica rapa L.’s Impact on Modulating Exercise-Induced Fatigue via Gut–Muscle Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Quantification of Samples
2.2.1. Preparation of Aqueous Brassica rapa L. Extract
2.2.2. Quantification of Constituents
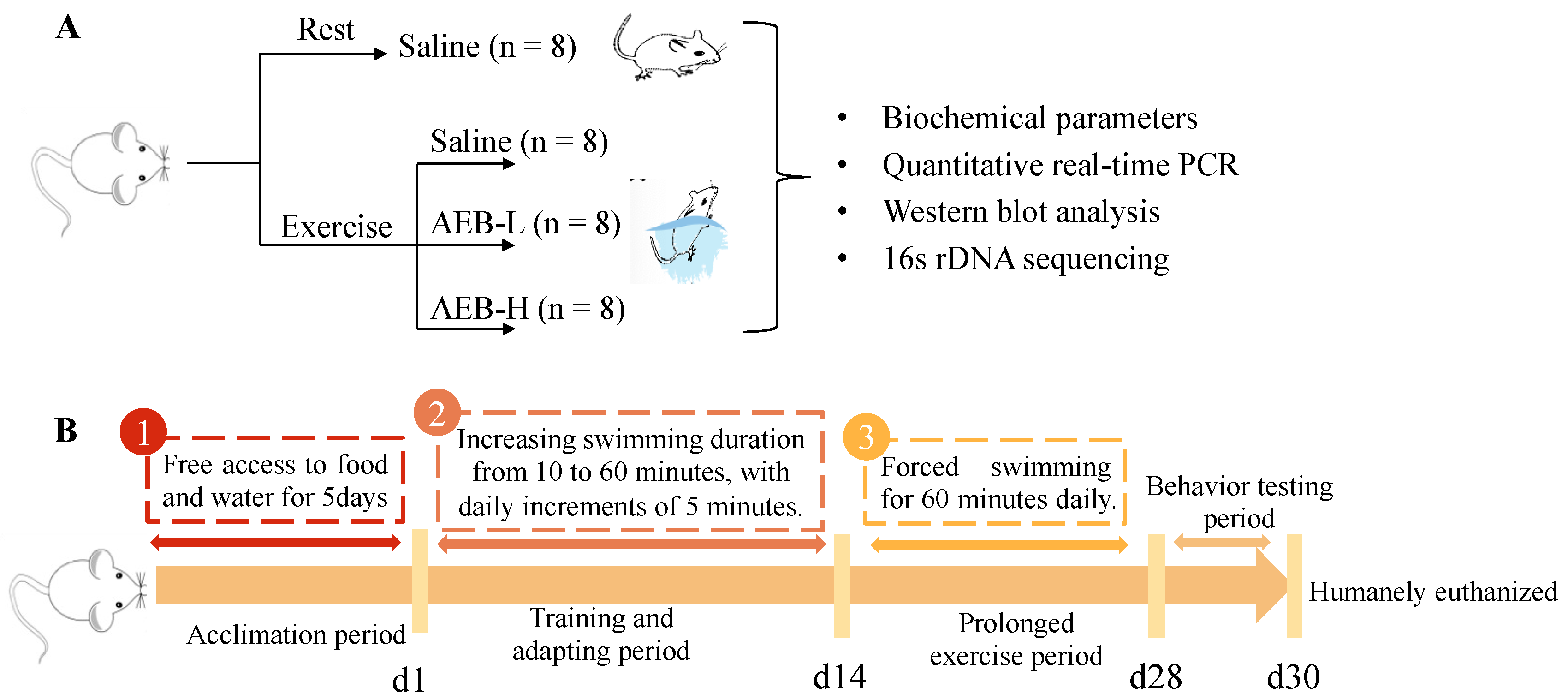
2.3. Animal Treatment
2.3.1. Experimental Animals
2.3.2. Experimental Groups and Treatments
- -
- Control group was gavaged with saline (Con);
- -
- Exercise group was subjected to swimming and gavaged with saline (Ex);
- -
- Exercise group was subjected to swimming and gavaged with AEB powder at a low dose of 0.5 g/kg body weight (AEB-L);
- -
- Exercise group was subjected to swimming and gavaged with AEB powder at a high dose of 1 g/kg body weight (AEB-H).
2.3.3. Exercise Protocol
2.3.4. Sample Collection
2.4. Biomarkers Determination
