Growth Profiles of Children and Adolescents Living with and without Perinatal HIV Infection in Southern Africa: A Secondary Analysis of Cohort Data
Abstract
1. Introduction
2. Materials and Methods
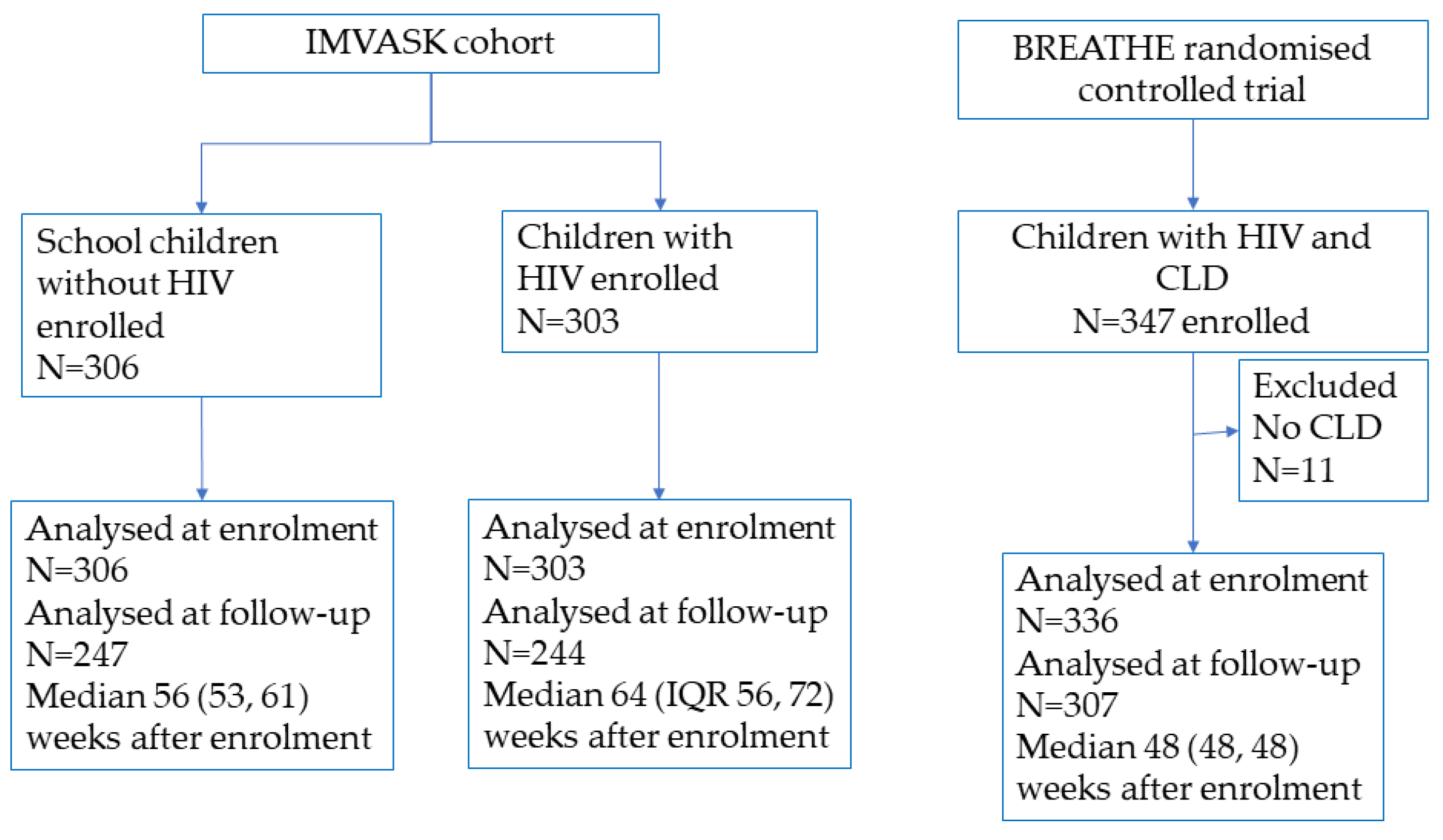
2.1. Study Population
2.2. Data Collection
2.3. Statistical Methods
3. Results
3.1. Characteristics of the Study Population
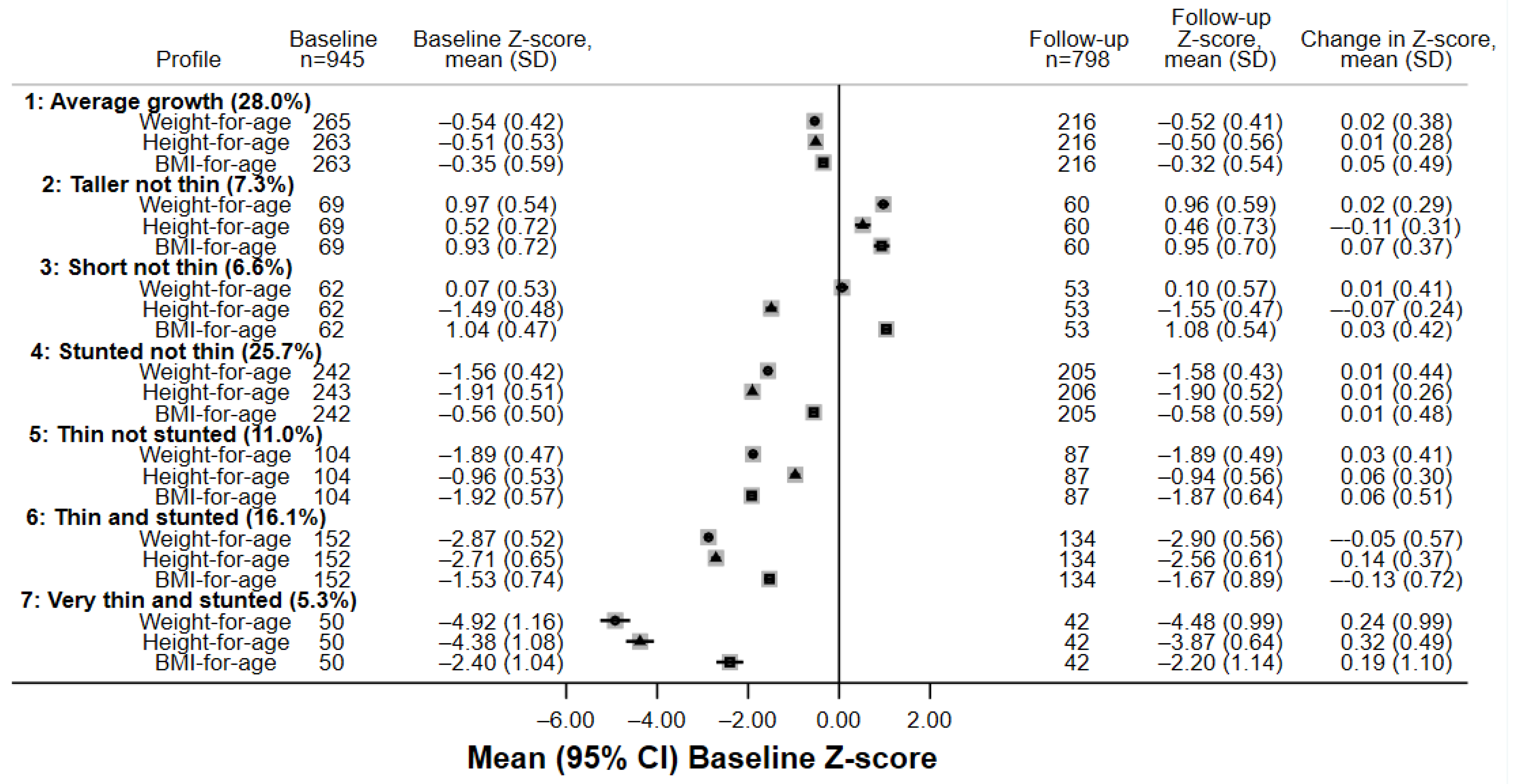
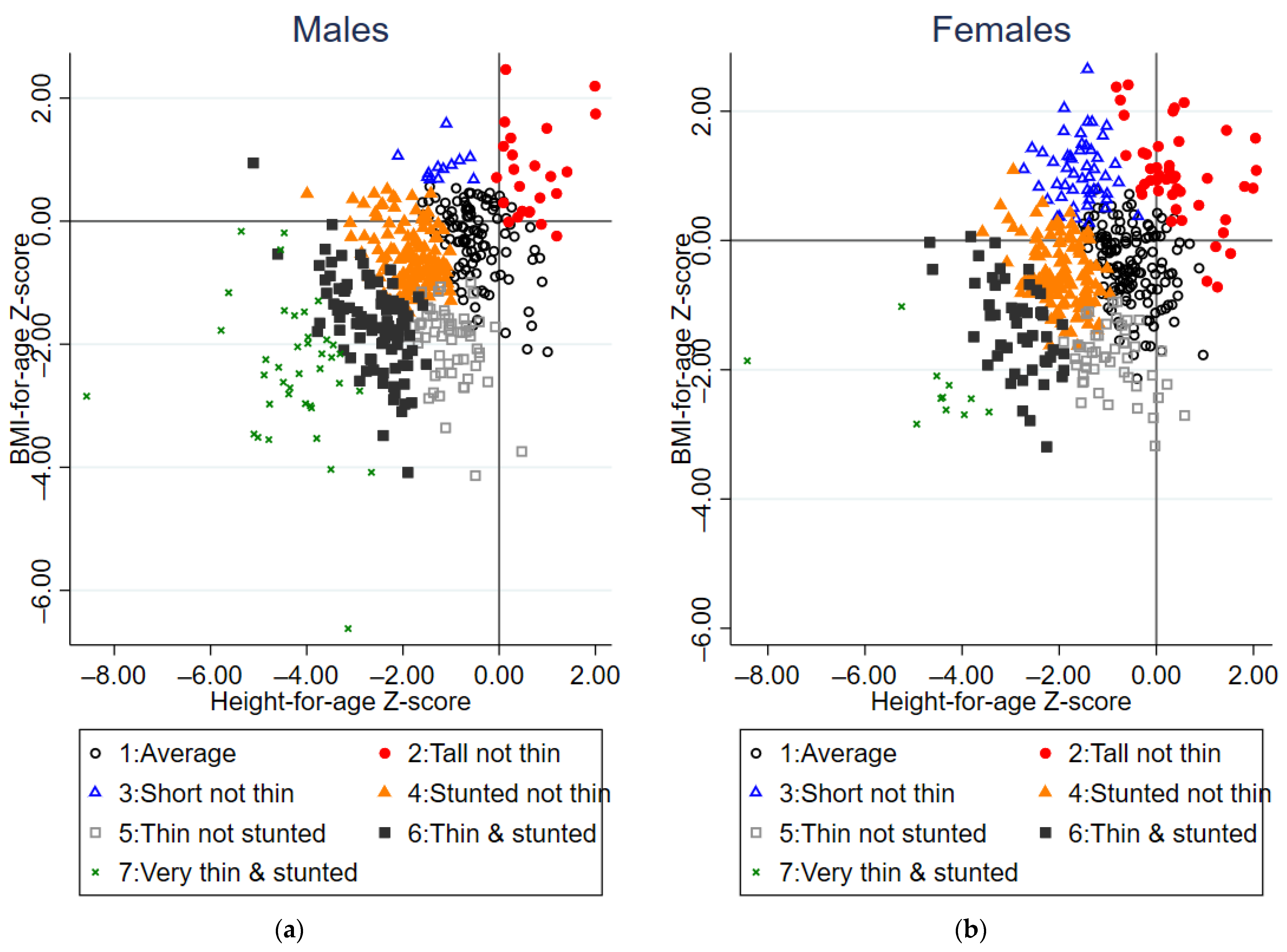
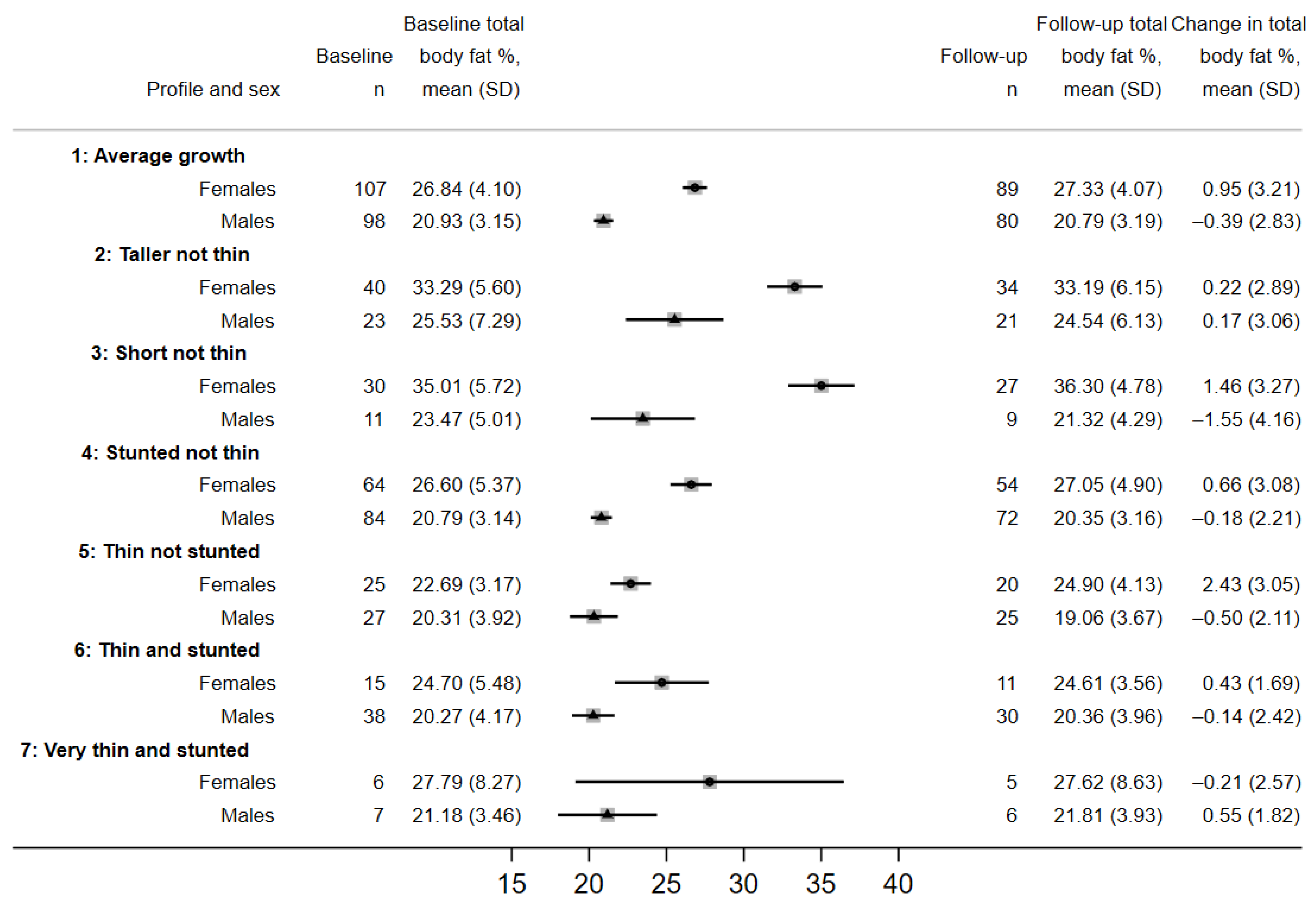
3.2. Identification and Summary of Growth Profiles
3.3. Factors Associated with Growth Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omoni, A.O.; Ntozini, R.; Evans, C.; Prendergast, A.J.; Moulton, L.H.; Christian, P.S.; Humphrey, J.H. Child Growth According to Maternal and Child HIV Status in Zimbabwe. Pediatr. Infect. Dis. J. 2017, 36, 869–876. [Google Scholar] [CrossRef]
- Williams, P.L.; Jesson, J. Growth and pubertal development in HIV-infected adolescents. Curr. Opin. HIV AIDS 2018, 13, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Chidumwa, G.; Said-Mohamed, R.; Nyati, L.H.; Mpondo, F.; Chikowore, T.; Prioreschi, A.; Kagura, J.; Ware, L.J.; Micklesfield, L.K.; Norris, S.A. Stunting in infancy, pubertal trajectories and adult body composition: The Birth to Twenty Plus cohort, South Africa. Eur. J. Clin. Nutr. 2021, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C. Maternal capital and the metabolic ghetto: An evolutionary perspective on the transgenerational basis of health inequalities. Am. J. Hum. Biol. 2010, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A.M.; Ward, K.A.; Goldberg, G.R.; Jarjou, L.M.; Moore, S.E.; Fulford, A.J.; Prentice, A. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr. 2013, 97, 911–918. [Google Scholar] [CrossRef]
