Lycium barbarum Polysaccharides Improved Glucose Metabolism in Prediabetic Mice by Regulating Duodenal Contraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Protocol
2.3. Oral Glucose Tolerance Test (OGTT) and Fasting Blood Glucose (FBG)
2.4. Biochemical Assays and Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Measurement of Duodenal Contraction
2.6. Analysis of Cecal Microbiota Composition
2.7. SCFAs Quantification Analysis
2.8. Statistical Analysis
3. Results
3.1. LBPs Improved Glucose Homeostasis in Prediabetic Mice
3.2. LBPs Regulated Duodenal Contraction via Gut Microbiota to Improve Glucose Homeostasis in Prediabetic Mice
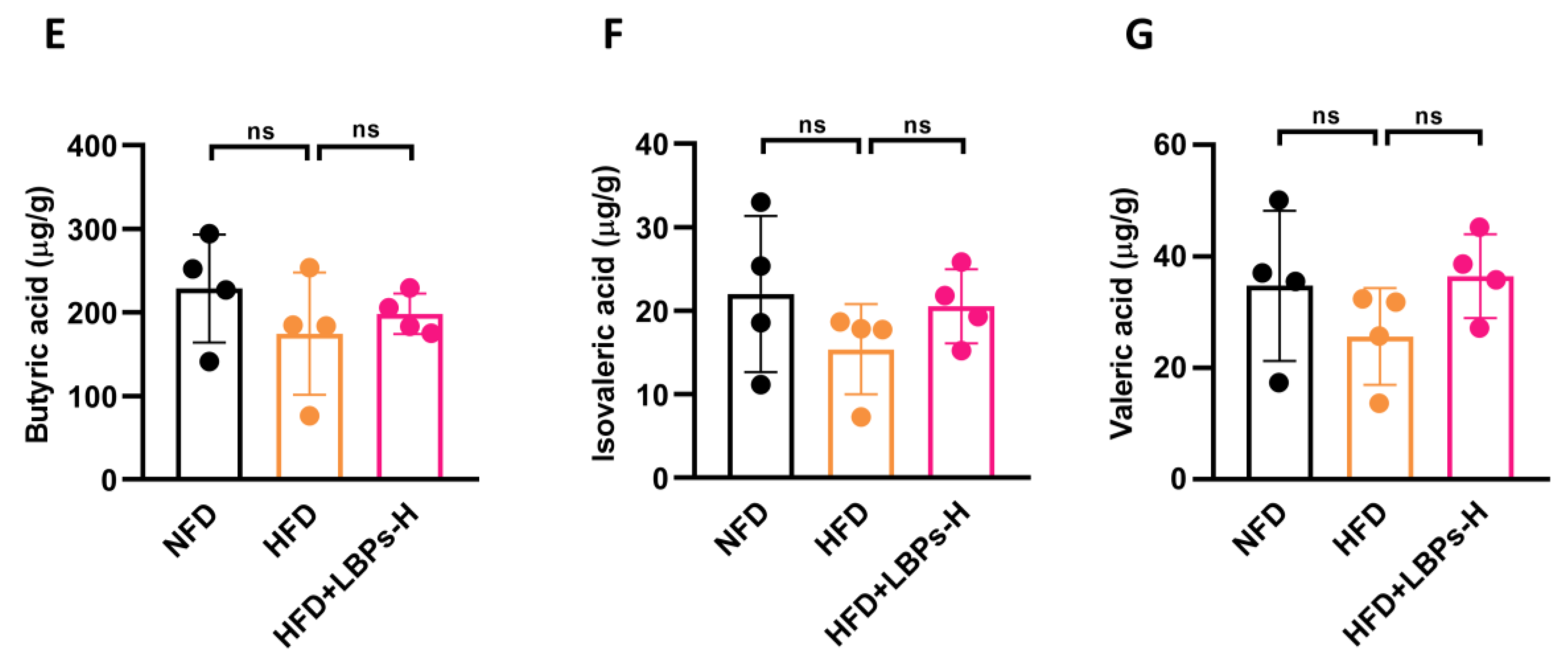
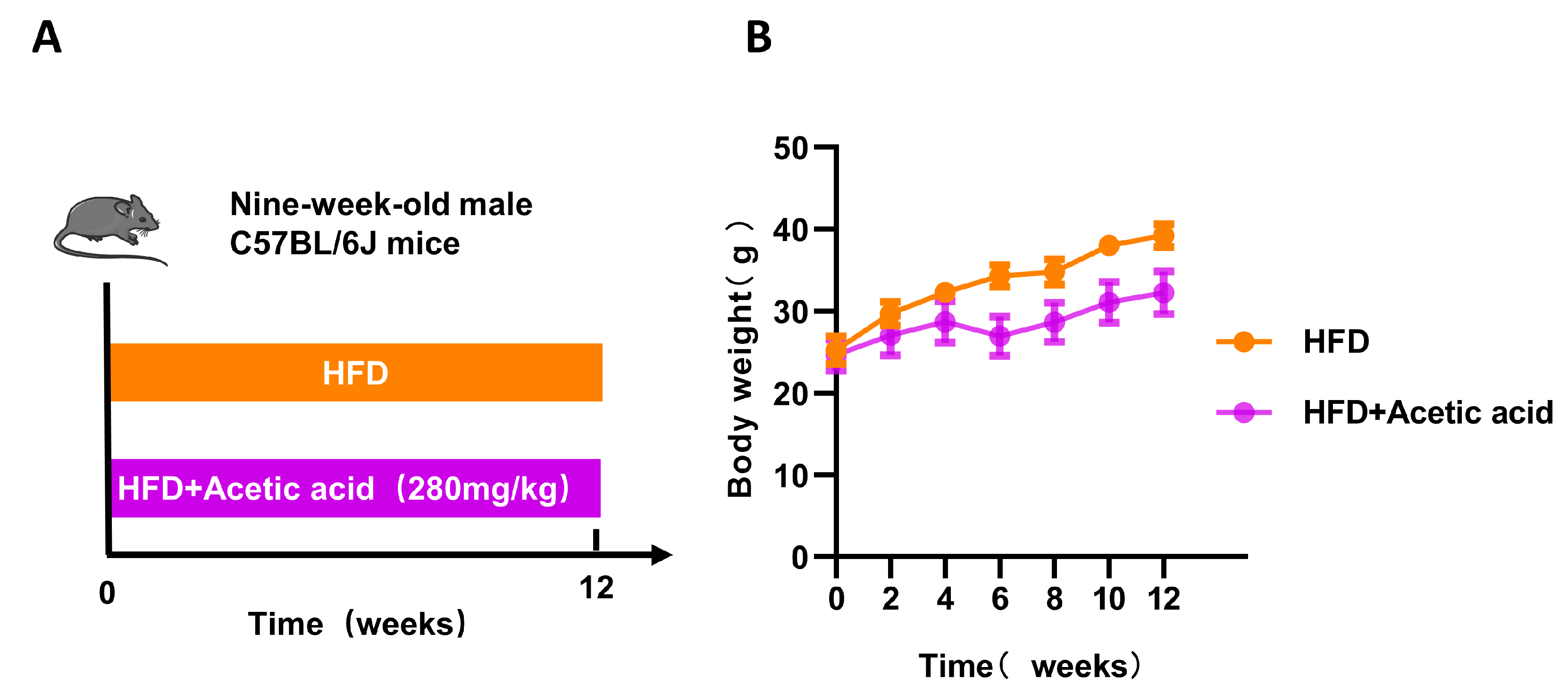
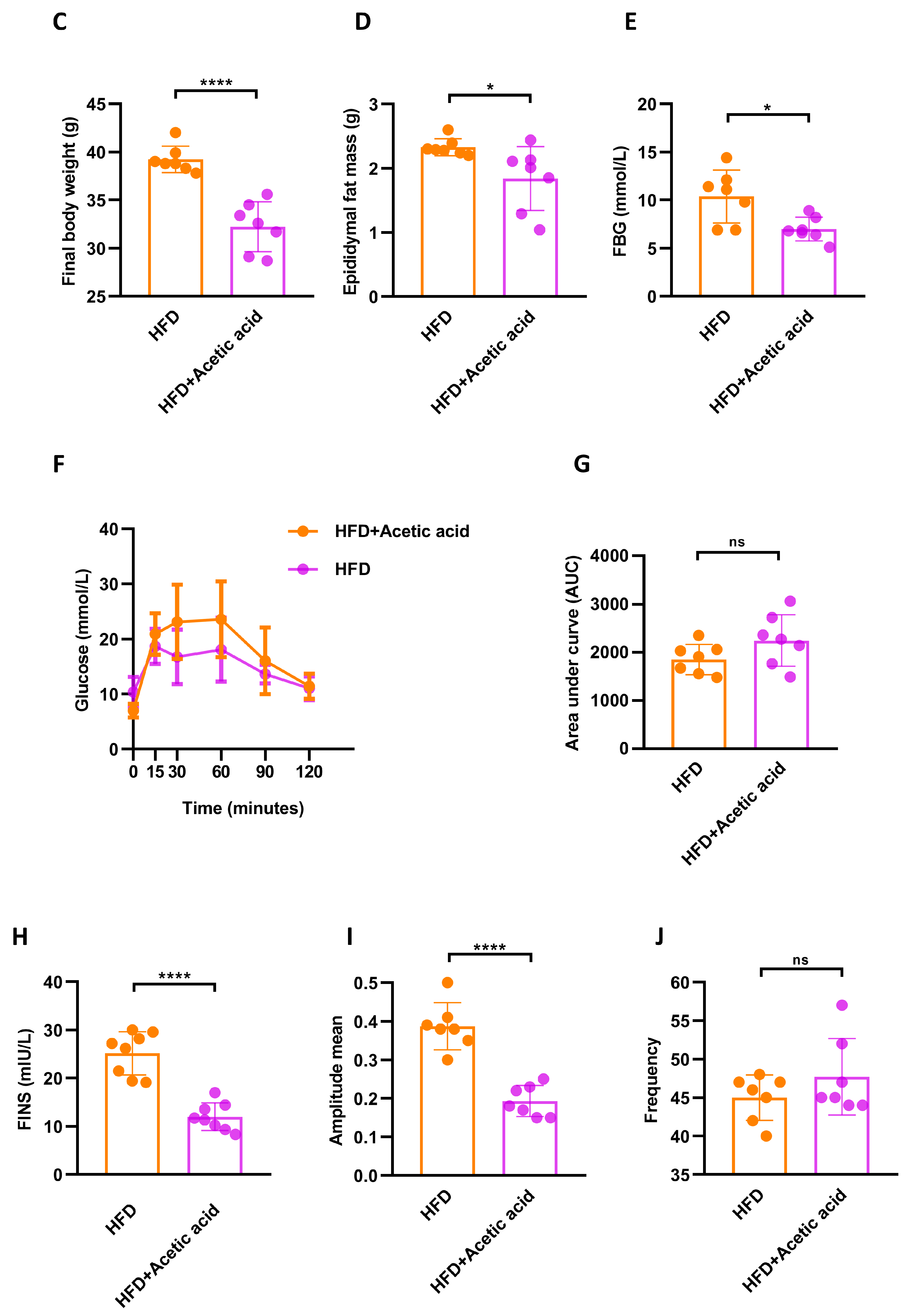
3.3. LBPs Regulated Duodenal Contraction via Acetic Acid to Improve Glucose Homeostasis in Prediabetic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Hu, H.; Mizoue, T.; Sasaki, N.; Ogasawara, T.; Tomita, K.; Nagahama, S.; Hori, A.; Nishihara, A.; Imai, T.; Yamamoto, M.; et al. Prediabetes and cardiovascular disease risk: A nested case-control study. Atherosclerosis 2018, 278, 1–6. [Google Scholar] [CrossRef]
- Romijn, J.A.; Corssmit, E.P.; Havekes, L.M.; Pijl, H. Gut-brain axis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 518–521. [Google Scholar] [CrossRef]
- Wachsmuth, H.R.; Weninger, S.N.; Duca, F.A. Role of the gut-brain axis in energy and glucose metabolism. Exp. Mol. Med. 2022, 54, 377–392. [Google Scholar] [CrossRef]
