Neuroimaging Insights: Kava’s (Piper methysticum) Effect on Dorsal Anterior Cingulate Cortex GABA in Generalized Anxiety Disorder
Abstract
1. Introduction
1.1. Gamma-Aminobutyric Acid (GABA) and Quantification in the CNS
1.2. The Dorsal Anterior Cingulate Cortex (dACC) as a Region of Interest
1.3. Kava for the Treatment of GAD Symptoms
2. Methods and Materials
2.1. Design
2.2. Participants
2.3. Sample Size
2.4. Measures
2.4.1. Screening and Eligibility
2.4.2. Assessment of Comorbidity in GAD
2.4.3. Assessment of Anxiety
2.5. H-MRS Protocol
2.5.1. Voxel Placement and Check
2.5.2. GABA Analysis
2.6. Treatment Handling
2.7. Statistical Analyses
3. Procedure
4. Results
4.1. Description of the Study Population
4.2. Adverse Events
4.3. Withdrawals
4.4. Structural Data and Metabolite Quantification
4.4.1. GABA Concentration Levels and HAMA at Baseline
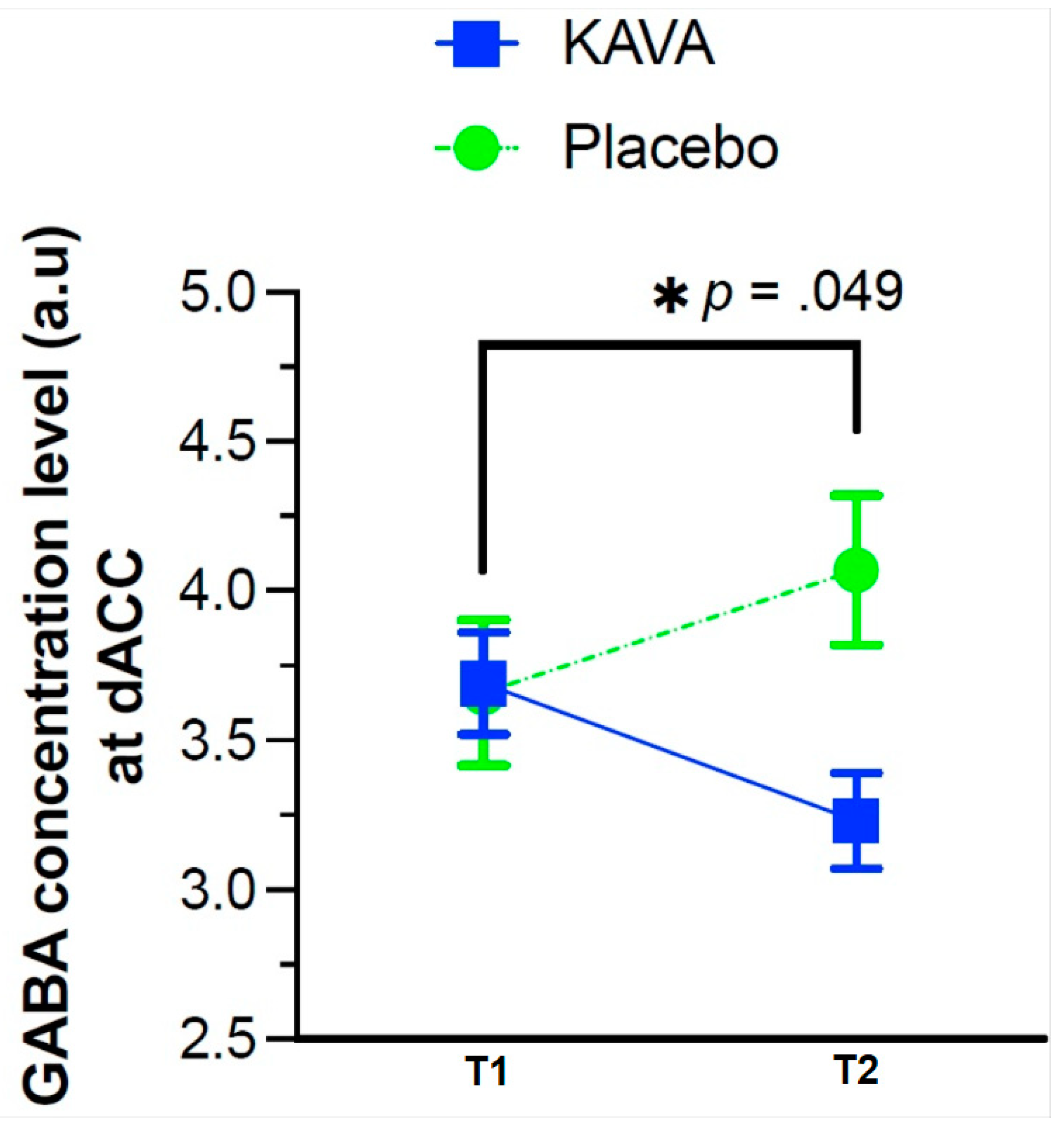
4.4.2. GABA Concentration Level Changes as a Function of the Eight-Week Kava Treatment
5. Discussion
5.1. Limitations in This Current Study
5.2. Future Research Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Enrolment Criteria |
| Generalised Anxiety Disorder Group |
| Inclusion Criteria |
|
|
| Exclusion Criteria |
|
| Imaging eligibility criteria—exclusions |
|
References
- Merikangas, K.R.; Zhang, H.; Avenevoli, S.; Acharyya, S.; Neuenschwander, M.; Angst, J. Longitudinal Trajectories of Depression and Anxiety in a Prospective Community Study: The Zurich Cohort Study. Arch. Gen. Psychiatry 2003, 60, 993–1000. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Zhao, S.Y.; Kessler, R.C.; Eaton, W.W. DSM-III-R Generalized Anxiety Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1994, 51, 355–364. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; Text Revision; American Psychiatric Press: Washington, DC, USA, 2022. [Google Scholar]
- Barlow, D.H.; Wincze, J. DSM-IV and beyond: What is generalized anxiety disorder? Acta Psychiatr. Scand. 1998, 98, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.; Lewis, D.A.; Michels, R.; Pine, D.S.; Schultz, S.K.; Tamminga, C.A.; Yager, J. The initial field trials of DSM-5: New blooms and old thorns. Am. J. Psychiatry 2013, 170, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Starcevic, V. Generalized anxiety disorder: Psychopharmacotherapy update on a common and commonly overlooked condition. Australas Psychiatry 2015, 23, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Sareen, J. Clinical Practice. Generalized Anxiety Disorder. New Engl. J. Med. 2015, 373, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Sijbrandij, M.; Koole, S.; Huibers, M.; Berking, M.; Andersson, G. Psychological treatment of generalized anxiety disorder: A meta-analysis. Clin. Psychol. Rev. 2014, 34, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.S.; Waldman, S.; Allgulander, C. Evidence-based pharmacological treatment of generalized anxiety disorder. Int. J. Neuropsychopharmacol. 2011, 14, 697–710. [Google Scholar] [CrossRef]
- Slee, A.; Nazareth, I.; Bondaronek, P.; Liu, Y.; Cheng, Z.; Freemantle, N. Pharmacological treatments for generalised anxiety disorder: A systematic review and network meta-analysis. Lancet 2019, 393, 768–777. [Google Scholar] [CrossRef]
- Gale, C.K.; Millichamp, J. Generalised anxiety disorder. BMJ Clin. Evid. 2011, 2011, 1002. [Google Scholar]