- Collaborative Initiative for Paediatric, H.I.V.E.; Research Global Cohort, C.; Jesson, J.; Crichton, S.; Quartagno, M.; Yotebieng, M.; Abrams, E.J.; Chokephaibulkit, K.; Le Coeur, S.; Ake-Assi, M.H.; et al. Growth and CD4 patterns of adolescents living with perinatally acquired HIV worldwide, a CIPHER cohort collaboration analysis. J. Int. AIDS Soc. 2022, 25, e25871. [Google Scholar] [CrossRef]
- Martin-Canavate, R.; Sonego, M.; Sagrado, M.J.; Escobar, G.; Rivas, E.; Ayala, S.; Castaneda, L.; Aparicio, P.; Custodio, E. Dietary patterns and nutritional status of HIV-infected children and adolescents in El Salvador: A cross-sectional study. PLoS ONE 2018, 13, e0196380. [Google Scholar] [CrossRef]
- Musoke, P.M.; Mudiope, P.; Barlow-Mosha, L.N.; Ajuna, P.; Bagenda, D.; Mubiru, M.M.; Tylleskar, T.; Fowler, M.G. Growth, immune and viral responses in HIV infected African children receiving highly active antiretroviral therapy: A prospective cohort study. BMC Pediatr. 2010, 10, 56. [Google Scholar] [CrossRef]
- Mwiru, R.S.; Spiegelman, D.; Duggan, C.; Seage, G.R., 3rd; Semu, H.; Chalamilla, G.; Kisenge, R.; Fawzi, W.W. Growth among HIV-infected children receiving antiretroviral therapy in Dar es Salaam, Tanzania. J. Trop. Pediatr. 