Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Blood Collection and Preparation
2.3. Mitochondrial Respiration
2.4. Western Blotting
2.5. RNA Sequencing
2.6. Tissue Preparation and Determination of Mitochondrial Fractional Synthesis Rate
2.7. Statistical Analysis
3. Results
3.1. Animal Characteristics
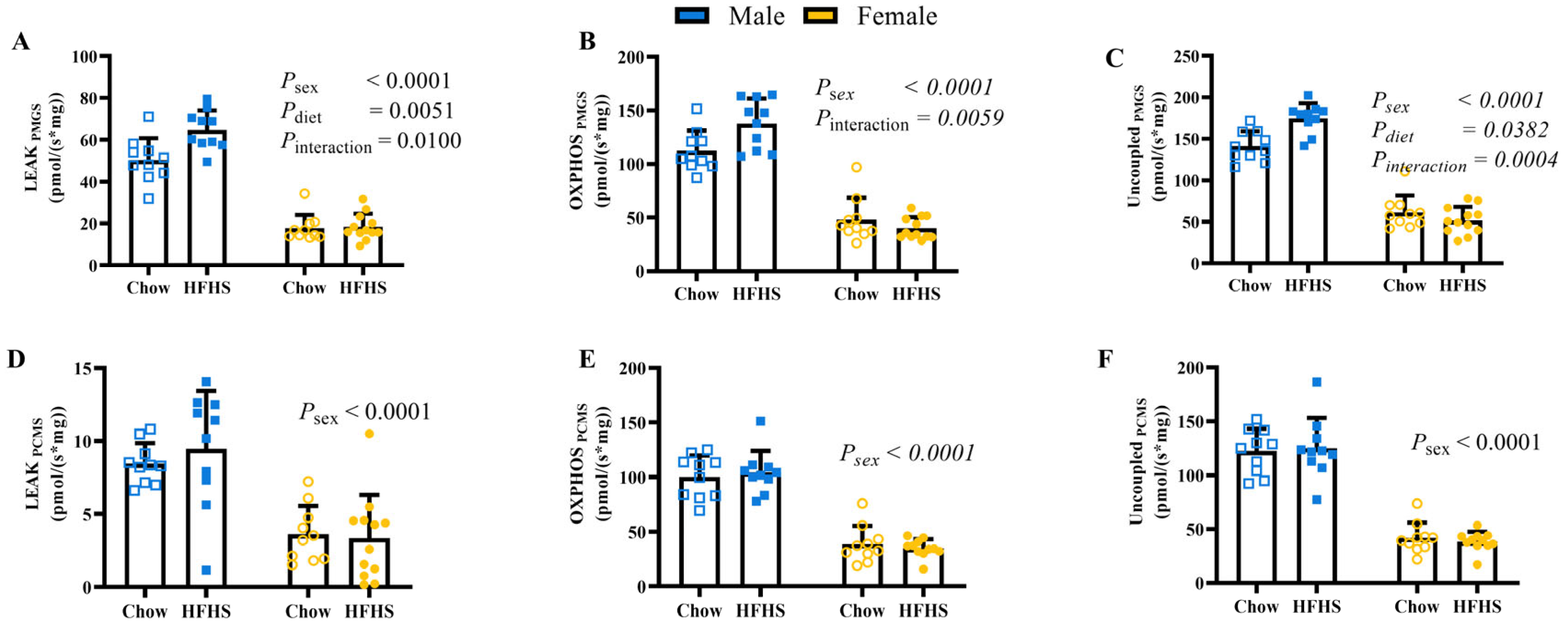
3.2. Skeletal Muscle Mitochondrial Respiration
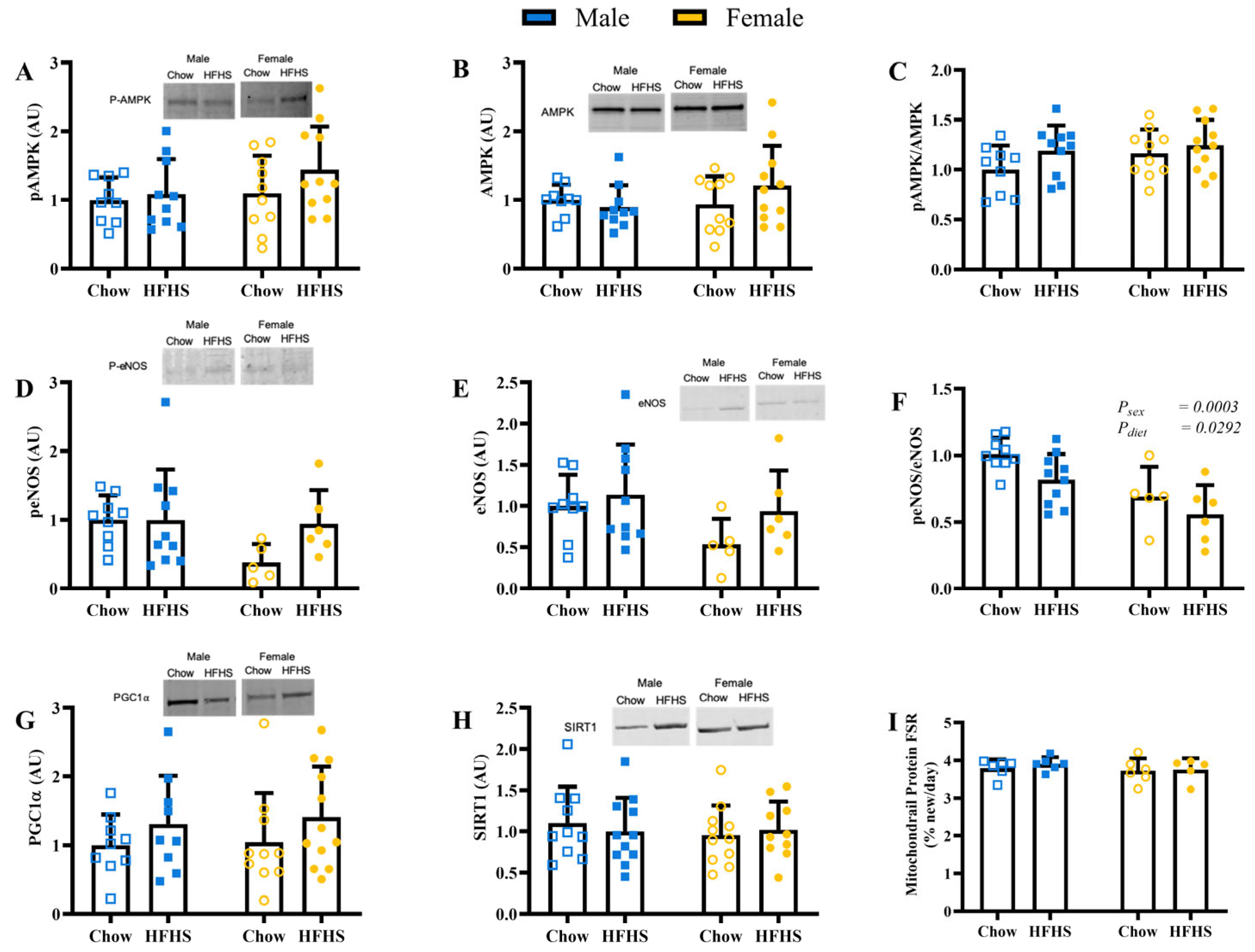
3.3. Skeletal Muscle Mitochondrial Biogenesis and Protein Content
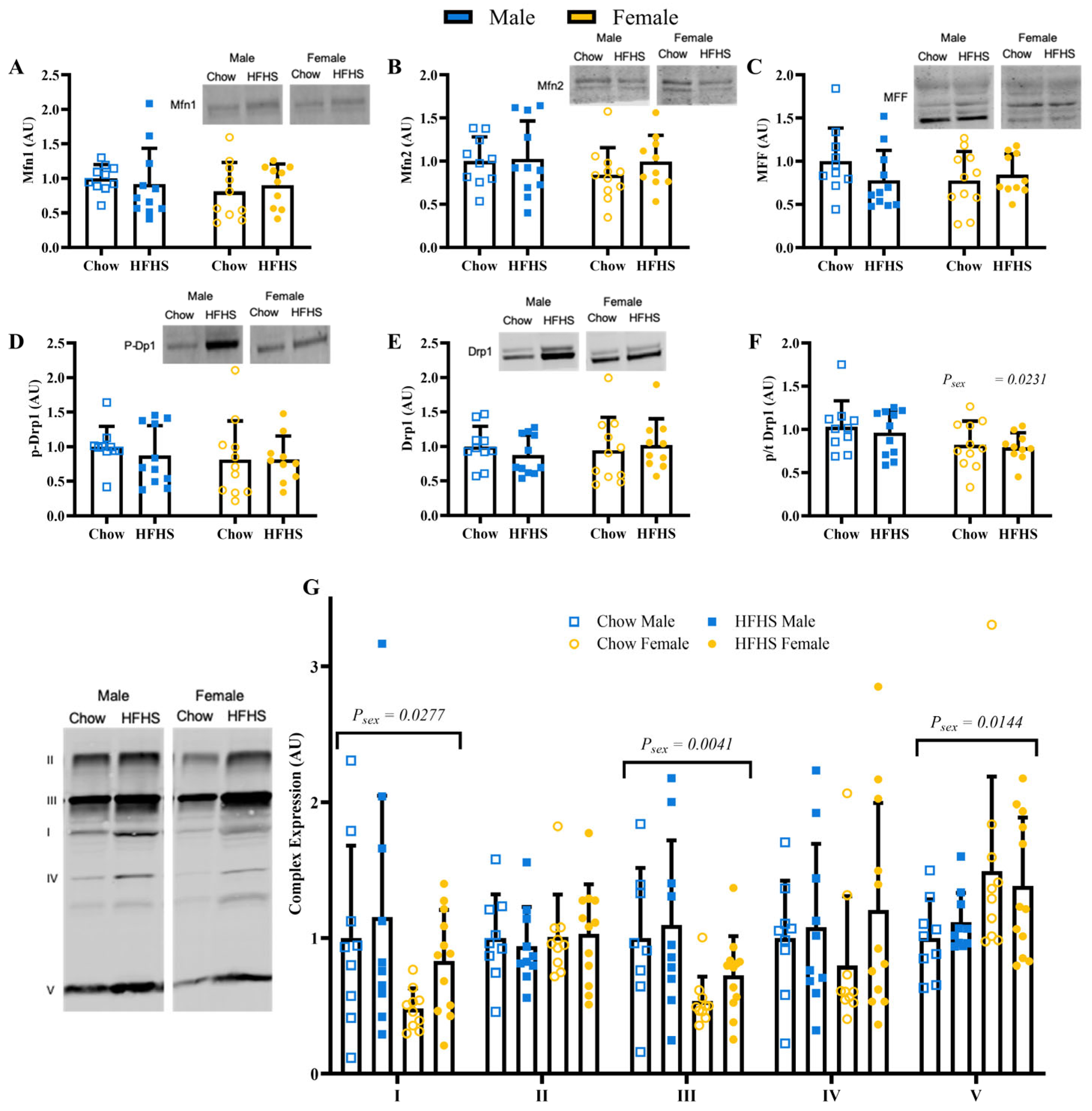
3.4. Skeletal Muscle Mitochondrial Quality
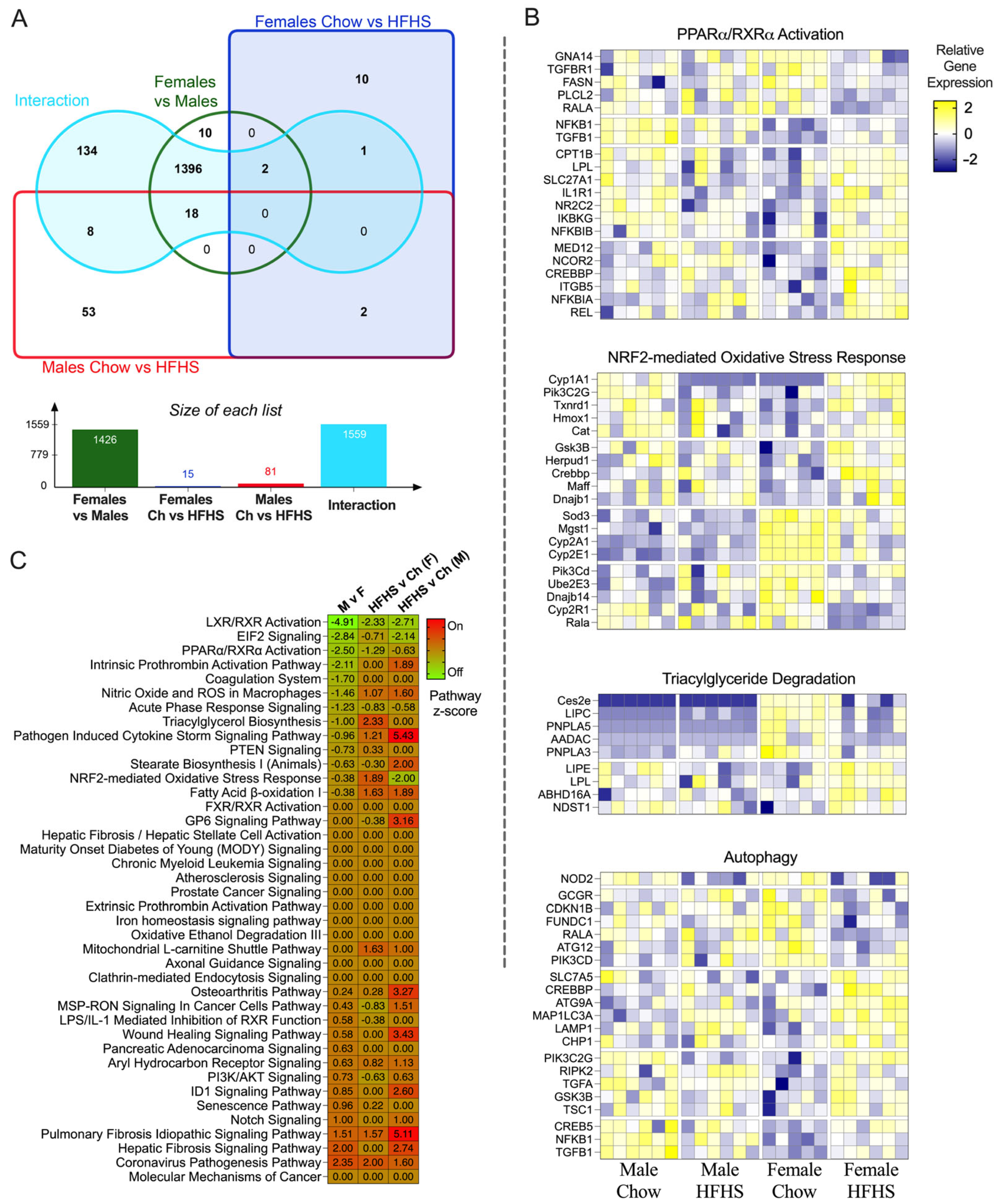
3.5. RNA Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017.
- Fletcher, B.; Gulanick, M.; Lamendola, C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 2002, 16, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.N. Sex does matter: Comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav. Pharmacol. 2007, 18, 583–589. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J. Sex and gender differences in therapy of type 2 diabetes. Diabetes Res. Clin. Pract. 2017, 131, 230–241. [Google Scholar] [CrossRef]
- Logue, J.; Walker, J.J.; Colhoun, H.M.; Leese, G.P.; Lindsay, R.S.; McKnight, J.A.; Morris, A.D.; Pearson, D.W.; Petrie, J.R.; Philip, S.; et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia 2011, 54, 3003–3006. [Google Scholar] [CrossRef]
- Casimiro, I.; Stull, N.D.; Tersey, S.A.; Mirmira, R.G. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J. Diabetes Complicat. 2021, 35, 107795. [Google Scholar] [CrossRef]
- Nordstrom, A.; Hadrevi, J.; Olsson, T.; Franks, P.W.; Nordstrom, P. Higher Prevalence of Type 2 Diabetes in Men Than in Women Is Associated with Differences in Visceral Fat Mass. J. Clin. Endocrinol. Metab. 2016, 101, 3740–3746. [Google Scholar] [CrossRef]