- Gale, C.K.; Millichamp, J. Generalised anxiety disorder in children and adolescents. BMJ Clin. Evid. 2016, 2016, 1002. [Google Scholar] [PubMed]
- Gunter, R.W.; Whittal, M.L. Dissemination of cognitive-behavioral treatments for anxiety disorders: Overcoming barriers and improving patient access. Clin. Psychol. Rev. 2010, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.J.; Mathew, S.J. Anxiety disorders: A comprehensive review of pharmacotherapies. Mt. Sinai J. Med. 2008, 75, 248–262. [Google Scholar] [CrossRef]
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate Systems in DSM-5 Anxiety Disorders: Their Role and a Review of Glutamate and GABA Psychopharmacology. Front. Psychiatry 2020, 11, 548505. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, R.B. Overview of generalized anxiety disorder: Epidemiology, presentation, and course. J. Clin. Psychiatry 2009, 70 (Suppl. 2), 4–9. [Google Scholar] [CrossRef] [PubMed]
- Farach, F.J.; Pruitt, L.D.; Jun, J.J.; Jerud, A.B.; Zoellner, L.A.; Roy-Byrne, P.P. Pharmacological treatment of anxiety disorders: Current treatments and future directions. J. Anxiety Disord. 2012, 26, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Phillips, A.G.; Insel, T.R. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol. Psychiatry 2012, 17, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Miller, G. Is pharma running out of brainy ideas? Science 2010, 329, 502–504. [Google Scholar] [CrossRef]
- Murrough, J.W.; Yaqubi, S.; Sayed, S.; Charney, D.S. Emerging drugs for the treatment of anxiety. Expert Opin. Emerg. Drugs 2015, 20, 393–406. [Google Scholar] [CrossRef]
- Sartori, S.B.; Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef]
- Sarris, J.; McIntyre, E.; Camfield, D.A. Plant-based medicines for anxiety disorders, part 1: A review of preclinical studies. CNS Drugs 2013, 27, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.; Firth, J.; Stough, C.K.; Sarris, J. GABA-modulating phytomedicines for anxiety: A systematic review of preclinical and clinical evidence. Phytother. Res. 2017, 32, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Riederer, P. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Garcia, R.; Mochcovitch, M.; do Cabo, M.C.; Nardi, A.E.; Freire, R.C. Predictors of pharmacotherapy response in generalized anxiety disorder: A systematic review. Harv. Rev. Psychiatry 2017, 25, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Maron, E.; Nutt, D. Biological Markers of Generalized Anxiety Disorder. Focus 2018, 16, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Levine, A. Treatment response biomarkers in anxiety disorders: From neuroimaging to neuronally-derived extracellular vesicles and beyond. Biomark. Neuropsychiatry 2020, 3, 100024. [Google Scholar] [CrossRef]
- Hayashi, T.; Nagai, K. Action of ω-amino acids on the motor cortex of higher animals, especially γ-amino-β-oxybutyric acid as the real inhibitory principle in brain. In Proceedings of the Twentieth International Physiological Congress, Brussels, Belgium, March 1955–January 1956. [Google Scholar]
- Killam, K.; Bain, J. Convulsant hydrazides I: In vitro and in vivo inhibition of vitamin B6 enzymes by convulsant hydrazides. J. Pharmacol. Exp. Ther. 1957, 119, 255–262. [Google Scholar]
- Etkin, A.; Wager, T.D. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef]