- Fournel, A.; Marlin, A.; Abot, A.; Pasquio, C.; Cirillo, C.; Cani, P.D.; Knauf, C. Glucosensing in the gastrointestinal tract: Impact on glucose metabolism. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G645–G658. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Srinivasan, S. Diabetes and the enteric nervous system. Neurogastroenterol. Motil. 2007, 19, 951–960. [Google Scholar] [CrossRef]
- Fournel, A.; Drougard, A.; Duparc, T.; Marlin, A.; Brierley, S.M.; Castro, J.; Le-Gonidec, S.; Masri, B.; Colom, A.; Lucas, A.; et al. Apelin targets gut contraction to control glucose metabolism via the brain. Gut 2017, 66, 258–269. [Google Scholar] [CrossRef]
- Abot, A.; Lucas, A.; Bautzova, T.; Bessac, A.; Fournel, A.; Le-Gonidec, S.; Valet, P.; Moro, C.; Cani, P.D.; Knauf, C. Galanin enhances systemic glucose metabolism through enteric Nitric Oxide Synthase-expressed neurons. Mol. Metab. 2018, 10, 100–108. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Gallego, D.; Mañé, N.; Gil, V.; Martínez-Cutillas, M.; Jiménez, M. Mechanisms responsible for neuromuscular relaxation in the gastrointestinal tract. Rev. Esp. Enferm. Dig. 2016, 108, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dong, H.; Zang, Z.; Wu, W.; Zhu, W.; Zhang, H.; Guan, Y. Kudzu Resistant Starch: An Effective Regulator of Type 2 Diabetes Mellitus. Oxid. Med. Cell. Longev. 2021, 2021, 4448048. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, B.; Zhang, Z. Mechanism of glycometabolism regulation by bioactive compounds from the fruits of Lycium barbarum: A review. Food Res. Int. 2022, 159, 111408. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- Xu, J.; Wang, R.; Liu, J.; Cheng, H.; Peng, D.; Xing, L.; Shi, S.; Yu, N. Determination of monosaccharides in Lycium barbarum fruit polysaccharide by an efficient UHPLC-QTRAP-MS/MS method. Phytochem. Anal. 2021, 32, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Xing, F.; Huo, J.; Fung, M.L.; Liong, E.C.; Ching, Y.P.; Xu, A.; Chang, R.C.; So, K.F.; Tipoe, G.L. Lycium barbarum polysaccharides therapeutically improve hepatic functions in non-alcoholic steatohepatitis rats and cellular steatosis model. Sci. Rep. 2014, 4, 5587. [Google Scholar] [CrossRef]
- Li, X.M. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int. J. Biol. Macromol. 2007, 40, 461–465. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Ahasan, M.T.; Sarkar, N.; Khan, H.; Hasan, A.M.; Cavalu, S.; Rauf, A. Microbiome in cancer: Role in carcinogenesis and impact in therapeutic strategies. Biomed. Pharmacother. 2022, 149, 112898. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Gao, L.L.; Ma, J.M.; Fan, Y.N.; Zhang, Y.N.; Ge, R.; Tao, X.J.; Zhang, M.W.; Gao, Q.H.; Yang, J.J. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int. J. Biol. Macromol. 2021, 183, 1379–1392. [Google Scholar] [CrossRef]
- Kaji, I.; Akiba, Y.; Konno, K.; Watanabe, M.; Kimura, S.; Iwanaga, T.; Kuri, A.; Iwamoto, K.; Kuwahara, A.; Kaunitz, J.D. Neural FFA3 activation inversely regulates anion secretion evoked by nicotinic ACh receptor activation in rat proximal colon. J. Physiol. 