- Von Schulze, A.; McCoin, C.S.; Onyekere, C.; Allen, J.; Geiger, P.; Dorn, G.W., 2nd; Morris, E.M.; Thyfault, J.P. Hepatic mitochondrial adaptations to physical activity: Impact of sexual dimorphism, PGC1alpha and BNIP3-mediated mitophagy. J. Physiol. 2018, 596, 6157–6171. [Google Scholar] [CrossRef]
- Estrany, M.E.; Proenza, A.M.; Llado, I.; Gianotti, M. Isocaloric intake of a high-fat diet modifies adiposity and lipid handling in a sex dependent manner in rats. Lipids Health Dis. 2011, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Fealy, C.E.; Grevendonk, L.; Hoeks, J.; Hesselink, M.K.C. Skeletal muscle mitochondrial network dynamics in metabolic disorders and aging. Trends Mol. Med. 2021, 27, 1033–1044. [Google Scholar] [CrossRef]
- Mogensen, M.; Sahlin, K.; Fernstrom, M.; Glintborg, D.; Vind, B.F.; Beck-Nielsen, H.; Hojlund, K. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 2007, 56, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- van Tienen, F.H.; Praet, S.F.; de Feyter, H.M.; van den Broek, N.M.; Lindsey, P.J.; Schoonderwoerd, K.G.; de Coo, I.F.; Nicolay, K.; Prompers, J.J.; Smeets, H.J.; et al. Physical activity is the key determinant of skeletal muscle mitochondrial function in type 2 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Asmann, Y.W.; Stump, C.S.; Short, K.R.; Coenen-Schimke, J.M.; Guo, Z.; Bigelow, M.L.; Nair, K.S. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 2006, 55, 3309–3319. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Jani, R.; Chavez, A.; Molina-Carrion, M.; Tripathy, D.; Defronzo, R.A. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 2009, 52, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R.; Gnaiger, E.; Schjerling, P.; Skovbro, M.; Kraunsoe, R.; Dela, F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 2007, 50, 790–796. [Google Scholar] [CrossRef]
- Phielix, E.; Schrauwen-Hinderling, V.B.; Mensink, M.; Lenaers, E.; Meex, R.; Hoeks, J.; Kooi, M.E.; Moonen-Kornips, E.; Sels, J.P.; Hesselink, M.K.; et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes 2008, 57, 2943–2949. [Google Scholar] [CrossRef]