- Northoff, G.; Walter, M.; Schulte, R.F.; Beck, J.; Dydak, U.; Henning, A.; Boesiger, P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat. Neurosci. 2007, 10, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Puts, N.A.J.; Edden, R.A.E. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Rae, C. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem. Res. 2014, 39, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Tasan, R.O.; Bukovac, A.; Peterschmitt, Y.N.; Sartori, S.B.; Landgraf, R.; Singewald, N.; Sperk, G. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience 2011, 183, 71–80. [Google Scholar] [CrossRef]
- Nemeroff, C.B. The role of GABA in the pathophysiology and treatment of anxiety disorders. Psychopharmacol. Bull. 2003, 37, 133–146. [Google Scholar] [PubMed]
- Nutt, D.J.; Malizia, A.L. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br. J. Psychiatry 2001, 179, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Schur, R.R.; Draisma, L.W.; Wijnen, J.P.; Boks, M.P.; Koevoets, M.G.; Joels, M.; Vinkers, C.H. Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of (1) H-MRS studies. Hum. Brain Mapp. 2016, 37, 3337–3352. [Google Scholar] [CrossRef] [PubMed]
- Delli Pizzi, S.; Padulo, C.; Brancucci, A.; Bubbico, G.; Edden, R.A.; Ferretti, A.; Bonanni, L. GABA content within the ventromedial prefrontal cortex is related to trait anxiety. Soc. Cogn. Affect. Neurosci. 2015, 11, 758–766. [Google Scholar] [CrossRef]
- Michels, L.; Schulte-Vels, T.; Schick, M.; O’Gorman, R.L.; Zeffiro, T.; Hasler, G.; Mueller-Pfeiffer, C. Prefrontal GABA and glutathione imbalance in posttraumatic stress disorder: Preliminary findings. Psychiatry Res. Neuroimaging 2014, 224, 288–295. [Google Scholar] [CrossRef]
- Pennington, D.L.; Abé, C.; Batki, S.L.; Meyerhoff, D.J. A preliminary examination of cortical neurotransmitter levels associated with heavy drinking in posttraumatic stress disorder. Psychiatry Res. 2014, 224, 281–287. [Google Scholar] [CrossRef]
- Rosso, I.M.; Weiner, M.R.; Crowley, D.J.; Silveri, M.M.; Rauch, S.L.; Jensen, J.E. Insula and anterior cingulate GABA levels in posttraumatic stress disorder: Preliminary findings using magnetic resonance spectroscopy. Depress. Anxiety 2014, 31, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.W.; Mason, G.F.; Almai, A.; Rothman, D.L.; Behar, K.L.; Petroff, O.A.; Krystal, J.H. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch. Gen. Psychiatry 2001, 58, 556–561. [Google Scholar] [CrossRef]
- Ham, B.-J.; Sung, Y.; Kim, N.; Kim, S.J.; Kim, J.E.; Kim, D.J.; Lyoo, I.K. Decreased GABA levels in anterior cingulate and basal ganglia in medicated subjects with panic disorder: A proton magnetic resonance spectroscopy (1H-MRS) study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hasler, G.; van der Veen, J.W.; Geraci, M.; Shen, J.; Pine, D.; Drevets, W.C. Prefrontal cortical gamma-aminobutyric Acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol. Psychiatry 2009, 65, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Pollack, M.H.; Jensen, J.E.; Simon, N.M.; Kaufman, R.E.; Renshaw, P.F. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: Response to treatment with levetiracetam. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Coplan, J.D.; Jackowski, A.; Sato, J.R.; Mao, X.; Shungu, D.C.; Mathew, S.J. A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur. Neuropsychopharmacol. 2013, 23, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.J.; Price, R.B.; Mao, X.; Smith, E.L.; Coplan, J.D.; Charney, D.S.; Shungu, D.C. Hippocampal N-acetylaspartate concentration and response to riluzole in generalized anxiety disorder. Biol. Psychiatry 2008, 63, 891–898. [Google Scholar] [CrossRef][Green Version]