2016, 594, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhou, X.; Li, X.; Zhu, Q.; Peng, J.; Zhu, B.; Zheng, X.; Lu, Y.; Yang, D.; Wang, B.; et al. Depletion of Gut Microbiota Impairs Gut Barrier Function and Antiviral Immune Defense in the Liver. Front. Immunol. 2021, 12, 636803. [Google Scholar] [CrossRef] [PubMed]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and Management of Prediabetes: A Review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, P.; Wang, J.; Ma, J.; An, Y.; Chen, Y.; Zhang, B.; Feng, X.; Li, H.; Chen, X.; et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019, 7, 452–461. [Google Scholar] [CrossRef]
- Hostalek, U.; Gwilt, M.; Hildemann, S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs 2015, 75, 1071–1094. [Google Scholar] [CrossRef]
- Davidson, M.B. Metformin Should Not Be Used to Treat Prediabetes. Diabetes Care 2020, 43, 1983–1987. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef]
- Zhou, B.; Xia, H.; Yang, L.; Wang, S.; Sun, G. The Effect of Lycium Barbarum Polysaccharide on the Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. J. Am. Nutr. Assoc. 2022, 41, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, F.; Zuo, P.; Huang, G.; Song, Z.; Wang, T.; Lu, H.; Guo, F.; Han, C.; Sun, G. Practical Application of Antidiabetic Efficacy of Lycium barbarum Polysaccharide in Patients with Type 2 Diabetes. Med. Chem. 2015, 11, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Yang, F.L.; Wei, Y.X.; Liao, B.Y.; Wei, G.J.; Qin, H.M.; Pang, X.X.; Wang, J.L. Effects of Lycium barbarum Polysaccharide on Endoplasmic Reticulum Stress and Oxidative Stress in Obese Mice. Front. Pharmacol. 