- Regensteiner, J.G.; Bauer, T.A.; Huebschmann, A.G.; Herlache, L.; Weinberger, H.D.; Wolfel, E.E.; Reusch, J.E. Sex differences in the effects of type 2 diabetes on exercise performance. Med. Sci. Sports Exerc. 2015, 47, 58–65. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Peltonen, G.L.; Binns, S.E.; Shankaran, M.; Giordano, G.R.; Hartley, D.A.; Klochak, A.L.; Lonac, M.C.; Paris, H.L.; Szallar, S.E.; et al. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J. 2014, 28, 2705–2714. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Foright, R.M.; Hull, S.E.; Knaub, L.A.; Johnson-Murguia, S.; Kinanee, F.; Kaplan, J.; Houck, J.A.; Johnson, G.; Sharp, R.R.; et al. Breast Cancer Endocrine Therapy Promotes Weight Gain with Distinct Adipose Tissue Effects in Lean and Obese Female Mice. Endocrinology 2021, 162, bqab174. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef]
- Scalzo, R.L.; Knaub, L.A.; Hull, S.E.; Keller, A.C.; Hunter, K.; Walker, L.A.; Reusch, J.E.B. Glucagon-like peptide-1 receptor antagonism impairs basal exercise capacity and vascular adaptation to aerobic exercise training in rats. Physiol. Rep. 2018, 6, e13754. [Google Scholar] [CrossRef]
- Batterson, P.M.; McGowan, E.M.; Stierwalt, H.D.; Ehrlicher, S.E.; Newsom, S.A.; Robinson, M.M. Two weeks of high-intensity interval training increases skeletal muscle mitochondrial respiration via complex-specific remodeling in sedentary humans. J. Appl. Physiol. 2023, 134, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Musci, R.V.; Andrie, K.M.; Walsh, M.A.; Valenti, Z.J.; Linden, M.A.; Afzali, M.F.; Bork, S.; Campbell, M.; Johnson, T.; Kail, T.E.; et al. Phytochemical compound PB125 attenuates skeletal muscle mitochondrial dysfunction and impaired proteostasis in a model of musculoskeletal decline. J. Physiol. 