- Mathew, S.; Price, R.; Shungu, D.; Mao, X.; Smith, E.; Amiel, J.; Coplan, J. A pilot study of the effects of chronic paroxetine administration on hippocampal N-acetylaspartate in generalized anxiety disorder. J. Psychopharmacol. 2010, 24, 1175–1181. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Critchley, H.D.; Mathias, C.J.; Josephs, O.; O’Doherty, J.; Zanini, S.; Dewar, B.K.; Dolan, R.J. Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 2003, 126, 2139–2152. [Google Scholar] [CrossRef]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Luu, P.; Pederson, S.M. The anterior cingulate cortex: Regulating actions in context. In Cognitive Neuroscience of Attention; Guilford Publication, Inc.: New York, NY, USA, 2004; pp. 232–242. [Google Scholar]
- Vogt, B.A.; Finch, D.M.; Olson, C.R. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative regions. Cereb. Cortex 1992, 2, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Botvinick, M.; Carter, C.S. Anterior cingulate and prefrontal cortex: Who’s in control? Nat. Neurosci. 2000, 3, 421. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Peccoralo, L.A.; Davidson, R.J.; Cohen, J.D. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Hum. Brain Mapp. 2006, 27, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 2005, 6, 533–544. [Google Scholar] [CrossRef]
- Levar, N.; van Leeuwen, J.; Puts, N.A.; Denys, D.; Van Wingen, G.A. GABA concentrations in the anterior cingulate cortex are associated with fear network function and fear recovery in humans. Front. Hum. Neurosci. 2017, 11, 202. [Google Scholar] [CrossRef]
- Makkar, S.R.; Zhang, S.Q.; Cranney, J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology 2010, 35, 1625–1652. [Google Scholar] [CrossRef]
- Phelps, E.A.; Delgado, M.R.; Nearing, K.I.; LeDoux, J.E. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 2004, 43, 897–905. [Google Scholar] [CrossRef]
- Shin, L.M.; Liberzon, I. The Neurocircuitry of Fear, Stress, and Anxiety Disorders. Neuropsychopharmacology 2010, 35, 169–191. [Google Scholar] [CrossRef] [PubMed]
- LaPorte, E.; Sarris, J.; Stough, C.; Scholey, A. Neurocognitive effects of kava (Piper methysticum): A systematic review. Hum. Psychopharmacol. 2011, 26, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Ernst, E. Kava extract for treating anxiety. Cochrane Database Syst. Rev. 2003, CD003383. [Google Scholar] [CrossRef]
- Singh, Y.N.; Singh, N.N. Therapeutic potential of kava in the treatment of anxiety disorders. CNS Drugs 2002, 16, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Stevinson, C.; Huntley, A.; Ernst, E. A systematic review of the safety of kava extract in the treatment of anxiety. Drug Saf. 2002, 25, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, A.; Myers, S.P.; Wu, S.M.; O’Connor, K. Naturopathic and Western herbal medicine practice in Australia—A workforce survey. Complement. Ther. Med. 2004, 12, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lebot, V.; Merlin, M.; Lindstrom, L. Kava: The Pacific Drug; Yale University Press: New Haven, CT, USA, 1992. [Google Scholar]
- Pittler, M.H.; Ernst, E. Efficacy of kava extract for treating anxiety: Systematic review and meta-analysis. J. Clin. Psychopharmacol. 2000, 20, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y. Kava: An overview. J. Ethnopharmacol. 1992, 37, 13–45. [Google Scholar] [CrossRef]