2020, 11, 742. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Li, Y.; Wang, Q.; Gao, L.; Zhao, J. Dietary Lycium barbarum polysaccharide induces Nrf2/ARE pathway and ameliorates insulin resistance induced by high-fat via activation of PI3K/AKT signaling. Oxid. Med. Cell. Longev. 2014, 2014, 145641. [Google Scholar] [CrossRef]
- Zhao, R.; Li, Q.; Xiao, B. Effect of Lycium barbarum polysaccharide on the improvement of insulin resistance in NIDDM rats. Yakugaku Zasshi 2005, 125, 981–988. [Google Scholar] [CrossRef]
- Zhao, R.; Qiu, B.; Li, Q.; Zhang, T.; Zhao, H.; Chen, Z.; Cai, Y.; Ruan, H.; Ge, W.; Zheng, X. LBP-4a improves insulin resistance via translocation and activation of GLUT4 in OLETF rats. Food Funct. 2014, 5, 811–820. [Google Scholar] [CrossRef]
- Zhao, C.; Zhao, H.; Zhang, C.C.; Yang, X.H.; Chen, K.; Xue, Y.; Li, Q.; Deng, S.Y.; Cai, H.Z. Impact of Lycium barbarum polysaccharide on the expression of glucagon-like peptide 1 in vitro and in vivo. Int. J. Biol. Macromol. 2023, 224, 908–918. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, T.; Xu, W.; Huang, Y.; Ran, L.; Yan, Y.; Mi, J.; Lu, L.; Sun, Y.; Zeng, X.; et al. The polysaccharides from the fruits of Lycium barbarum L. confer anti-diabetic effect by regulating gut microbiota and intestinal barrier. Carbohydr. Polym. 2022, 291, 119626. [Google Scholar] [CrossRef] [PubMed]