2.5. Quantitative Real-Time PCR (q Rt-PCR)
2.6. Western Blot Analysis
2.7. Colon Histological Analysis
2.8. 16S rDNA Gene Sequencing and Analysis
2.9. Data Analysis
3. Results and Discussion
3.1. Nutrient Intake of Mice
Measurement of AEB Main Components
3.2. Anti-Fatigue Effect of Brassica rapa L. (AEB)
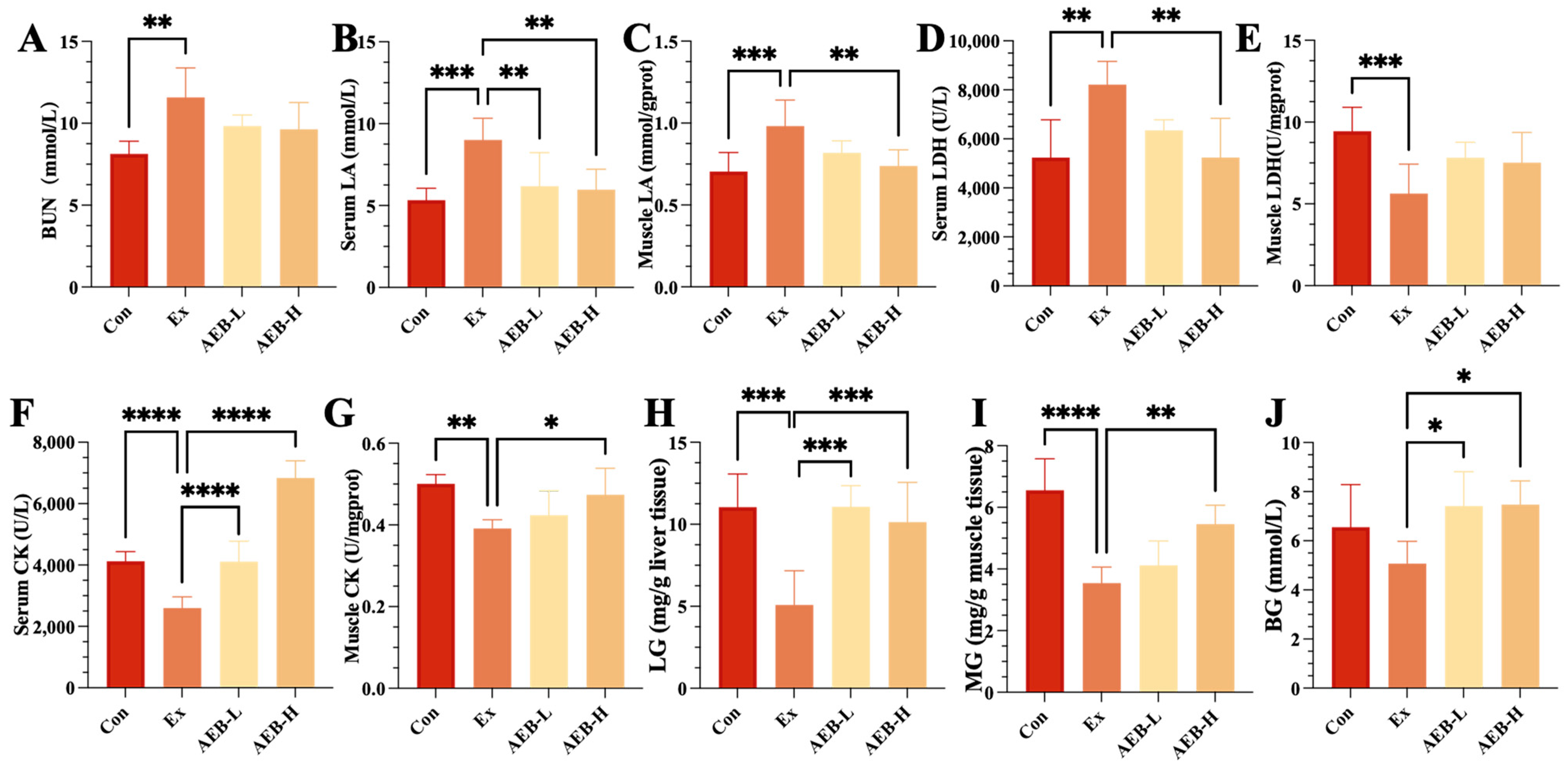
3.2.1. Effect on Fatigue-Related Biomarkers
3.2.2. Effect on Glycogen Storage
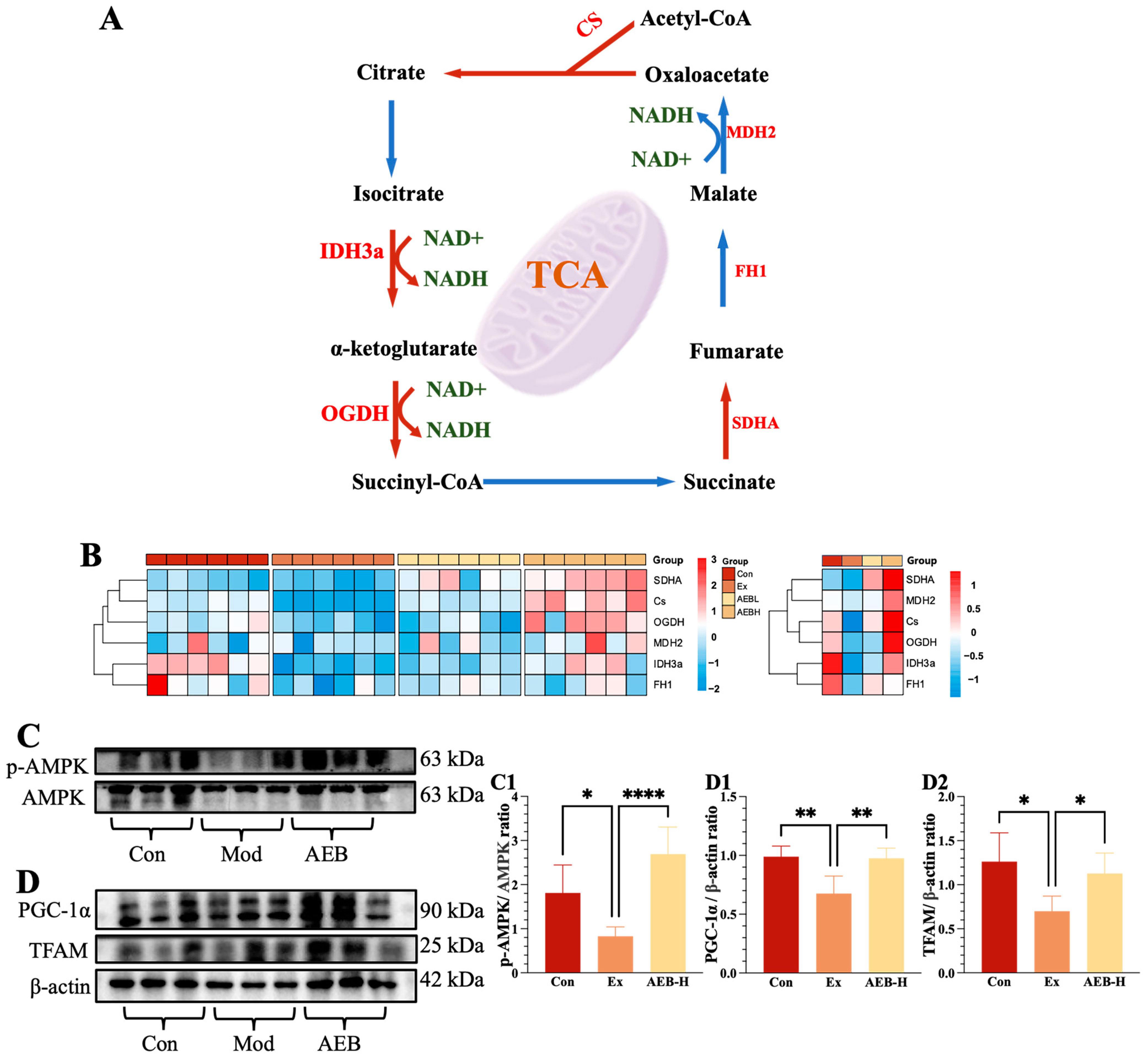
3.3. Effect of AEB on Energy Metabolism Function in Muscle
3.3.1. Effect on Glycolysis Process
3.3.2. Effect on Mitochondrial Function of Muscle
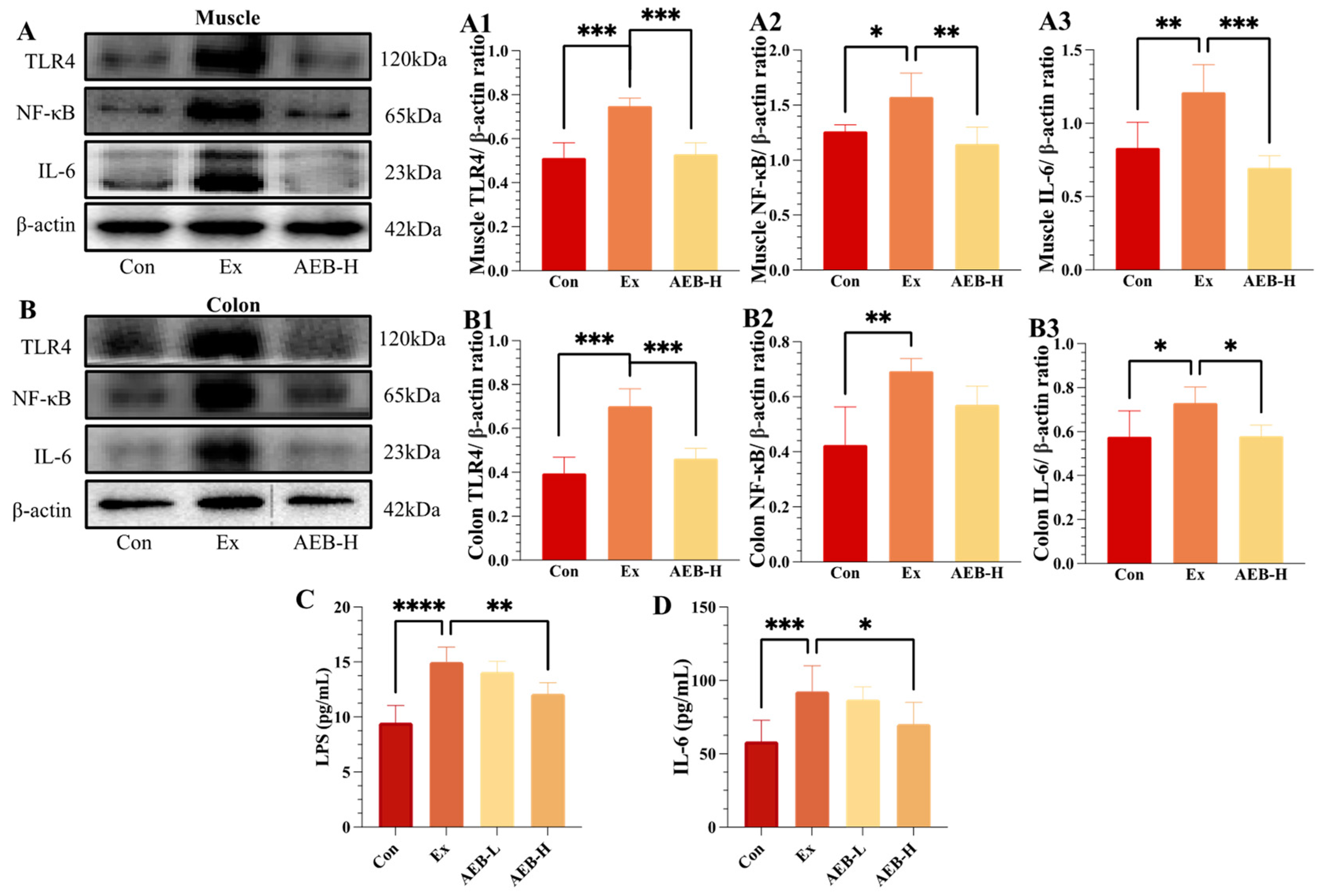
3.4. Effect of AEB on Inflammatory Response
3.4.1. Effect on TLR4/NF-κB Pathways
3.4.2. Effect on LPS Levels
3.5. Effect of AEB on Intestinal Integrity