2014, 60, 179–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ramteke, S.M.; Shiau, S.; Foca, M.; Strehlau, R.; Pinillos, F.; Patel, F.; Violari, A.; Liberty, A.; Coovadia, A.; Kuhn, L.; et al. Patterns of Growth, Body Composition, and Lipid Profiles in a South African Cohort of Human Immunodeficiency Virus-Infected and Uninfected Children: A Cross-Sectional Study. J. Pediatr. Infect. Dis. Soc. 2018, 7, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, H.; Gebremedhin, S. Undernutrition Among HIV-Positive Adolescents on Antiretroviral Therapy in Southern Ethiopia. Adolesc. Health Med. Ther. 2020, 11, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rukuni, R.; Gregson, C.; Kahari, C.; Kowo, F.; McHugh, G.; Munyati, S.; Mujuru, H.; Ward, K.; Filteau, S.; Rehman, A.M.; et al. The IMpact of Vertical HIV infection on child and Adolescent SKeletal development in Harare, Zimbabwe (IMVASK Study): A protocol for a prospective cohort study. BMJ Open 2020, 10, e031792. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Martinez, C.; Kranzer, K.; McHugh, G.; Corbett, E.L.; Mujuru, H.; Nicol, M.P.; Rowland-Jones, S.; Rehman, A.M.; Gutteberg, T.J.; Flaegstad, T.; et al. Azithromycin versus placebo for the treatment of HIV-associated chronic lung disease in children and adolescents (BREATHE trial): Study protocol for a randomised controlled trial. Trials 2017, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, R.A.; McHugh, G.; Rehman, A.M.; Mujuru, H.; Simms, V.; Majonga, E.D.; Nicol, M.P.; Flaegstad, T.; Gutteberg, T.J.; Gonzalez-Martinez, C.; et al. Effect of Once-Weekly Azithromycin vs Placebo in Children With HIV-Associated Chronic Lung Disease: The BREATHE Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2028484. [Google Scholar] [CrossRef]