- Weber, C. Neurogastroenterology: Improving glucose tolerance via the gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 4. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef]
- Sanna, S.; van Zuydam, N.R.; Mahajan, A.; Kurilshikov, A.; Vich Vila, A.; Võsa, U.; Mujagic, Z.; Masclee, A.A.M.; Jonkers, D.; Oosting, M.; et al. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 2019, 51, 600–605. [Google Scholar] [CrossRef]
- Reitmeier, S.; Kiessling, S.; Clavel, T.; List, M.; Almeida, E.L.; Ghosh, T.S.; Neuhaus, K.; Grallert, H.; Linseisen, J.; Skurk, T.; et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe 2020, 28, 258–272.e256. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Abdugheni, R.; Wang, W.-Z.; Wang, Y.-J.; Du, M.-X.; Liu, F.-L.; Zhou, N.; Jiang, C.-Y.; Wang, C.-Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, H.; Xiao, X.; Hu, L.; Xin, F.; Yu, X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 2018, 6, e4446. [Google Scholar] [CrossRef]
- Santos, H.O.; de Moraes, W.; da Silva, G.A.R.; Prestes, J.; Schoenfeld, B.J. Vinegar (acetic acid) intake on glucose metabolism: A narrative review. Clin. Nutr. ESPEN 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Launholt, T.L.; Kristiansen, C.B.; Hjorth, P. Safety and side effects of apple vinegar intake and its effect on metabolic parameters and body weight: A systematic review. Eur. J. Nutr. 2020, 59, 2273–2289. [Google Scholar] [CrossRef]