2023, 601, 2189–2216. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Zhong, Y.; Gomez-Acevedo, H.; Thakali, K.M.; Borengasser, S.J.; Andres, A.; Shankar, K. Early growth response protein-1 mediates lipotoxicity-associated placental inflammation: Role in maternal obesity. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1–E14. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Newsom, S.A.; Miller, B.F.; Hamilton, K.L.; Ehrlicher, S.E.; Stierwalt, H.D.; Robinson, M.M. Long-term rates of mitochondrial protein synthesis are increased in mouse skeletal muscle with high-fat feeding regardless of insulin-sensitizing treatment. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E552–E562. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- D’Amico, D.; Marino Gammazza, A.; Macaluso, F.; Paladino, L.; Scalia, F.; Spinoso, G.; Dimauro, I.; Caporossi, D.; Cappello, F.; Di Felice, V.; et al. Sex-based differences after a single bout of exercise on PGC1alpha isoforms in skeletal muscle: A pilot study. FASEB J. 2021, 35, e21328. [Google Scholar] [CrossRef]
- Gomez-Perez, Y.; Capllonch-Amer, G.; Gianotti, M.; Llado, I.; Proenza, A.M. Long-term high-fat-diet feeding induces skeletal muscle mitochondrial biogenesis in rats in a sex-dependent and muscle-type specific manner. Nutr. Metab. 2012, 9, 15. [Google Scholar] [CrossRef]
- Chun, J.H.; Henckel, M.M.; Knaub, L.A.; Hull, S.E.; Pott, G.B.; Ramirez, D.G.; Reusch, J.E.; Keller, A.C. (−)-Epicatechin Reverses Glucose Intolerance in Rats Housed at Thermoneutrality. Planta Med. 2022, 88, 735–744. [Google Scholar] [CrossRef]
- Islam, H.; Edgett, B.A.; Gurd, B.J. Coordination of mitochondrial biogenesis by PGC-1alpha in human skeletal muscle: A re-evaluation. Metabolism 2018, 79, 42–51. [Google Scholar] [CrossRef]
