Characteristics of Metabolites in the Development of Atherosclerosis in Tibetan Minipigs Determined Using Untargeted Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
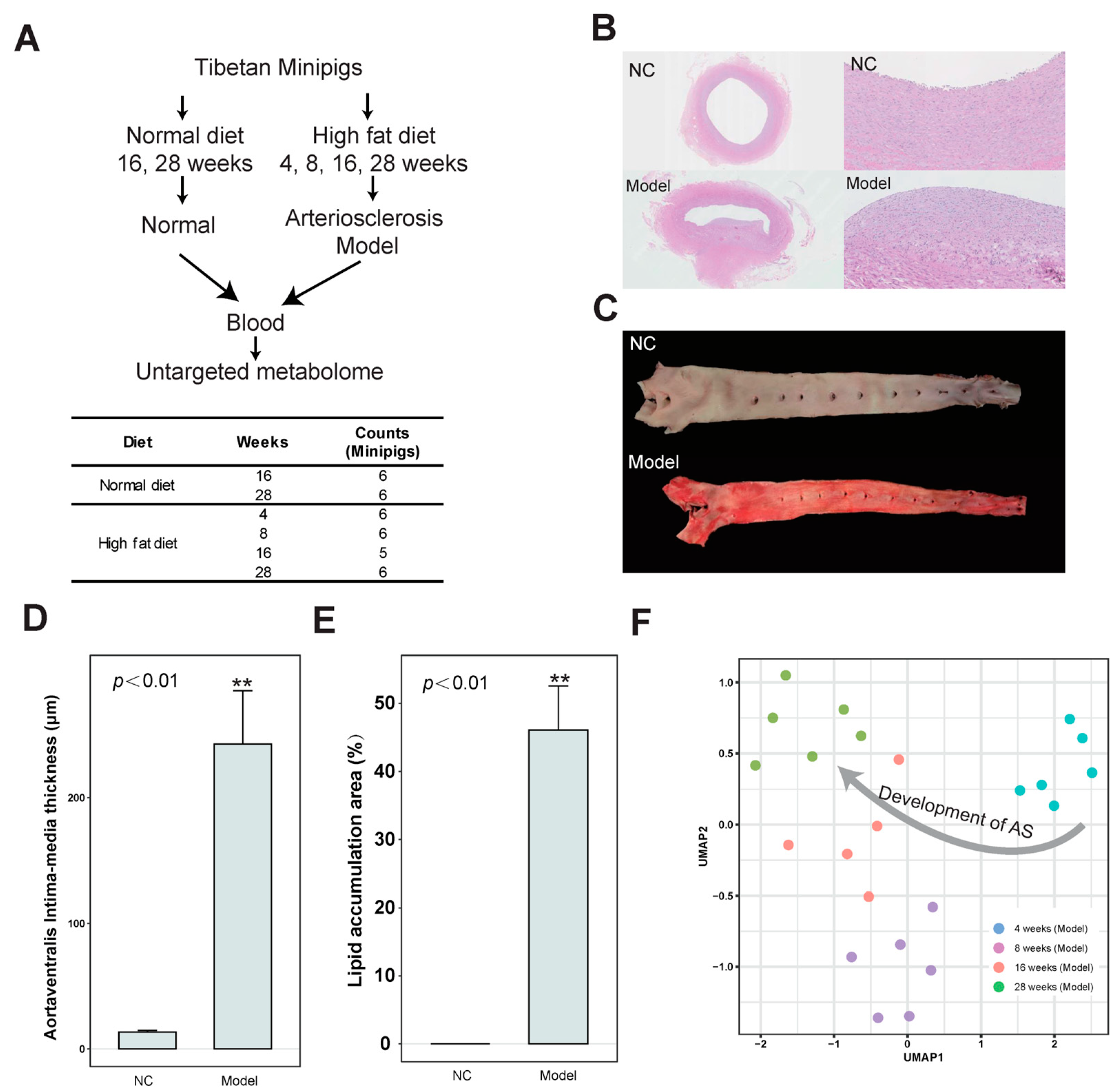
2.1. Laboratory Animal
2.2. Animal Serum and Aortic Vessel
2.2.1. Sudan IV Staining to Observe Lipid Deposition in the Aorta
2.2.2. HE Staining to Observe the Pathological Morphology of Coronary Artery Tissue
2.2.3. Blood Lipid Detection
2.2.4. Detection of Serum Inflammation, Oxidative Stress, and Vascular Endothelial Injury-Related Indicators
2.3. Non-Targeted Metabolomics Analysis
2.4. Data Analysis
3. Results
3.1. Identification of Differential Abundance Metabolites in the Development of AS Based on the Untargeted Metabolome
3.2. Characteristics of Metabolites Enriched in the AS Model Group
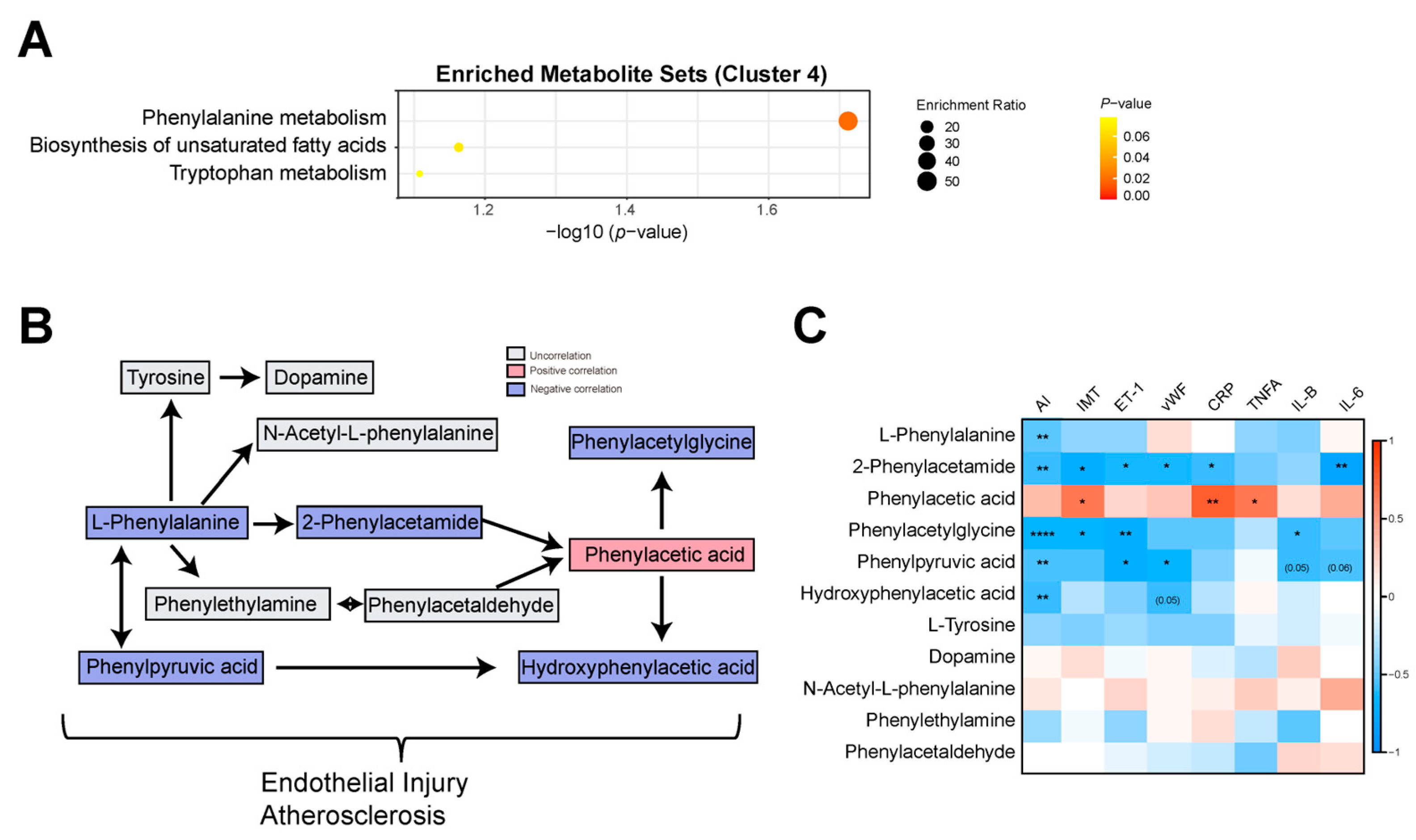
3.3. Phenylalanine Metabolism Was Reduced in the AS Model Group
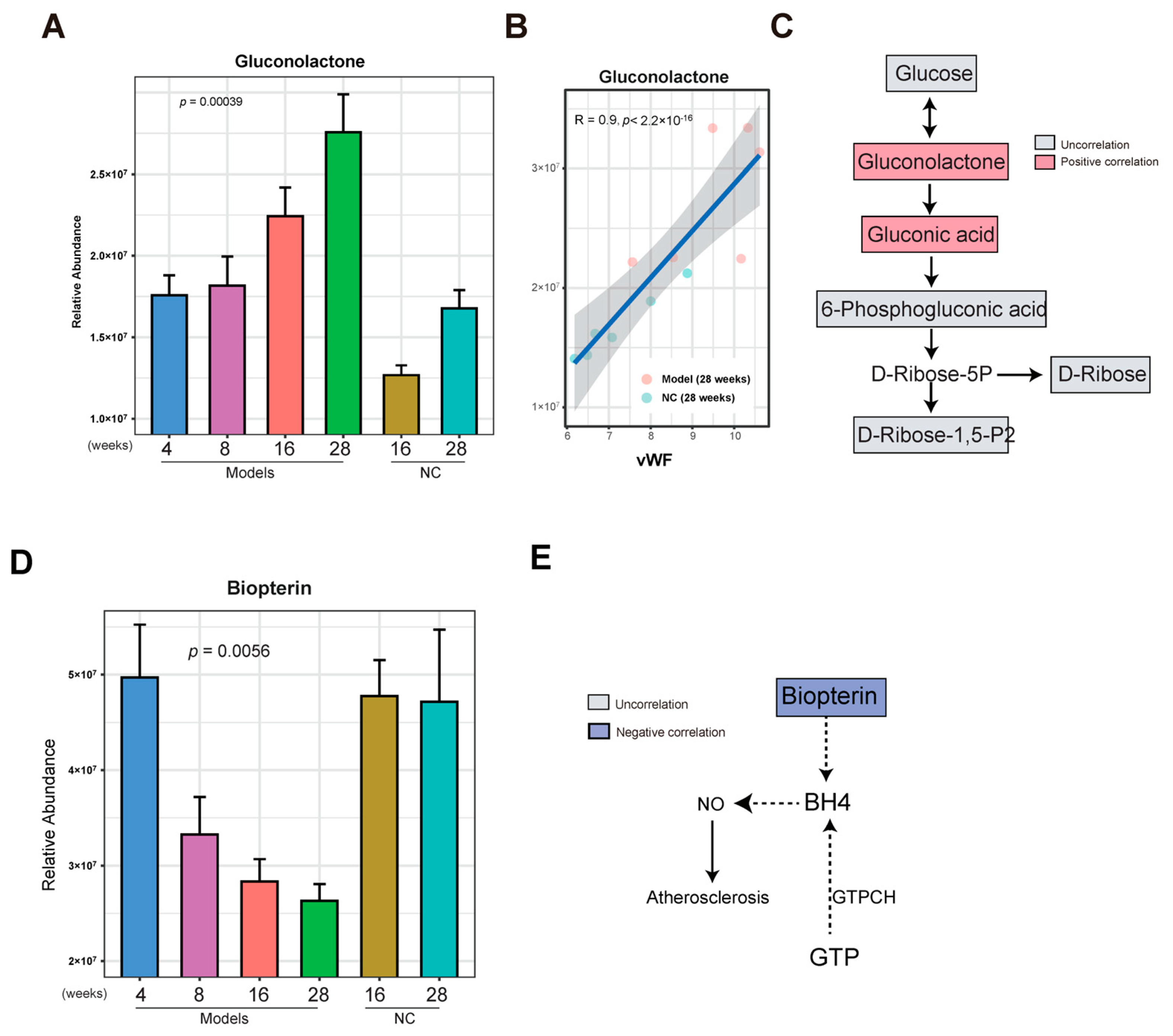
3.4. Gluconolactone Was Enriched in the Late Stage of AS
3.5. Biopterin Was Reduced in the Late Stage of AS