- Tzeng, Y.M.; Lee, M.J. Neuroprotective properties of kavalactones. Neural Regen. Res. 2015, 10, 875–877. [Google Scholar]
- Bilia, A.R.; Bergonzi, M.C.; Lazari, D.; Vincieri, F.F. Characterization of commercial kava-kava herbal drug and herbal drug preparations by means of nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2002, 50, 5016–5025. [Google Scholar] [CrossRef]
- Chua, H.C.; Christensen, E.T.; Hoestgaard-Jensen, K.; Hartiadi, L.Y.; Ramzan, I.; Jensen, A.A.; Chebib, M. Kavain, the Major Constituent of the Anxiolytic Kava Extract, Potentiates GABAA Receptors: Functional Characteristics and Molecular Mechanism. PLoS ONE 2016, 11, e0157700. [Google Scholar] [CrossRef]
- Duffield, A.; Jamieson, D.; Lidgard, R.; Duffield, P.; Bourne, D. Identification of some human urinary metabolites of the intoxicating beverage kava. J. Chromatogr. A 1989, 475, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.J. Pharmacology of kava. In Ethnopharmacologic Search for Psychoactive Drugs; Raven Press: New York, NY, USA, 1979. [Google Scholar]
- Boonen, G.; Haberlein, H. Influence of genuine kavapyrone enantiomers on the GABA-A binding site. Planta Med. 1998, 64, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.P.; Drew, C.A.; Duffield, P.; Johnston, G.A.; Jamieson, D.D. Kava pyrones and resin: Studies on GABAA, GABAB and benzodiazepine binding sites in rodent brain. Pharmacol. Toxicol. 1992, 71, 120–126. [Google Scholar] [CrossRef]
- Yuan, C.S.; Dey, L.; Wang, A.; Mehendale, S.; Xie, J.T.; Aung, H.H.; Ang-Lee, M.K. Kavalactones and dihydrokavain modulate GABAergic activity in a rat gastric-brainstem preparation. Planta Medica 2002, 68, 1092–1096. [Google Scholar] [CrossRef] [PubMed]
- Boonen, G.; Ferger, B.; Kuschinsky, K.; Haberlein, H. In Vivo effects of the kavapyrones (+)-dihydromethysticin (+/−)-kavain on dopamine, 3,4-dihydroxyphenylacetic acid, serotonin and 5-hydroxyindoleacetic acid levels in striatal and cortical brain regions. Planta Medica 1998, 64, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Gleitz, J.; Beile, A.; Peters, T. (+/−)-Kavain inhibits veratridine-activated voltage dependent Na(+)-channels in synaptosomes prepared from rat cerebral cortex. Neuropharmacology 1995, 34, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.D.; Duffield, P.H. Positive interaction of ethanol and kava resin in mice. Clin. Exp. Pharmacol. Physiol. 1990, 17, 509–514. [Google Scholar] [CrossRef]
- Lehmann, E.; Klieser, E.; Klimke, A.; Krach, H.; Spatz, R. The efficacy of Cavain in patients suffering from anxiety. Pharmacopsychiatry 1989, 22, 258–262. [Google Scholar] [CrossRef]
- Sarris, J.; Kavanagh, D.J.; Adams, J.; Bone, K.; Byrne, G. Kava Anxiety Depression Spectrum Study (KADSS): A mixed methods RCT using an aqueous extract of Piper methysticum. Complement. Ther. Med. 2009, 17, 176–178. [Google Scholar] [CrossRef][Green Version]
- Saletu, B.; Grünberger, J.; Linzmayer, L.; Anderer, P. EEG-Brain mapping, psychometric and psychophysiological studies on central effects of kavain—A kava plant derivative. Hum. Psychopharmacol.: Clin. Exp. 1989, 4, 169–190. [Google Scholar] [CrossRef]