- Islam, H.; Hood, D.A.; Gurd, B.J. Looking beyond PGC-1alpha: Emerging regulators of exercise-induced skeletal muscle mitochondrial biogenesis and their activation by dietary compounds. Appl. Physiol. Nutr. Metab. 2020, 45, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Leduc-Gaudet, J.P.; Hussain, S.N.A.; Barreiro, E.; Gouspillou, G. Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Health and Aging. Int. J. Mol. Sci. 2021, 22, 8179. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef]
- Suen, D.F.; Norris, K.L.; Youle, R.J. Mitochondrial dynamics and apoptosis. Genes Dev. 2008, 22, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.B.; Bush, Z.; McGinnis, G.R.; Rowe, G.C. Adult skeletal muscle deletion of Mitofusin 1 and 2 impedes exercise performance and training capacity. J. Appl. Physiol. 2019, 126, 341–353. [Google Scholar] [CrossRef]
- Chen, H.; Vermulst, M.; Wang, Y.E.; Chomyn, A.; Prolla, T.A.; McCaffery, J.M.; Chan, D.C. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 2010, 141, 280–289. [Google Scholar] [CrossRef]
- Sebastian, D.; Sorianello, E.; Segales, J.; Irazoki, A.; Ruiz-Bonilla, V.; Sala, D.; Planet, E.; Berenguer-Llergo, A.; Munoz, J.P.; Sanchez-Feutrie, M.; et al. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J. 2016, 35, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, I.D.; Vassi, E.; Alvanou, M.; Angelakis, C.; Skaperda, Z.; Tekos, F.; Garikipati, V.N.S.; Spandidos, D.A.; Kouretas, D. The impact of diet upon mitochondrial physiology (Review). Int. J. Mol. Med. 2022, 50, 135. [Google Scholar] [CrossRef]
- Luk, H.Y.; Jiwan, N.C.; Appell, C.R.; Levitt, D.E.; Vingren, J.L. Sex-specific mitochondrial dynamics and mitophagy response to muscle damage. Physiol. Rep. 2022, 10, e15230. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Alcolea, M.P.; Valle, A.; Oliver, J.; Roca, P.; Garcia-Palmer, F.J. Skeletal muscle of female rats exhibit higher mitochondrial mass and oxidative-phosphorylative capacities compared to males. Cell Physiol. Biochem. 2007, 19, 205–212. [Google Scholar] [CrossRef]