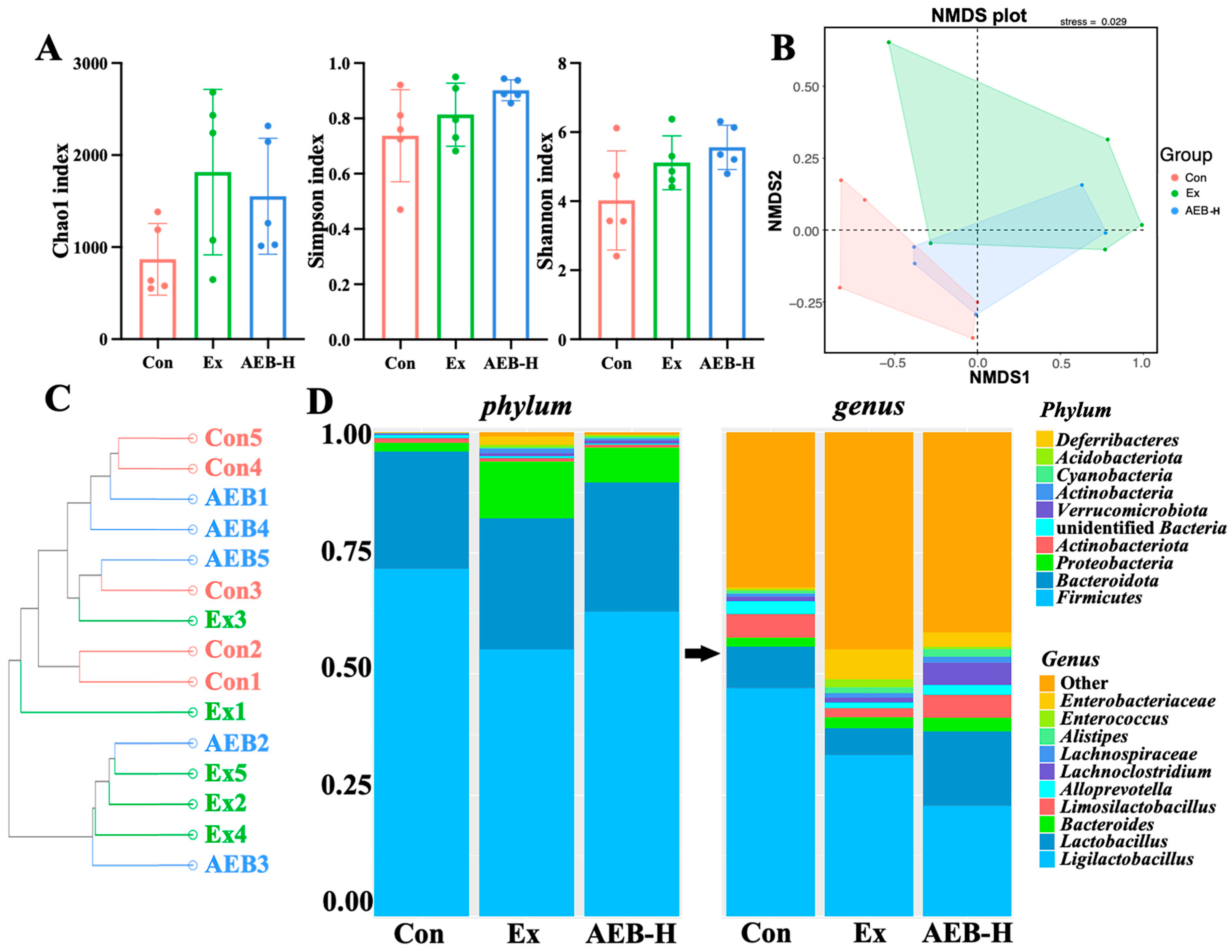
3.6. Effect of AEB on Gut Microbiome
3.6.1. Changes in Microbial Diversity
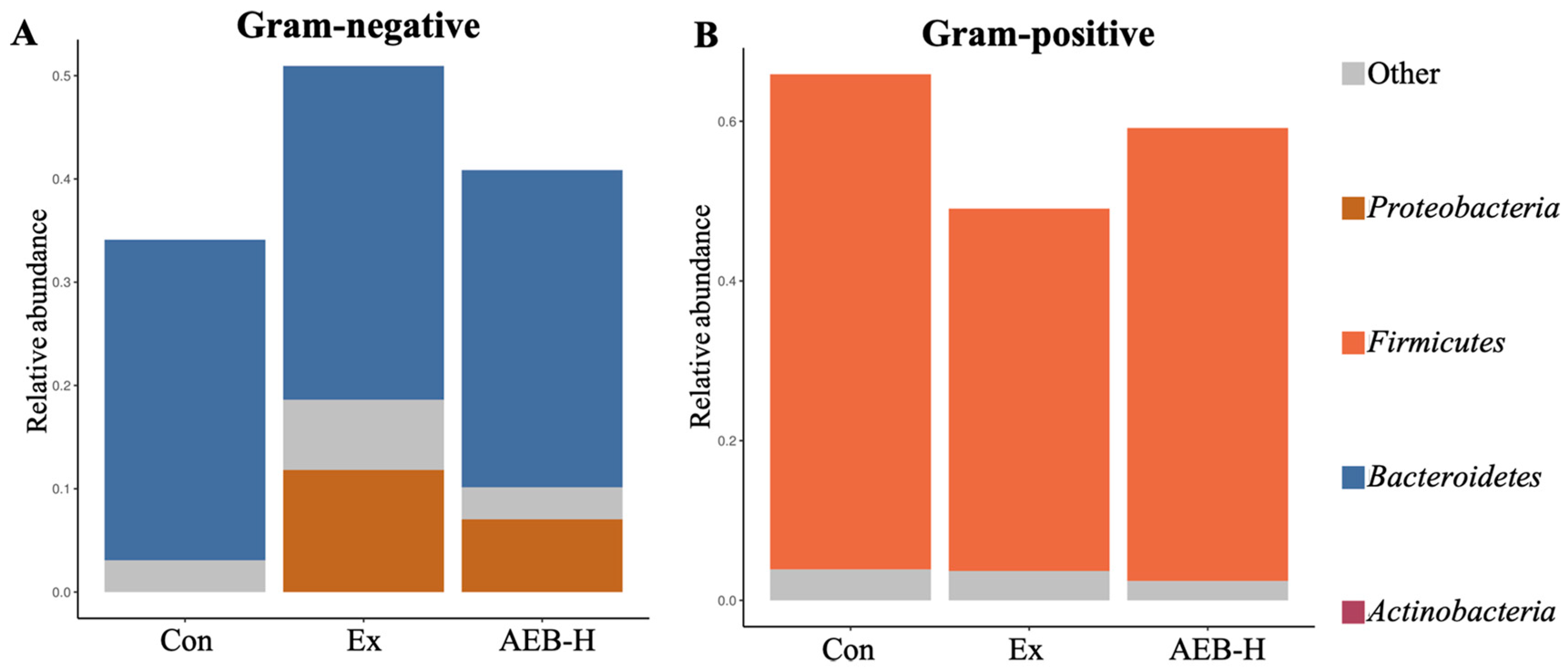
3.6.2. The Phenotypes of Microbial
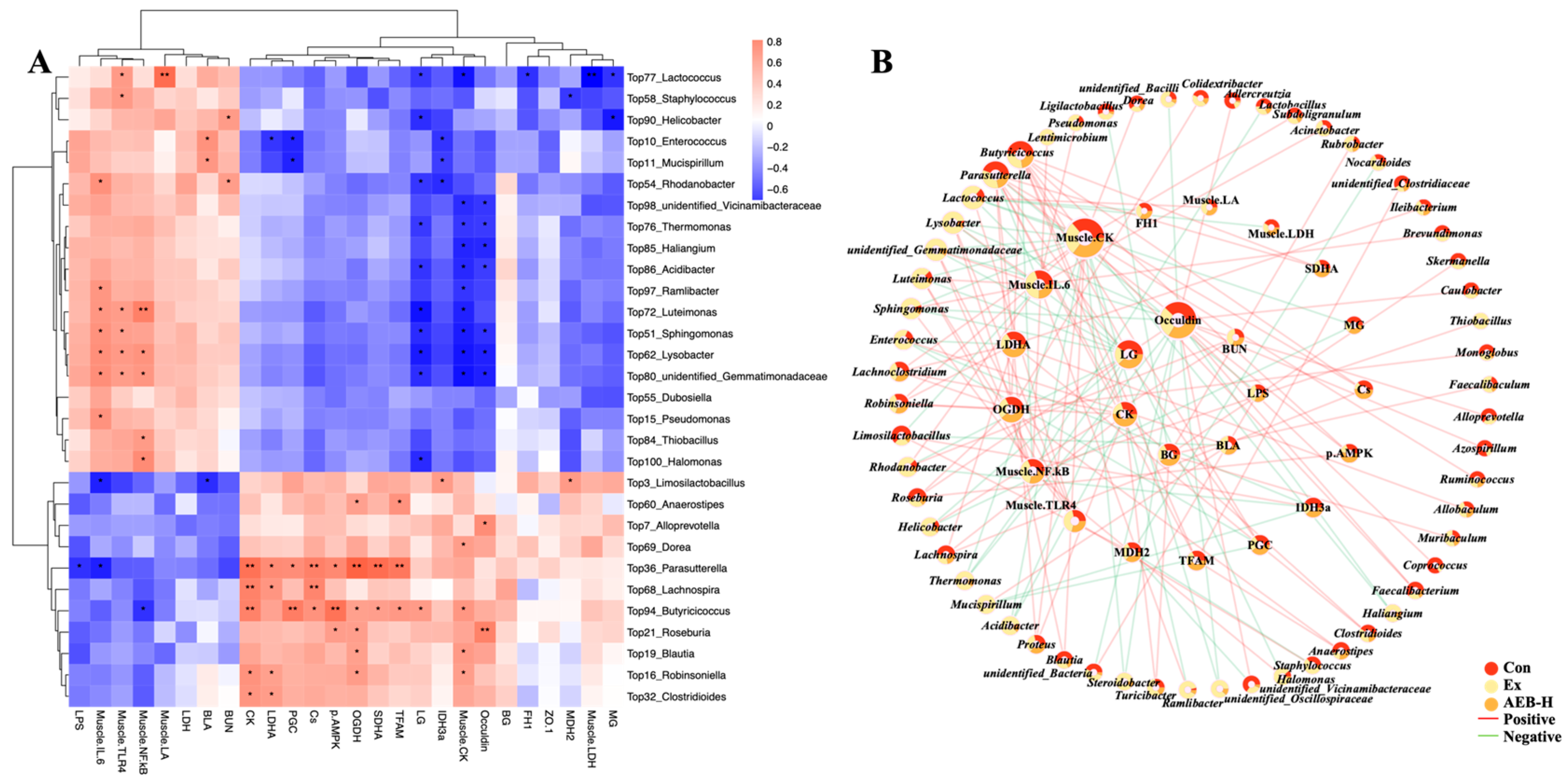
3.6.3. Correlation Analysis of Gut Microbiome and Biochemical Parameters
4. Discussion
- (1)
- Enterococcus leads to serious infection in the body, and inducing the inflammatory response. As previously reported, an increase in Enterococcus abundance has been found in patients with Parkinson’s disease, suggesting a possible link to neuroinflammation [48]. Furthermore, another study found that inhibiting Enterococcus could alleviate hepatic inflammation [49]. Enterococcus, among the top ten most abundant genera in the microbiome, significantly correlated with increased lactic acid during exercise