- Savage, K.M.; Stough, C.K.; Byrne, G.J.; Scholey, A.; Bousman, C.; Murphy, J.; Sarris, J. Kava for the treatment of generalised anxiety disorder (K-GAD): Study protocol for a randomised controlled trial. Trials 2015, 16, 493. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Bousman, C.A.; Cribb, L.; Savage, K.M.; Holmes, O.; Stough, C. Kava for generalised anxiety disorder: A 16-week double-blind, randomised, placebo-controlled study. Aust. New Zealand J. Psychiatry 2020, 54, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59, 22–33. [Google Scholar] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Press: Washington, DC, USA, 2013. [Google Scholar]
- Hamilton, M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959, 32, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.A.; Åsberg, M. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 1979, 134, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Kobak, K. Structured Interview Guide for the Montgomery Asberg Depression Rating Scale (SIG-MA); Biometrics Research Department: New York, NY, USA, 1996. [Google Scholar]
- Noyes, R., Jr. Comorbidity in generalized anxiety disorder. Psychiatr. Clin. North Am. 2001, 24, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J.; Ballenger, J.C.; Sheehan, D.; Wittchen, H.-U. Generalized anxiety disorder: Comorbidity, comparative biology and treatment. Int. J. Neuropsychopharmacol. 2002, 5, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Shear, M.K.; Vander Bilt, J.; Rucci, P.; Endicott, J.; Lydiard, B.; Otto, M.W.; Frank, D.M. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A). Depress. Anxiety 2001, 13, 166–178. [Google Scholar] [CrossRef]
- Mescher, M.; Merkle, H.; Kirsch, J.; Garwood, M.; Gruetter, R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998, 11, 266–272. [Google Scholar] [CrossRef]
- Rorden, C.; Brett, M. Stereotaxic display of brain lesions. Behav. Neurol. 2000, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Edden, R.A.; Puts, N.A.; Harris, A.D.; Barker, P.B.; Evans, C.J. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J. Magn. Reson. Imaging 2014, 40, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Provencher, S.W. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001, 14, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.K.; An, Z.; Banerjee, A.; Madan, A.; Hulsey, K.M.; Choi, C. Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7 T in vivo. NMR Biomed. 2014, 27, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Puts, N.A.; Edden, R.A. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J. Magn. Reson. Imaging 2015, 42, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Kreis, R.; Ernst, T.; Ross, B.D. Development of the human brain: In vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn. Reson. Med. 1993, 30, 424–437. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Singh, K.D.; Brealy, J.A.; Linden, D.E.; Evans, C.J. Quantification of γ-aminobutyric acid (GABA) in 1H MRS volumes composed heterogeneously of grey and white matter. NMR Biomed. 2016, 29, 1644–1655. [Google Scholar] [CrossRef]
- Kirkovski, M.; Suo, C.; Enticott, P.G.; Yucel, M.; Fitzgerald, P.B. Short communication: Sex-linked differences in gamma-aminobutyric acid (GABA) are related to social functioning in autism spectrum disorder. Psychiatry. Res. Neuroimaging 2018, 274, 19–22. [Google Scholar] [CrossRef]