- Gibson, R.S. Principles of Nutritional Assessment; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Cole, T.J.; Freeman, J.V.; Preece, M.A. British 1990 growth reference centiles for weight, height, body mass index and head circumference fitted by maximum penalized likelihood. Stat. Med. 1998, 17, 407–429. [Google Scholar] [CrossRef]
- Working Group on Infant and Young Child Feeding Indicators. Developing and Validating Simple Indicators of Dietary Quality and Energy Intake of Infants and Young Children in Developing Countries: Summary of Findings from Analysis of 10 Data Sets; USAID: Washington, DC, USA, 2006; p. 112. Available online: https://www.fantaproject.org/research/indicators-dietary-quality-intake-children (accessed on 22 September 2022).
- Weller, B.E.; Bowen, N.K.; Faubert, S.J. Latent Class Analysis: A Guide to Best Practice. J. Black Psychol. 2020, 46, 231–287. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guilford Press: New York, NY, US, 2005; 366p. [Google Scholar]
- Nyati, L.H.; Pettifor, J.M.; Ong, K.K.; Norris, S.A. Adolescent growth and BMI and their associations with early childhood growth in an urban South African cohort. Am. J. Hum. Biol. 2021, 33, e23469. [Google Scholar] [CrossRef]
- Chiabi, A.; Lebela, J.; Kobela, M.; Mbuagbaw, L.; Obama, M.T.; Ekoe, T. The frequency and magnitude of growth failure in a group of HIV-infected children in Cameroon. Pan Afr. Med. J. 2012, 11, 15. [Google Scholar]
- Feucht, U.D.; Van Bruwaene, L.; Becker, P.J.; Kruger, M. Growth in HIV-infected children on long-term antiretroviral therapy. Trop. Med. Int. Health 2016, 21, 619–629. [Google Scholar] [CrossRef]