and inhibited PGC-1α expression, potentially disrupting muscle energy metabolism. Furthermore, it positively correlated with inflammatory markers. These suggests that Enterococcus can impact exercise physiology and inflammation.
- (2)
- As reported, Sphingomonas was enriched in the feces of PD patients, suggesting a potential role in PD pathogenesis or response [50]. This bacterium can cause infections in immunocompromised individuals. In this research, it showed significant correlation with inflammatory responses and was associated with a strain on intestinal integrity. These findings suggest that Sphingomonas may contribute to inflammation and disrupt gut health.
- (3)
- Alterations in Mucispirillum’s abundance and function have been observed in certain disease conditions, such as inflammatory bowel disease and obesity [51]. In this research, it showed a positive correlation with the inflammation indices and was associated with mitochondrial disorders.
- (4)
- Pseudomonas, a known pathogenic bacterium, is often associated with certain neural diseases and is considered a trigger for neural inflammation [52]. In our research, we also observed its significant correlation with inflammation.
- (1)
- Parasutterella can link fatty acid biosynthesis pathways, enhancing the energy metabolizing progress and reducing the risk of obesity [53]. In this study, Parasutterella showed a significant correlation with these energy metabolism pathways. Additionally, it was found to inhibit the progression of infections towards inflammation.
- (2)
- (3)
- Roseburia is also a bacterium that can induce the production of butyrate [56].