- Sarris, J.; LaPorte, E.; Schweitzer, I. Kava: A comprehensive review of efficacy, safety, and psychopharmacology. Aust. N. Z. J. Psychiatry 2011, 45, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Witte, S.; Loew, D.; Gaus, W. Meta-analysis of the efficacy of the acetonic kava-kava extract WS® 1490 in patients with non-psychotic anxiety disorders. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Krum, B.N.; de Freitas, C.M.; Busanello, A.; Schaffer, L.F.; Fachinetto, R. Ex vivo and in vitro inhibitory potential of Kava extract on monoamine oxidase B activity in mice. J. Tradit. Complement. Med. 2022, 12, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, M.; Barker, P.B.; Bhattacharyya, P.K.; Brix, M.K.; Buur, P.F.; Cecil, K.M.; Edden, R.A. Big GABA: Edited MR spectroscopy at 24 research sites. Neuroimage 2017, 159, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Mullins, P.G.; McGonigle, D.J.; O’Gorman, R.L.; Puts, N.A.; Vidyasagar, R.; Evans, C.J.; Edden, R.A. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 2014, 86, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Bachtiar, V.; Johansen-Berg, H. What are we measuring with GABA magnetic resonance spectroscopy? Commun. Integr. Biol. 2011, 4, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.R. The NIMH Research Domain Criteria (RDoC) Project: Precision medicine for psychiatry. Am. J. Psychiatry 2014, 171, 395–397. [Google Scholar] [CrossRef]
- Williams, L.M.; Goldstein-Piekarski, A.N.; Chowdhry, N.; Grisanzio, K.A.; Haug, N.A.; Samara, Z.; Yesavage, J. Developing a clinical translational neuroscience taxonomy for anxiety and mood disorder: Protocol for the baseline-follow up Research domain criteria Anxiety and Depression (“RAD”) project. BMC Psychiatry 2016, 16, 68. [Google Scholar] [CrossRef]
- McNaughton, N. Development of a theoretically-derived human anxiety syndrome biomarker. Transl. Neurosci. 2014, 5, 137–146. [Google Scholar] [CrossRef]
- Sanislow, C.A.; Pine, D.S.; Quinn, K.J.; Kozak, M.J.; Garvey, M.A.; Heinssen, R.K.; Cuthbert, B.N. Developing constructs for psychopathology research: Research domain criteria. J. Abnorm. Psychol. 2010, 119, 631–639. [Google Scholar] [CrossRef]
- Cuthbert, B.N.; Insel, T.R. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013, 11, 126. [Google Scholar] [CrossRef]
- Fernandes, B.S.; Williams, L.M.; Steiner, J.; Leboyer, M.; Carvalho, A.F.; Berk, M. The new field of ‘precision psychiatry’. BMC Med. 2017, 15, 80. [Google Scholar] [CrossRef]
- Singh, I.; Rose, N. Biomarkers in psychiatry. Nature 2009, 460, 202–207. [Google Scholar] [CrossRef]
- Lueken, U.; Zierhut, K.C.; Hahn, T.; Straube, B.; Kircher, T.; Reif, A.; Domschke, K. Neurobiological markers predicting treatment response in anxiety disorders: A systematic review and implications for clinical application. Neurosci. Biobehav. Rev. 2016, 66, 143–162. [Google Scholar] [CrossRef]

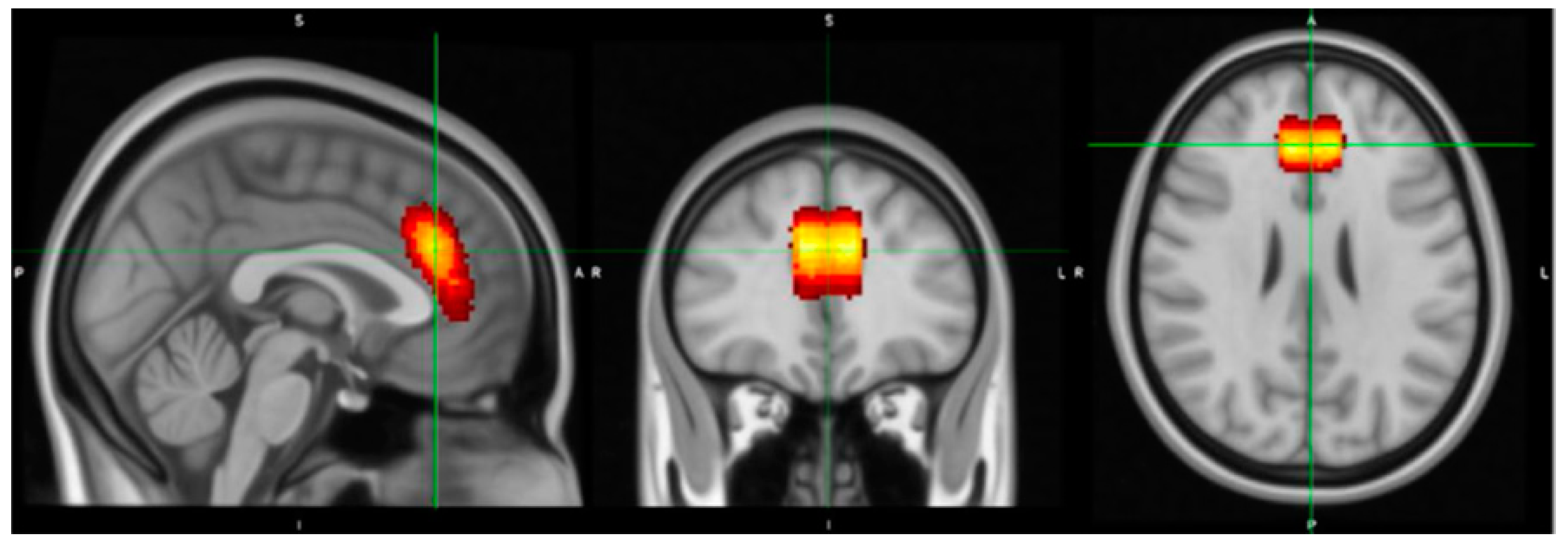
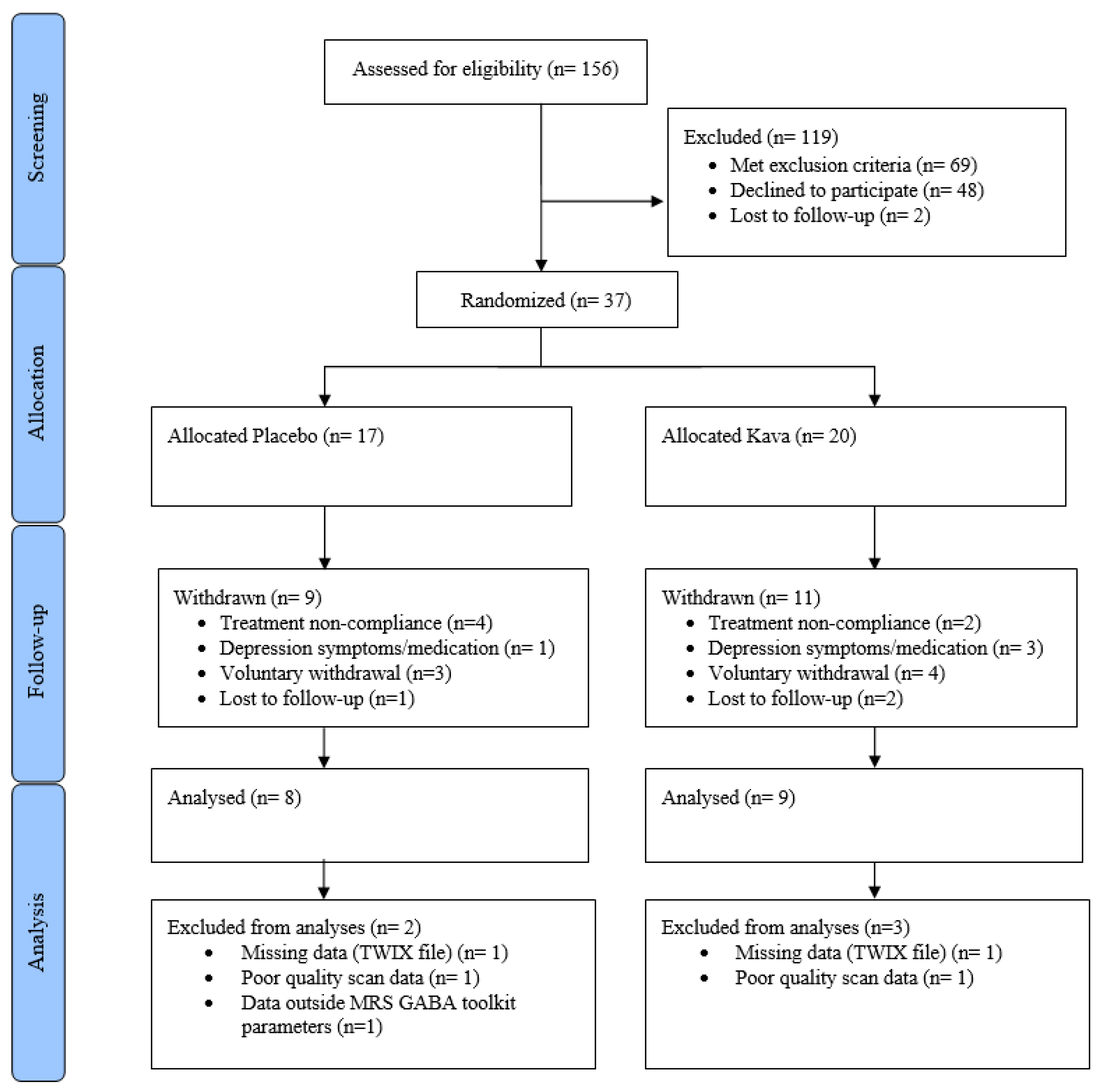
| Demographics Mean (S.D.), n or % where relevant | Baseline Whole Group | Baseline Placebo | Baseline Kava | Tmt p-Value * | Week 8 Whole Group | Week 8 Placebo | Week 8 Kava |
|---|---|---|---|---|---|---|---|
| Participants (n) | 37 | 17 (9 male) | 20 (11 male) | 0.653 | 20 | 9 | 11 |
| Age | 36.16 (13.09) | 36.06 (11.31) | 36.25 (14.73) | 0.965 | - | - | - |
| Education (years) | 17.65 (3.91) | 18.59 (4.70) | 16.85 (2.98) | 0.181 | - | - | - |
| Psychiatric | |||||||
| Comorbid condition | 21 | 12 | 9 | 0.110 | - | - | - |
| Comorbid: SAD | 11 | 6 | 5 | 0.395 | - | - | - |
| Comorbid: PD | 9 | 7 | 2 | 0.034 * | - | - | - |