- Anand, P.; Behrman, J.R.; Dang, H.H.; Jones, S. Varied patterns of catch-up in child growth: Evidence from Young Lives. Soc. Sci. Med. 2018, 214, 206–213. [Google Scholar] [CrossRef]
- Isanaka, S.; Duggan, C.; Fawzi, W.W. Patterns of postnatal growth in HIV-infected and HIV-exposed children. Nutr. Rev. 2009, 67, 343–359. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Filteau, S.; Kasonka, L.; Wells, J.C.; Munthali, G.; Chisenga, M.; Rehman, A.M. Anthropometry, body composition, early growth, and chronic disease risk factors among Zambian adolescents exposed or not to perinatal maternal HIV. Br. J. Nutr. 2022, 129, 678–689. [Google Scholar] [CrossRef]
- Millward, D.J. Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev. 2017, 30, 50–72. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Soliman, A.; Alaaraj, N.; Ahmed, S.; Alyafei, F.; Hamed, N. Early and Long-term Consequences of Nutritional Stunting: From Childhood to Adulthood. Acta Biomed. 2021, 92, e2021168. [Google Scholar] [CrossRef]
- Dewey, K.G.; Begum, K. Long-term consequences of stunting in early life. Matern. Child. Nutr. 2011, 7 (Suppl. S3), 5–18. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child. Health 2014, 34, 250–265. [Google Scholar] [CrossRef]
- Sudfeld, C.R.; McCoy, D.C.; Danaei, G.; Fink, G.; Ezzati, M.; Andrews, K.G.; Fawzi, W.W. Linear growth and child development in low- and middle-income countries: A meta-analysis. Pediatrics 2015, 135, e1266–e1275. [Google Scholar] [CrossRef]
| Characteristics * | HIV− IMVASK Cohort | HIV+ IMVASK Cohort | HIV+ and CLD+ BREATHE RCT |