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamilio, D.M.; Scifres, C.M. Extreme Obesity and Postcesarean Maternal Complications. Obstet. Gynecol. 2014, 124, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risks: A compass for global action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef]
- Xu, S.; Kamato, D.; Little, P.J.; Nakagawa, S.; Pelisek, J.; Jin, Z.G. Targeting epigenetics and non-coding RNAs in atherosclerosis: From mechanisms to therapeutics. Pharmacol. Ther. 2019, 196, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pelisek, J.; Jin, Z.G. Atherosclerosis Is an Epigenetic Disease. Trends Endocrinol. Metab. 2018, 29, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, T.T.; Nuotio, J.; Rovio, S.P.; Niinikoski, H.; Juonala, M.; Magnussen, C.G.; Jokinen, E.; Lagström, H.; Jula, A.; Viikari, J.S.; et al. Dietary Fats and Atherosclerosis From Childhood to Adulthood. Pediatrics 2020, 145, e20192786. [Google Scholar] [CrossRef]
- Acosta, S.; Johansson, A.; Drake, I. Diet and Lifestyle Factors and Risk of Atherosclerotic Cardiovascular Disease—A Prospective Cohort Study. Nutrients 2021, 13, 3822. [Google Scholar] [CrossRef]
- Zhu, Y.; Xian, X.; Wang, Z.; Bi, Y.; Chen, Q.; Han, X.; Tang, D.; Chen, R. Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8, 80. [Google Scholar] [CrossRef]