- (4)
- Lachnospira can help to metabolize dietary carbohydrates and produce SCFAs such as acetate and butyrate [57].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Liu, C.; Qian, H. Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review. Plants 2022, 11, 2004. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef]
- Hou, Y.; Tang, Y.; Wang, X.; Ai, X.; Wang, H.; Li, X.; Chen, X.; Zhang, Y.; Hu, Y.; Meng, X.; et al. Rhodiola Crenulata ameliorates exhaustive exercise-induced fatigue in mice by suppressing mitophagy in skeletal muscle. Exp. Ther. Med. 2020, 20, 3161–3173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Liang, S.; Xu, J.; Wei, M.; Du, Z.; Qiang, S. Guben Xiezhuo Decoction inhibits M1 polarization through the Raf1/p-Elk1 signaling axis to attenuate renal interstitial fibrosis. J. Ethnopharmacol. 2024, 319, 117189. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.-J.; Yang, H.-Y.; He, Y.-L.; Li, Y.; Gao, K. New acylglycosides from the roots of Rheum palmatum L. Phytochem. Lett. 2022, 49, 83–87. [Google Scholar] [CrossRef]
- Paul, S.; Geng, C.-A.; Yang, T.-H.; Yang, Y.-P.; Chen, J.-J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa). J. Food Sci. 2019, 84, 19–30. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, G.; Peng, Y. A Critical Review on Phytochemical Profile and Biological Effects of Turnip (Brassica rapa L.). Front. Nutr. 2021, 8, 721733. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Li, Z.; Wang, X.; Qian, H. An integrated network pharmacology and metabolomics approach to reveal the immunomodulatory mechanism of Brassica rapa L. (Tibetan Turnip) in fatigue mice. Food Funct. 2022, 13, 11097–11110. [Google Scholar] [CrossRef]
- Finsterer, J.; Mahjoub, S.Z. Fatigue in Healthy and Diseased Individuals. Am. J. Hosp. Palliat. Med. 2013, 31, 562–575. [Google Scholar] [CrossRef]
- Matura, L.A.; Malone, S.; Jaime-Lara, R.; Riegel, B. A Systematic Review of Biological Mechanisms of Fatigue in Chronic Illness. Biol. Res. Nurs. 2018, 20, 410–421. [Google Scholar] [CrossRef]
- Kizina, K.; Stolte, B.; Totzeck, A.; Bolz, S.; Schlag, M.; Ose, C.; von Velsen, O.; Kleinschnitz, C.; Hagenacker, T. Fatigue in adults with spinal muscular atrophy under treatment with nusinersen. Sci. Rep. 2020, 10, 11069. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Xu, F.; Liu, G.; Pang, B.; Liao, N.; Li, H.; Shi, J. Gut microbiota as a potential target for developing anti-fatigue foods. Crit. Rev. Food Sci. Nutr. 2023, 63, 3065–3080. [Google Scholar] [CrossRef]
- Yeh, W.-L.; Hsu, Y.-J.; Ho, C.-S.; Ho, H.-H.; Kuo, Y.-W.; Tsai, S.-Y.; Huang, C.-C.; Lee, M.-C. Lactobacillus plantarum PL-02 Supplementation Combined With Resistance Training Improved Muscle Mass, Force, and Exercise Performance in Mice. Front. Nutr. 2022, 9, 896503. [Google Scholar] [CrossRef]
- Chaffer, T.J.; Jafarnezhad-Ansariha, F. The gut–muscle axis shapes exercise performance. J. Physiol. 2023, 601, 3445–3446. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, C.; Hua, H.; Zhao, W.; Zhu, H.; Cheng, Y.; Guo, Y.; Qian, H. Effect of polysaccharides from Tibetan turnip (Brassica rapa L.) on the gut microbiome after in vitro fermentation and in vivo metabolism. Food Funct. 2022, 13, 3063–3076. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.5-2016; Determination of Protein in Foods. Ministry of Health of the People’s Republic of China: Beijing, China, 2016.
- T/AHFIA 005-2018; Determination of Total Polyphenol in Plant Extracts and Its Products. Spectrophotometric Method. Anhui Food Industry Association: Hefei, China, 2018.
- GB/T 20574-2006; Method for Determination of Total Flavonoids in Propolis. Colorimetric Method. Insitute of Apicultural Research: Beijing, China, 2006.
- NY/T 2332-2013; Determination of Total Sugars in Red Ginseng—Spectrophotometry. Jilin Agricultural University: Changchun, China, 2013.
- DB22/T 1668-2012; Determination of Total Ginseng Saponin Component in Ginseng Foods. Spectrophotometric Method. Health Commission of Jilin Province: Changchun, China, 2012.
- GB 5009.168-2016; Determination of Eicosapentaenoic Acid and Docosahexaenoic Acid in Foods. National Health Comission of the People’s Republic of China: Beijing, China, 2016.
- GB/T 5009.10-2003; Determination of Crude Fiber in Vegetable Foods. Ministry of Health of the People’s Republic of China: Beijing, China, 2003.
- GB 5009.124-2016; Determination of Amino Acid in Food. National Health Comission of the People’s Republic of China: Beijing, China, 2016.