|---|---|---|---|
| n | 306 | 303 | 336 |
| Mean age, years (SD) | 12.5 (2.5) | 12.4 (2.5) | 15.0 (3.2) |
| Female sex, n (%) | 155 (50.7) | 151 (49.8) | 166 (49.4) |
| Zimbabwean, n (%) | 306 (100) | 303 (100) | 241 (71.7) |
| Malawian, n (%) | - | - | 95 (28.3) |
| Median age at ART initiation, years (IQR) | - | 3.8 (1.8, 6.9) | 8.5 (5.7, 11.6) |
| HIV viral load <1000 copies/mL, n (%) | - | 212 (79.1) | 187 (56.0) |
| CD4 cell count ≥500 cells/µL, n (%) | - | 230 (79.9) | 208 (61.9) |
| Mean weight (SD), kg | 40.2 (12.2) | 34.0 (9.4) | 38.5 (11.1) |
| Mean Weight-for-age Z-score (SD) | −0.50 (1.09) | −1.47 (1.19) | −2.17 (1.46) |
| Underweight (WA Z-score < −2), n (%) | 23 (7.5) | 80 (26.4) | 176 (52.4) |
| Mean height (SD), cm | 147.5 (13.5) | 140.0 (12.8) | 146.0 (14.1) |
| Mean Height-for-age Z-score (SD) | −0.59 (1.02) | −1.65 (1.12) | −2.11 (1.22) |
| Stunted (HA Z-score < −2), n (%) | 22 (7.2) | 95 (31.6) | 168 (50.0) |
| Mean BMI (SD) | 18.0 (3.1) | 17.0 (2.2) | 17.6 (2.8) |
| Mean BMI Z-score (SD) | −0.27 (1.09) | −0.68 (0.94) | −1.09 (1.15) |
| Thin (BMI Z-score < −2), n (%) | 16 (5.3) | 28 (9.3) | 67 (19.9) |
| Overweight (BMI Z-Score > 1), n (%) | 36 (11.8) | 9 (3.0) | 12 (3.6) |
| Number of Classes | Degrees of Freedom | Log Likelihood | Bayesian Information Criteria | Entropy | Smallest Class Size, n (%) | Mean Class Posterior Probability |
|---|---|---|---|---|---|---|
| 1 | 12 | −8634 | 17,350 | - | 945 (100%) | - |
| 2 | 19 | −7587 | 15,304 | 0.832 | 402 (42.5%) | 0.95 |
| 3 | 26 | −7000 | 14,178 | 0.834 | 116 (12.3%) | 0.94 |
| 4 | 33 | −6655 | 13,537 | 0.907 | 81 (8.6%) | 0.93 |