- Chweih, H.; Castilho, R.F.; Figueira, T.R. Tissue and sex specificities in Ca2+ handling by isolated mitochondria in conditions avoiding the permeability transition. Exp. Physiol. 2015, 100, 1073–1092. [Google Scholar] [CrossRef]
- Rosa-Caldwell, M.E.; Greene, N.P. Muscle metabolism and atrophy: Let’s talk about sex. Biol. Sex Differ. 2019, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Guevara, R.; Garcia-Palmer, F.J.; Roca, P.; Oliver, J. Sexual dimorphism in liver mitochondrial oxidative capacity is conserved under caloric restriction conditions. Am. J. Physiol. Cell Physiol. 2007, 293, C1302–C1308. [Google Scholar] [CrossRef] [PubMed]
- Gormsen, L.C.; Jensen, M.D.; Schmitz, O.; Moller, N.; Christiansen, J.S.; Nielsen, S. Energy expenditure, insulin, and VLDL-triglyceride production in humans. J. Lipid Res. 2006, 47, 2325–2332. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Mohammed, B.S.; Mittendorfer, B. Plasma lipid transfer enzymes in non-diabetic lean and obese men and women. Lipids 2009, 44, 459–464. [Google Scholar] [CrossRef]
- Miotto, P.M.; McGlory, C.; Holloway, T.M.; Phillips, S.M.; Holloway, G.P. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R909–R915. [Google Scholar] [CrossRef]
- Lark, D.S.; Wasserman, D.H. Meta-fibrosis links positive energy balance and mitochondrial metabolism to insulin resistance. F1000Research 2017, 6, 1758. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Zacharova, G.; Soukup, T. Individual, age and sex differences in fiber type composition of slow and fast muscles of adult Lewis rats: Comparison with other rat strains. Physiol. Res. 2010, 59, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Eiben, R.; Bomhard, E.M. Trends in mortality, body weights and tumor incidences of Wistar rats over 20 years. Exp. Toxicol. Pathol. 1999, 51, 523–536. [Google Scholar] [CrossRef] [PubMed]


| Male | Female | |||