- Yan, K.; Gao, H.; Liu, X.; Zhao, Z.; Gao, B.; Zhang, L. Establishment and identification of an animal model of long-term exercise-induced fatigue. Front. Endocrinol. 2022, 13, 915937. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Feng, B.-N.; Hu, B.; Cheng, Y.-L.; Guo, Y.-H.; Qian, H. Neuroprotection of chicoric acid in a mouse model of Parkinson’s disease involves gut microbiota and TLR4 signaling pathway. Food Funct. 2022, 13, 2019–2032. [Google Scholar] [CrossRef]
- Ward, T.; Larson, J.; Meulemans, J.; Hillmann, B.; Lynch, J.; Sidiropoulos, D.; Spear, J.R.; Caporaso, G.; Blekhman, R.; Knight, R.; et al. BugBase predicts organism-level microbiome phenotypes. bioRxiv 2017, 133462. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, H.; Hua, H.; Liu, C.; Cheng, Y.; Guo, Y.; Du, P.; Qian, H. Anti-fatigue activity of Brassica rapa L. extract and correlation among biochemical changes in forced swimming mice. Food Biosci. 2022, 47, 101633. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. The macamide relieves fatigue by acting as inhibitor of inflammatory response in exercising mice: From central to peripheral. Eur. J. Pharmacol. 2022, 917, 174758. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Suzuki, M.; Saito, T.; Miyado, K. Emerging Role of TCA Cycle-Related Enzymes in Human Diseases. Int. J. Mol. Sci. 2021, 22, 13057. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Wei, Z.; Wang, X.; Gao, Y.; Wang, D.; Liu, X.; Bai, C.; Su, G.; Yang, L.; Li, G. Myostatin Knockout Affects Mitochondrial Function by Inhibiting the AMPK/SIRT1/PGC1α Pathway in Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 13703. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heredero, G.; Gómez de las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.; Tiwari-Pandey, R.; Pandey, N.R. Mitochondria: The indispensable players in innate immunity and guardians of the inflammatory response. J. Cell Commun. Signal. 2019, 13, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, Z.; Luo, Y.; Yang, F.; Cao, F.; Luo, F.; Lin, Q. Dietary Polysaccharides Exert Anti-Fatigue Functions via the Gut-Muscle Axis: Advances and Prospectives. Foods 2023, 12, 3083. [Google Scholar] [CrossRef]
- Cook, M.D.; Martin, S.A.; Williams, C.; Whitlock, K.; Wallig, M.A.; Pence, B.D.; Woods, J.A. Forced treadmill exercise training exacerbates inflammation and causes mortality while voluntary wheel training is protective in a mouse model of colitis. Brain Behav. Immun. 2013, 33, 46–56. [Google Scholar] [CrossRef]
- Neumann, J.; Ziegler, K.; Gelléri, M.; Fröhlich-Nowoisky, J.; Liu, F.; Bellinghausen, I.; Schuppan, D.; Birk, U.; Pöschl, U.; Cremer, C.; et al. Nanoscale distribution of TLR4 on primary human macrophages stimulated with LPS and ATI. Nanoscale 2019, 11, 9769–9779. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Twisk, F.N.M.; Kubera, M.; Ringel, K.; Leunis, J.-C.; Geffard, M. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J. Affect. Disord. 2012, 136, 909–917. [Google Scholar] [CrossRef]
- McGregor, N.R.; Armstrong, C.W.; Lewis, D.P.; Gooley, P.R. Post-Exertional Malaise Is Associated with Hypermetabolism, Hypoacetylation and Purine Metabolism Deregulation in ME/CFS Cases. Diagnostics 2019, 9, 70. [Google Scholar] [CrossRef]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal Barrier Dysfunction, LPS Translocation, and Disease Development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef]
- Barger, K.; Langsetmo, L.; Orwoll, E.S.; Lustgarten, M.S.; Osteoporotic Fractures in Men Research, G. Investigation of the Diet-Gut-Muscle Axis in the Osteoporotic Fractures in Men Study. J. Nutr. Health Aging 2020, 24, 445–452. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging Gut Microbiota at the Cross-Road between Nutrition, Physical Frailty, and Sarcopenia: Is There a Gut–Muscle Axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Wang, N.; Li, R.; Feng, B.; Cheng, Y.; Guo, Y.; Qian, H. Chicoric Acid Prevents Neuroinflammation and Neurodegeneration in a Mouse Parkinson’s Disease Model: Immune Response and Transcriptome Profile of the Spleen and Colon. Int. J. Mol. Sci. 2022, 23, 2031. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Chen, C.; Li, J.; Chen, X.; Li, Y.; Tang, W.; Jin, L.; Zhang, Y. Effects of Tibetan turnip (Brassica rapa L.) on promoting hypoxia-tolerance in healthy humans. J. Ethnopharmacol. 2017, 195, 246–254. [Google Scholar] [CrossRef]
- Young, A.; Oldford, C.; Mailloux, R.J. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2020, 28, 101339. [Google Scholar] [CrossRef] [PubMed]
- Nacul, L.; de Barros, B.; Kingdon, C.C.; Cliff, J.M.; Clark, T.G.; Mudie, K.; Dockrell, H.M.; Lacerda, E.M. Evidence of Clinical Pathology Abnormalities in People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) from an Analytic Cross-Sectional Study. Diagnostics 2019, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.B.; Dolezal, B.A.; Neufeld, E.V.; Shieh, P.; Jenner, J.R.; Riley, M. Exercise responses in patients with chronically high creatine kinase levels. Muscle Nerve 2017, 56, 264–270. [Google Scholar] [CrossRef]