| 5 | 40 | −6433 | 13,140 | 0.936 | 32 (3.4%) | 0.93 |
| 6 | 47 | −6292 | 12,905 | 0.912 | 48 (5.1%) | 0.91 |
| 7 | 54 | −6147 | 12,663 | 0.946 | 50 (5.3%) | 0.91 |
| 8 | 61 | −6003 | 12,425 | 0.955 | 38 (4.0%) | 0.91 |
| Factors | Profile 2: Tall not Thin | Profile 3: Short not Thin | Profile 4: Stunted not Thin | Profile 5: Thin not Stunted | Profile 6: Thin and Stunted | Profile 7: Very Thin and Stunted |
|---|---|---|---|---|---|---|
| RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | RRR (95% CI) | |
| Sex | ||||||
| Male | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Female | 1.70 (0.97, 3.00) | 2.86 (1.44, 5.68) | 0.58 (0.40, 0.84) | 0.56 (0.35, 0.90) | 0.30 (0.19, 0.48) | 0.15 (0.07, 0.31) |
| Age, years | ||||||
| 6 to 10 | 1.58 (0.71, 3.55) | 0.22 (0.09, 0.54) | 0.78 (0.47, 1.29) | 0.55 (0.28, 1.10) | 0.65 (0.35, 1.21) | 0.06 (0.01, 0.29) |
| 11 to 14 | 1.29 (0.59, 2.81) | 0.43 (0.22, 0.83) | 0.69 (0.44, 1.10) | 0.96 (0.54, 1.70) | 0.76 (0.44, 1.30) | 0.30 (0.14, 0.64) |
| 15 to 19 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| HIV status | ||||||
| HIV− | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| HIV+ | 0.18 (0.08, 0.41) | 1.37 (0.69, 2.73) | 3.30 (2.13, 5.10) | 1.75 (0.98, 3.15) | 8.26 (3.92, 17.4) | 28.8 (3.67, 226.8) |
| HIV+ CLD+ | 0.20 (0.06, 0.70) | 1.64 (0.75, 3.60) | 4.02 (2.33, 6.93) | 4.46 (2.35, 8.49) | 25.5 (11.6, 56.1) | 57.3 (7.30, 449.7) |
| Country | ||||||