|---|---|---|---|---|
| Chow | HFHS | Chow | HFHS | |
| LPMs,d,i | 15.48 ± 5.42 | 24.80 ± 7.04 | 3.06 ± 2.04 | 2.64 ± 2.17 |
| PPMs,d | 75.76 ± 8.39 | 91.96 ± 11.18 | 30.48 ± 7.38 | 21.82 ± 7.94 |
| LPCMs | 8.46 ± 1.39 | 9.47 ± 3.97 | 3.62 ± 1.93 | 3.36 ± 2.95 |
| PPCMs | 100.32 ± 19.59 | 104.37 ± 19.64 | 38.58 ± 16.80 | 34.93 ± 8.24 |
| L/PPMs | 0.20 ± 0.07 | 0.27 ± 0.08 | 0.10 ± 0.07 | 0.11 ± 0.07 |
| L/PPCM | 0.45 ± 0.06 | 0.47 ± 0.15 | 0.45 ± 0.27 | 0.65 ± 0.68 |
| L/PPMGS | 0.45 ± 0.08 | 0.48 ± 0.07 | 0.39 ± 0.09 | 0.46 ± 0.08 |
| L/PPCMSs | 0.65 ± 0.10 | 0.67 ± 0.07 | 0.82 ± 0.14 | 0.84 ± 0.08 |
| P/EPMGS | 0.80 ± 0.07 | 0.79 ± 0.10 | 0.80 ± 0.30 | 0.80 ± 0.32 |
| P/EPCMSs | 0.82 ± 0.06 | 0.84 ± 0.08 | 0.90 ± 0.10 | 0.91 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hulett, N.A.; Knaub, L.A.; Hull, S.E.; Pott, G.B.; Peelor, R.; Miller, B.F.; Shankar, K.; Rudolph, M.C.; Reusch, J.E.B.; Scalzo, R.L. Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats. Nutrients 2023, 15, 4438. https://doi.org/10.3390/nu15204438
Hulett NA, Knaub LA, Hull SE, Pott GB, Peelor R, Miller BF, Shankar K, Rudolph MC, Reusch JEB, Scalzo RL. Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats. Nutrients. 2023; 15(20):4438. https://doi.org/10.3390/nu15204438
Chicago/Turabian StyleHulett, Nicholas A., Leslie A. Knaub, Sara E. Hull, Gregory B. Pott, Rick Peelor, Benjamin F. Miller, Kartik Shankar, Michael C. Rudolph, Jane E. B. Reusch, and Rebecca L. Scalzo. 2023. "Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats" Nutrients 15, no. 20: 4438. https://doi.org/10.3390/nu15204438
APA StyleHulett, N. A., Knaub, L. A., Hull, S. E., Pott, G. B., Peelor, R., Miller, B. F., Shankar, K., Rudolph, M. C., Reusch, J. E. B., & Scalzo, R. L. (2023). Sex Differences in the Skeletal Muscle Response to a High Fat, High Sucrose Diet in Rats. Nutrients, 15(20), 4438. https://doi.org/10.3390/nu15204438