- Shen, W.D.; Lin, X.; Liu, H.M.; Li, B.Y.; Qiu, X.; Lv, W.Q.; Zhu, X.Z.; Greenbaum, J.; Liu, R.K.; Shen, J.; et al. Gut microbiota accelerates obesity in peri-/post-menopausal women via Bacteroides fragilis and acetic acid. Int. J. Obes. 2022, 46, 1918–1924. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Shishehbor, F.; Mansoori, A.; Shirani, F. Vinegar consumption can attenuate postprandial glucose and insulin responses; a systematic review and meta-analysis of clinical trials. Diabetes Res. Clin. Pract. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Khezri, S.S.; Saidpour, A.; Hosseinzadeh, N.; Amiri, Z. Beneficial effects of apple cider vinegar on weight management, visceral adiposity index and lipid profile in overweight or obese subjects receiving restricted calorie diet: A randomized clinical trial. J. Funct. Foods 2018, 43, 95–102. [Google Scholar] [CrossRef]
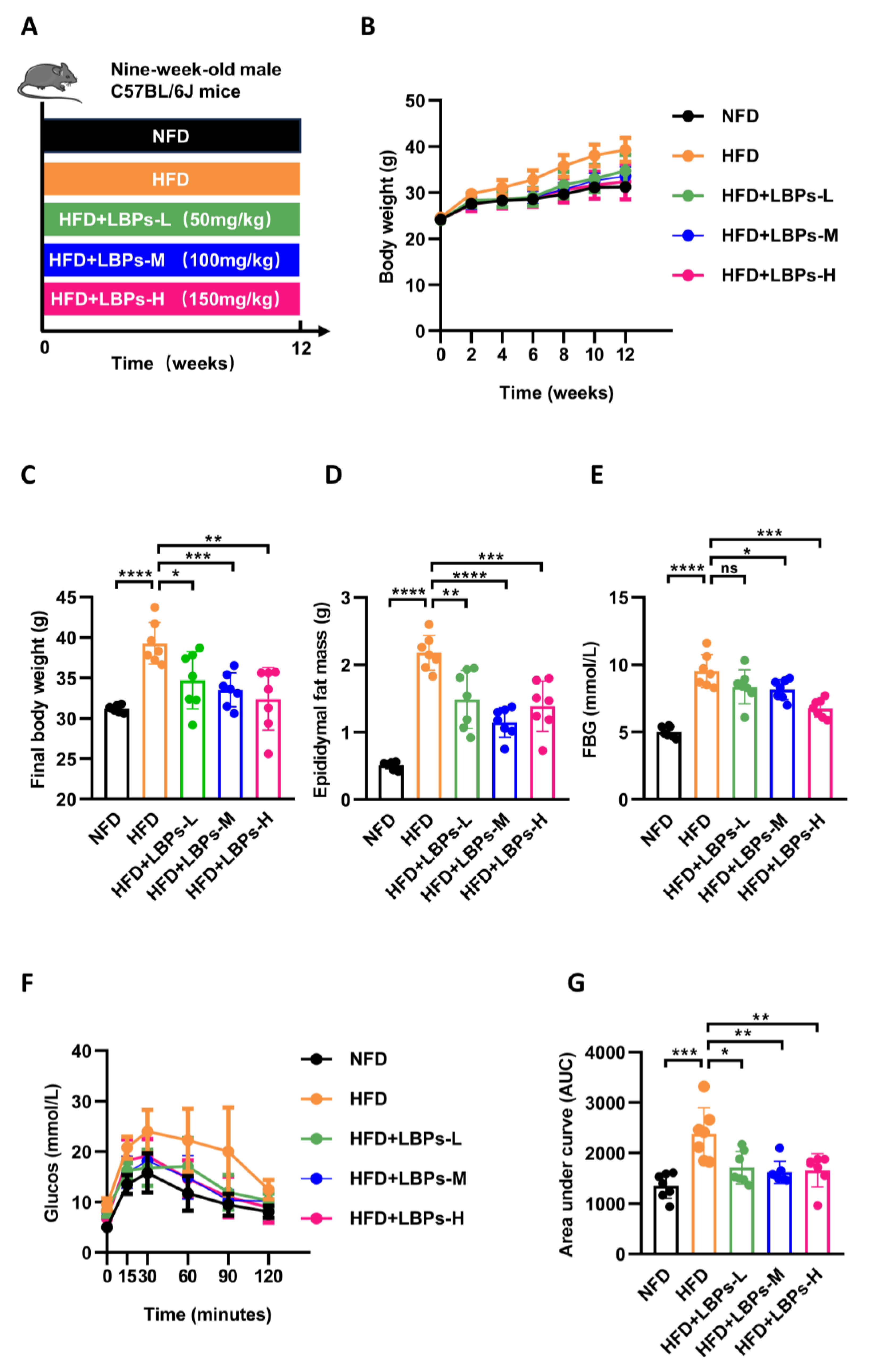

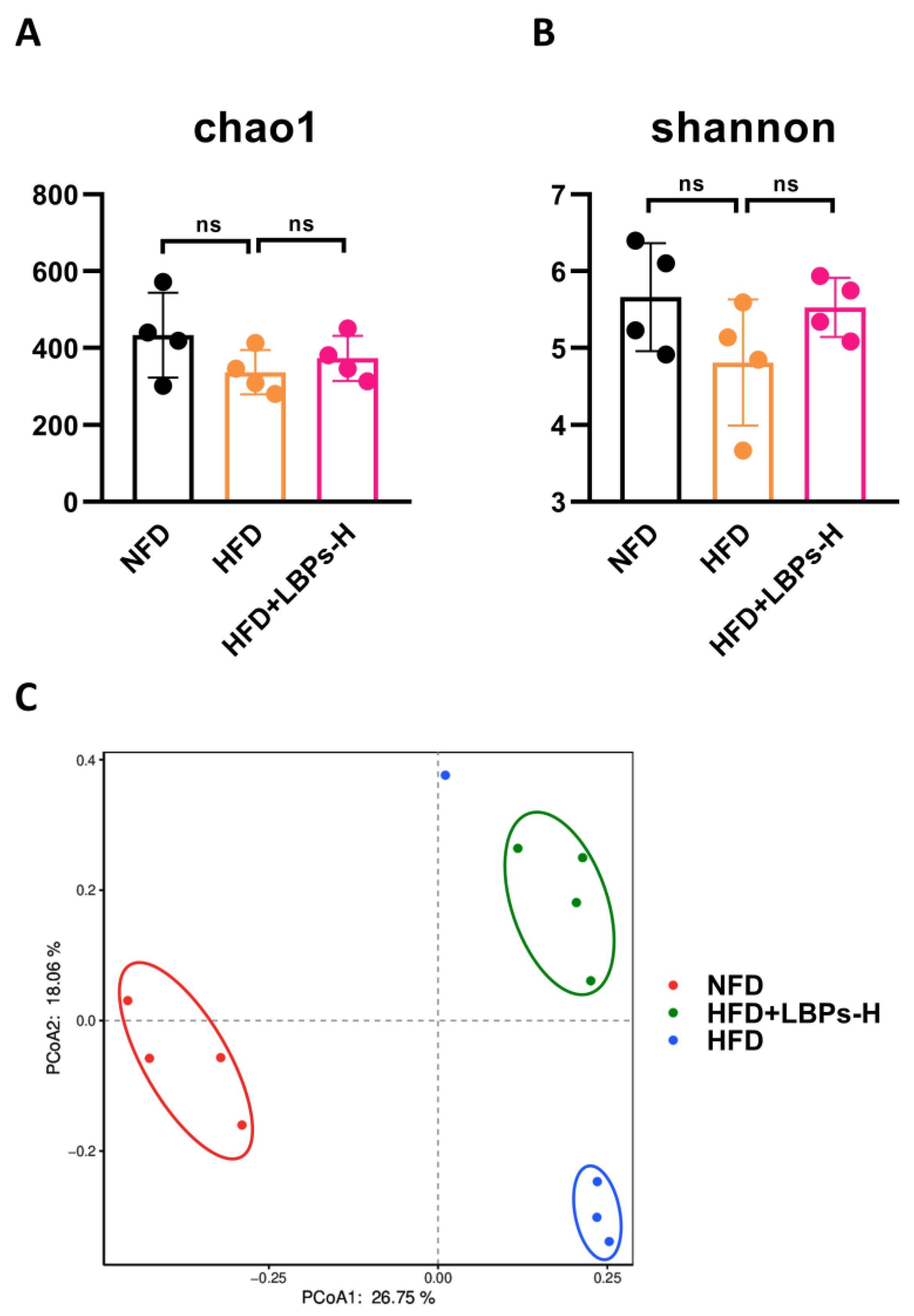
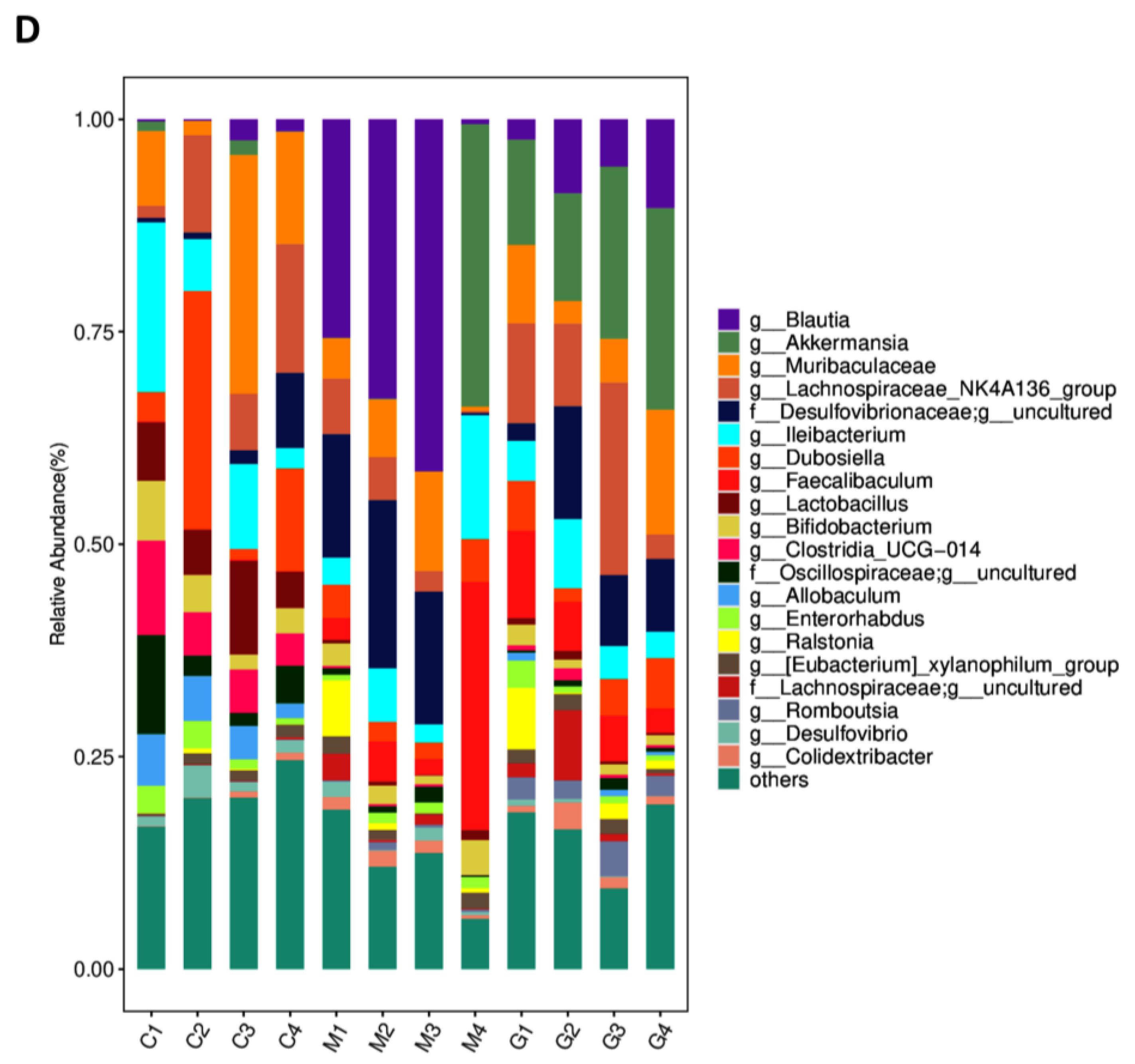


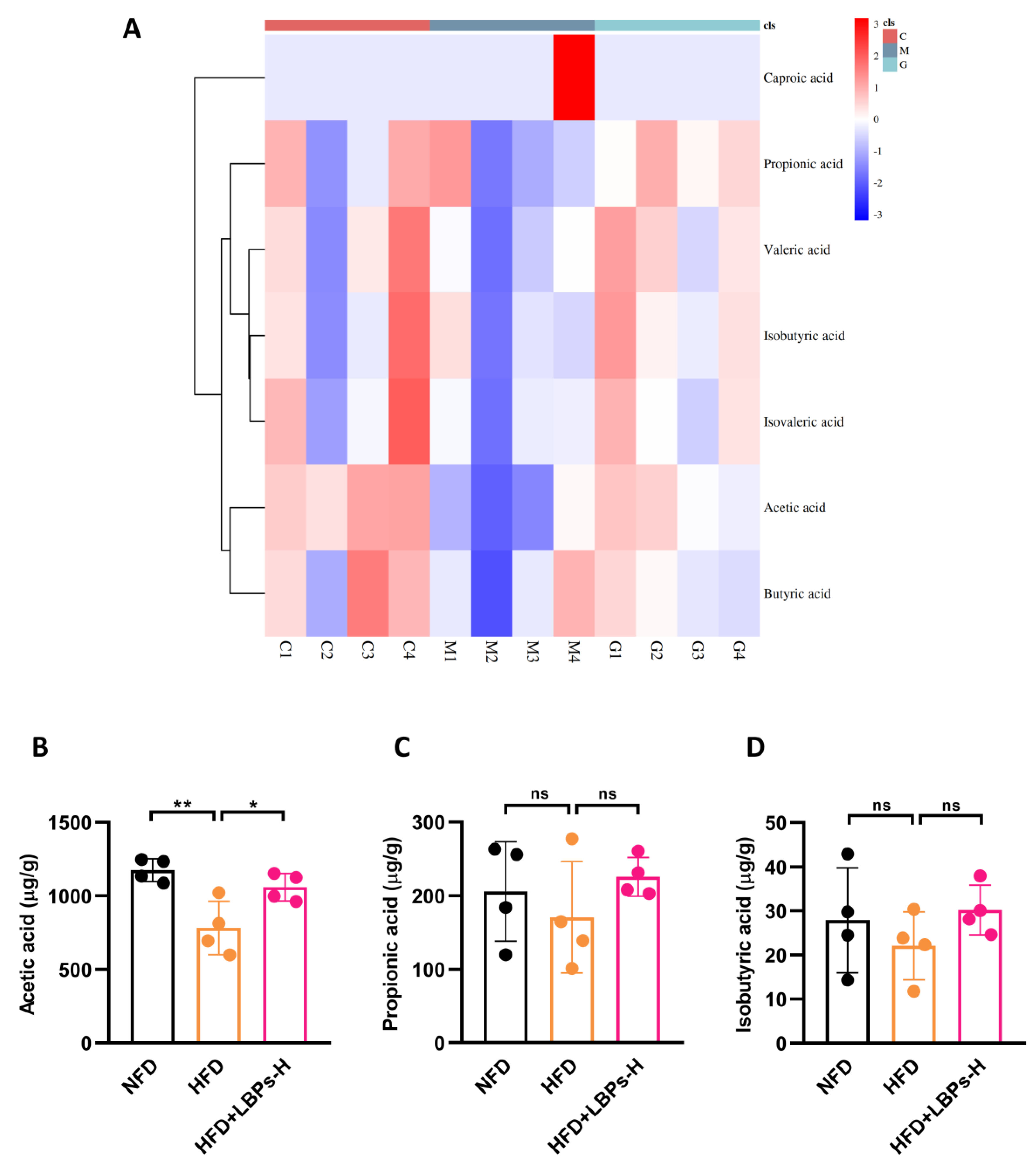
| Parameters | NFD | HFD | HFD + LBPs-L | HFD + LBPs-M | HFD + LBPs-H |
|---|---|---|---|---|---|
| TG (mmol/L) | 1.08 ± 0.12 | 1.07 ± 0.15 | 1.28 ± 0.19 * | 1.04 ± 0.27 | 0.99 ± 0.19 |
| TC (mmol/L) | 2.83 ± 0.37 | 6.61 ± 0.93 #### | 3.92 ± 0.84 *** | 5.01 ± 0.35 ** | 4.74 ± 1.03 ** |
| HDL-C (mmol/L) | 6.44 ± 2.63 | 5.46 ± 0.44 | 3.36 ± 0.92 *** | 3.90 ± 0.58 **** | 5.48 ± 0.71 |
| LDL-C (mmol/L) | 0.15 ± 0.04 | 1.23 ± 0.31 #### | 0.43 ± 0.21 *** | 0.34 ± 0.14 **** | 0.46 ± 0.21 *** |
| LDL-C/HDL-C | 0.02 ± 0.01 | 0.23 ± 0.07 #### | 0.13 ± 0.06 * | 0.08 ± 0.03 *** | 0.08 ± 0.04 *** |
| Parameters | ABx | ABx + LBPs-H |
|---|---|---|
| TG (mmol/L) | 1.66 ± 0.27 | 0.75 ± 0.31 **** |