- dos Santos, R.S.; Veras, F.P.; Ferreira, D.W.; Sant’Anna, M.B.; Lollo, P.C.B.; Cunha, T.M.; Galdino, G. Involvement of the Hsp70/TLR4/IL-6 and TNF-α pathways in delayed-onset muscle soreness. J. Neurochem. 2020, 155, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Gaab, J.; Rohleder, N.; Heitz, V.; Engert, V.; Schad, T.; Schurmeyer, T.H.; Ehlert, U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology 2005, 30, 188–198. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Mo, C.; Liu, X.; Li, J.; Yan, Z.; Qian, Y.; Lai, Y.; Xu, S.; Yang, X.; et al. Association Between Microbial Tyrosine Decarboxylase Gene and Levodopa Responsiveness in Patients With Parkinson Disease. Neurology 2022, 99, e2443–e2453. [Google Scholar] [CrossRef]
- Tan, J.; Hu, R.; Gong, J.; Fang, C.; Li, Y.; Liu, M.; He, Z.; Hou, D.-X.; Zhang, H.; He, J.; et al. Protection against Metabolic Associated Fatty Liver Disease by Protocatechuic Acid. Gut Microbes 2023, 15, 2238959. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Dikiy, S.; Bhattarai, S.K.; Chinen, T.; Matheis, F.; Calafiore, M.; Hoyos, B.; Hanash, A.; Mucida, D.; Bucci, V.; et al. Extrathymically Generated Regulatory T Cells Establish a Niche for Intestinal Border-Dwelling Bacteria and Affect Physiologic Metabolite Balance. Immunity 2018, 48, 1245–1257.e9. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Zheng, Z.; Afridi, M.I.; Zhang, S.; Wan, Z.; Zhang, X.; Stingelin, L.; Wang, Y.; Tu, H. GABAergic signaling between enteric neurons and intestinal smooth muscle promotes innate immunity and gut defense in Caenorhabditis elegans. Immunity 2023, 56, 1515–1532.e9. [Google Scholar] [CrossRef]
- Henneke, L.; Schlicht, K.; Andreani, N.A.; Hollstein, T.; Demetrowitsch, T.; Knappe, C.; Hartmann, K.; Jensen-Kroll, J.; Rohmann, N.; Pohlschneider, D.; et al. A dietary carbohydrate—Gut Parasutterella—Human fatty acid biosynthesis metabolic axis in obesity and type 2 diabetes. Gut Microbes 2022, 14, 2057778. [Google Scholar] [CrossRef]
- Li, Q.; Liu, W.; Zhang, H.; Chen, C.; Liu, R.; Hou, H.; Luo, Q.; Yu, Q.; Ouyang, H.; Feng, Y.; et al. α-D-1,3-glucan from Radix Puerariae thomsonii improves NAFLD by regulating the intestinal flora and metabolites. Carbohydr. Polym. 2023, 299, 120197. [Google Scholar] [CrossRef]
- Engels, C.; Ruscheweyh, H.J.; Beerenwinkel, N.; Lacroix, C.; Schwab, C. The Common Gut Microbe Eubacterium hallii also Contributes to Intestinal Propionate Formation. Front. Microbiol. 2016, 7, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, S.; Liu, Z.; Wei, H.; Zhang, L.; He, M.; Pei, F.; Zhang, J.; Sun, Q.; Duan, L. Increased Expression of Colonic Mucosal Melatonin in Patients with Irritable Bowel Syndrome Correlated with Gut Dysbiosis. Genom. Proteom. Bioinform. 2020, 18, 708–720. [Google Scholar] [CrossRef]
- Yang, B.; Ding, M.; Chen, Y.; Han, F.; Yang, C.; Zhao, J.; Malard, P.; Stanton, C.; Ross, R.P.; Zhang, H.; et al. Development of gut microbiota and bifidobacterial communities of neonates in the first 6 weeks and their inheritance from mother. Gut Microbes 2021, 13, 1908100. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sense Primer 5′-3′ | Anti-Sense Primer 5′-3′ |
|---|---|---|
| CS | CAGAATTAAGACCTTCAAGCAGCAACA | TCAAGAACCGAAGTCTCATACACAAGT |
| IDH3a | GCAATGTCACAGCAATTCAAGGA | ACGAAGCAACAGATTCATAGATGGA |
| OGDH | GGAGGAGGAGGTGGCTATTAC | CCTTGGTTCTTGTGCTCTTCC |
| SDHA | GTGGACATCAAGACTGGCAAGGT | GTAGGAGCGGATAGCAGGAGGTA |
| FH1 | TGTTACCGTTGGAGGCAGCAATG | GTCTGTGAAGGACACTGAAGCATCTC |
| MDH2 | GGAAGGAAGGAGTCGTTGAGTGTT | ATCTTGCCAATGCCCAGGTTCTT |
| Primary Antibodies | Dilution Ratio | Manufacturer |
|---|---|---|
| p-AMPK | 1:1000 | Abcam, Cambridge, UK |
| AMPK | 1:1000 | Abcam, Cambridge, UK |
| PGC-1α | 1:5000 | Abcam, Cambridge, UK |
| TFAM | 1:1000 | Beyotime, Shanghai, China |
| TLR4 | 1:1000 | Proteintech, Chicago, USA |
| NF-κB | 1:2000 | Cell Signaling Technology, Beverly, USA |
| IL-6 | 1:1000 | Abcam, Cambridge, UK |
| ZO-1 | 1:2000 | Abcam, Cambridge, UK |
| Occludin | 1:2000 | Proteintech, Chicago, USA |
| β-actin | 1:1000 | Proteintech, Chicago, USA |
| Main Components | Dehydrated Brassica rapa L. (g/100 g) | AEB (g/100 g) |
|---|---|---|
| Soluble sugar | 12.364 ± 1.233 | 27.754 ± 1.764 |
| Total protein | 7.098 ± 0.616 | 12.764 ± 1.426 |
| Total lipid | 0.135 ± 0.007 | 0.234 ± 0.002 |
| Total amino acid | 4.171 ± 0.056 | 7.983 ± 0.168 |
| Total Fiber | 10.345 ± 0.732 | 2.432 ± 0.543 |
| Total flavonoids | 0.460 ± 0.089 | 1.343 ± 0.324 |
| Total polyphenols | 0.304 ± 0.071 | 2.533 ± 0.134 |
| Total triterpenes | 0.386 ± 0.059 | 3.412 ± 0.243 |
| Total saponin | 0.396 ± 0.025 | 2.754 ± 0.342 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhu, H.; Cheng, Y.; Guo, Y.; Zhao, Y.; Qian, H. Aqueous Extract of Brassica rapa L.’s Impact on Modulating Exercise-Induced Fatigue via Gut–Muscle Axis. Nutrients 2023, 15, 4737. https://doi.org/10.3390/nu15224737
Wang C, Zhu H, Cheng Y, Guo Y, Zhao Y, Qian H. Aqueous Extract of Brassica rapa L.’s Impact on Modulating Exercise-Induced Fatigue via Gut–Muscle Axis. Nutrients. 2023; 15(22):4737. https://doi.org/10.3390/nu15224737
Chicago/Turabian StyleWang, Cheng, Hongkang Zhu, Yuliang Cheng, Yahui Guo, Yong Zhao, and He Qian. 2023. "Aqueous Extract of Brassica rapa L.’s Impact on Modulating Exercise-Induced Fatigue via Gut–Muscle Axis" Nutrients 15, no. 22: 4737. https://doi.org/10.3390/nu15224737
APA StyleWang, C., Zhu, H., Cheng, Y., Guo, Y., Zhao, Y., & Qian, H. (2023). Aqueous Extract of Brassica rapa L.’s Impact on Modulating Exercise-Induced Fatigue via Gut–Muscle Axis. Nutrients, 15(22), 4737. https://doi.org/10.3390/nu15224737