| Zimbabwe | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Malawi | 0.89 (0.08, 9.46) | 1.10 (0.32, 3.74) | 1.58 (0.70, 3.55) | 0.81 (0.30, 2.20) | 1.54 (0.68, 3.48) | 2.78 (1.01, 7.60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, A.M.; Sekitoleko, I.; Rukuni, R.; Webb, E.L.; McHugh, G.; Bandason, T.; Moyo, B.; Ngwira, L.G.; Mukwasi-Kahari, C.; Gregson, C.L.; et al. Growth Profiles of Children and Adolescents Living with and without Perinatal HIV Infection in Southern Africa: A Secondary Analysis of Cohort Data. Nutrients 2023, 15, 4589. https://doi.org/10.3390/nu15214589
Rehman AM, Sekitoleko I, Rukuni R, Webb EL, McHugh G, Bandason T, Moyo B, Ngwira LG, Mukwasi-Kahari C, Gregson CL, et al. Growth Profiles of Children and Adolescents Living with and without Perinatal HIV Infection in Southern Africa: A Secondary Analysis of Cohort Data. Nutrients. 2023; 15(21):4589. https://doi.org/10.3390/nu15214589
Chicago/Turabian StyleRehman, Andrea M., Isaac Sekitoleko, Ruramayi Rukuni, Emily L. Webb, Grace McHugh, Tsitsi Bandason, Brewster Moyo, Lucky Gift Ngwira, Cynthia Mukwasi-Kahari, Celia L. Gregson, and et al. 2023. "Growth Profiles of Children and Adolescents Living with and without Perinatal HIV Infection in Southern Africa: A Secondary Analysis of Cohort Data" Nutrients 15, no. 21: 4589. https://doi.org/10.3390/nu15214589
APA StyleRehman, A. M., Sekitoleko, I., Rukuni, R., Webb, E. L., McHugh, G., Bandason, T., Moyo, B., Ngwira, L. G., Mukwasi-Kahari, C., Gregson, C. L., Simms, V., Filteau, S., & Ferrand, R. A. (2023). Growth Profiles of Children and Adolescents Living with and without Perinatal HIV Infection in Southern Africa: A Secondary Analysis of Cohort Data. Nutrients, 15(21), 4589. https://doi.org/10.3390/nu15214589