| TC (mmol/L) | 2.86 ± 0.26 | 2.60 ± 0.43 |
| HDL-C (mmol/L) | 1.77 ± 0.38 | 1.31 ± 0.34 * |
| LDL-C (mmol/L) | 23.99 ± 3.66 | 20.92 ± 5.41 |
| LDL-C/HDL-C | 14.21 ± 4.00 | 16.65 ± 5.52 |
| Parameters | HFD | HFD + Acetic Acid |
|---|---|---|
| TG (mmol/L) | 1.43 ± 0.28 | 1.17 ± 0.22 |
| TC (mmol/L) | 3.84 ± 0.75 | 3.47 ± 0.67 |
| HDL-C (mmol/L) | 1.73 ± 0.36 | 2.58 ± 0.84 * |
| LDL-C (mmol/L) | 49.65 ± 7.75 | 38.04 ± 11.87 |
| LDL-C/HDL-C | 29.53 ± 7.07 | 17.44 ± 10.73 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Zhang, X.; Fan, Y.; Zhang, Y.; Tao, X.; Yang, J. Lycium barbarum Polysaccharides Improved Glucose Metabolism in Prediabetic Mice by Regulating Duodenal Contraction. Nutrients 2023, 15, 4437. https://doi.org/10.3390/nu15204437
Li D, Zhang X, Fan Y, Zhang Y, Tao X, Yang J. Lycium barbarum Polysaccharides Improved Glucose Metabolism in Prediabetic Mice by Regulating Duodenal Contraction. Nutrients. 2023; 15(20):4437. https://doi.org/10.3390/nu15204437
Chicago/Turabian StyleLi, Doudou, Xiaoke Zhang, Yanna Fan, Yannan Zhang, Xiujuan Tao, and Jianjun Yang. 2023. "Lycium barbarum Polysaccharides Improved Glucose Metabolism in Prediabetic Mice by Regulating Duodenal Contraction" Nutrients 15, no. 20: 4437. https://doi.org/10.3390/nu15204437
APA StyleLi, D., Zhang, X., Fan, Y., Zhang, Y., Tao, X., & Yang, J. (2023). Lycium barbarum Polysaccharides Improved Glucose Metabolism in Prediabetic Mice by Regulating Duodenal Contraction. Nutrients, 15(20), 4437. https://doi.org/10.3390/nu15204437