| Comorbid: AGO | 17 | 9 | 8 | 0.324 | - | - | - |
| Comorbid: PTSD | 1 | 1 | 0 | 0.460 | - | - | - |
| Comorbid: MDD | 20 | 9 | 11 | 0.581 | - | - | - |
| HAM-A a | 23.05 (3.60) | 23.29 (4.19) | 22.85 (3.10) | 0.714 | 15.55 (5.69) | 14.00 (6.20) | 16.82 (5.17) |
| MADRS b | 13.59 (2.99) | 14.00 (2.91) | 13.25 (3.09) | 0.456 | 10.38 (5.30) | 10.67 (4.82) | 10.17 (5.84) |
| Medical | |||||||
| Medications | 15 | 7 | 6 | 0.357 | - | - | - |
| Supplements | 9 | 2 | 7 | 0.103 | - | - | - |
| Substance | |||||||
| Caffeine (mg/daily) | 135.54 (127.64) | 156.47 (132.96) | 117.75 (123.51) | 0.365 | 100.55 (83.71) | 107.27 (68.93) | 94.50 (98.52) |
| Alcohol (SD/weekly) | 2.86 (3.08) | 1.88 (2.02) | 3.70 (3.60) | 0.073 | 4.10 (3.65) | 1.67 (1.50) | 6.09 (3.73) |
| Volumetric | |||||||
| dACC GM (%, mm3) | 60.29 (13.20) | 59.41 (12.22) | 61.05 (14.25) | 0.712 | 58.35 (12.33) | 53.11 (15.89) | 62.64 (6.45) |
| dACC WM (%, mm3) | 23.48 (11.46) | 24.58 (11.86) | 22.55 (11.34) | 0.597 | 23.40 (10.78) | 27.67 (13.90) | 19.91 (6.02) |
| dACC CSF (%, mm3) | 15.57 (5.04) | 14.53 (3.93) | 16.47 (5.77) | 0.249 | 18.01 (6.18) | 19.44 (4.56) | 16.84 (7.26) |
| GABA toolkit | |||||||
| LCModel GABA/GM | 3.67 (0.88) | 3.66 (1.01) | 3.69 (0.77) | 0.932 | 3.59 (0.74) | 4.07 (0.75) | 3.23 (0.53) |
| LCModel SD (%) | 9.39 (2.30) | 9.47 (1.58) | 9.31 (2.83) | 0.843 | 9.50 (2.68) | 8.89 (0.78) | 10.00 (3.55) |
| LCModel SNR | 9.00 (2.58) | 9.70 (2.26) | 8.37 (2.75) | 0.123 | 9.05 (2.42) | 9.44 (2.13) | 8.73 (2.69) |
| LCModel FWHM | 0.08 (0.04) | 0.06 (0.02) | 0.09 (0.05) | 0.025 * | 0.07 (0.03) | 0.06 (0.02) | 0.08 (0.04) |
| GABA Model | B/Estimate | 95% CI | t | df | p-Value |
|---|---|---|---|---|---|
| Corrected GABA concentration | |||||
| Time | 0.48 | −0.09, 1.06 | 1.74 | 21 | 0.097 a |
| Treatment | 1.06 | 0.28, 1.83 | 2.75 | 46 | 0 0.008 * |
| Sex | −0.28 | −0.78, 0.23 | −1.14 | 21 | 0 0.268 |
| Age | 0.01 | 0.00, 0.03 | 1.58 | 19 | 0.129 |
| Comorbid | 0.13 | −0.39, 0.65 | 0.53 | 24 | 0.603 |
| Baseline alcohol | 0.05 | −0.04, 0.03 | 1.58 | 21 | 0.129 |
| Time*Treatment | −0.87 | −1.74, 0.00 | −2.09 | 21 | 0 0.049 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savage, K.; Sarris, J.; Hughes, M.; Bousman, C.A.; Rossell, S.; Scholey, A.; Stough, C.; Suo, C. Neuroimaging Insights: Kava’s (Piper methysticum) Effect on Dorsal Anterior Cingulate Cortex GABA in Generalized Anxiety Disorder. Nutrients 2023, 15, 4586. https://doi.org/10.3390/nu15214586
Savage K, Sarris J, Hughes M, Bousman CA, Rossell S, Scholey A, Stough C, Suo C. Neuroimaging Insights: Kava’s (Piper methysticum) Effect on Dorsal Anterior Cingulate Cortex GABA in Generalized Anxiety Disorder. Nutrients. 2023; 15(21):4586. https://doi.org/10.3390/nu15214586
Chicago/Turabian StyleSavage, Karen, Jerome Sarris, Matthew Hughes, Chad A. Bousman, Susan Rossell, Andrew Scholey, Con Stough, and Chao Suo. 2023. "Neuroimaging Insights: Kava’s (Piper methysticum) Effect on Dorsal Anterior Cingulate Cortex GABA in Generalized Anxiety Disorder" Nutrients 15, no. 21: 4586. https://doi.org/10.3390/nu15214586
APA StyleSavage, K., Sarris, J., Hughes, M., Bousman, C. A., Rossell, S., Scholey, A., Stough, C., & Suo, C. (2023). Neuroimaging Insights: Kava’s (Piper methysticum) Effect on Dorsal Anterior Cingulate Cortex GABA in Generalized Anxiety Disorder. Nutrients, 15(21), 4586. https://doi.org/10.3390/nu15214586