- Lecce, L.; Xu, Y.; V’gangula, B.; Chandel, N.; Pothula, V.; Caudrillier, A.; Santini, M.P.; D’escamard, V.; Ceholski, D.K.; Gorski, P.A.; et al. Histone deacetylase 9 promotes endothelial-mesenchymal transition and an unfavorable atherosclerotic plaque phenotype. J. Clin. Investig. 2021, 131, e131178. [Google Scholar] [CrossRef]
- Iida, M.; Harada, S.; Takebayashi, T. Application of Metabolomics to Epidemiological Studies of Atherosclerosis and Cardiovascular Disease. J. Atheroscler. Thromb. 2019, 26, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.; Al-Mashhadi, R.; Sørensen, C.; Bentzon, J. Large animal models of atherosclerosis—New tools for persistent problems in cardiovascular medicine. J. Pathol. 2016, 238, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Xu, Y.; Shen, L.; Pan, Y.; Huang, J.; Ma, Q.; Yu, C.; Chen, J.; Chen, Y.; Chen, M. Guanxinning Tablet Attenuates Coronary Atherosclerosis via Regulating the Gut Microbiota and Their Metabolites in Tibetan Minipigs Induced by a High-Fat Diet. J. Immunol. Res. 2022, 2022, 7128230. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Yu, C.; Fu, D.; Pan, Y.; Huang, J.; Rong, Y.; Deng, L.; Chen, J.; Chen, M. Differential metabolic and hepatic transcriptome responses of two miniature pig breeds to high dietary cholesterol. Life Sci. 2020, 250, 117514. [Google Scholar] [CrossRef]
- Pan, Y.; Rong, Y.; Huang, J.; Zhu, K.; Chen, J.; Yu, C.; Chen, M. Lower cardiovagal tone and baroreflex sensitivity associated with hepatic insulin resistance and promote cardiovascular disorders in Tibetan minipigs induced by a high fat and high cholesterol diet. J. Diabetes Its Complicat. 2019, 33, 278–288. [Google Scholar] [CrossRef]
- Becht, E.; McInnes, L.; Healy, J.; Dutertre, C.-A.; Kwok, I.W.H.; Ng, L.G.; Ginhoux, F.; Newell, E.W. Dimensionality reduction for visualizing single-cell data using UMAP. Nat. Biotechnol. 2018, 37, 38–44. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Skoura, A.; Michaud, J.; Im, D.-S.; Thangada, S.; Xiong, Y.; Smith, J.D.; Hla, T. Sphingosine-1-Phosphate Receptor-2 Function in Myeloid Cells Regulates Vascular Inflammation and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 81–85. [Google Scholar] [CrossRef]
- Christoffersen, C.; Obinata, H.; Kumaraswamy, S.B.; Galvani, S.; Ahnström, J.; Sevvana, M.; Egerer-Sieber, C.; Muller, Y.A.; Hla, T.; Nielsen, L.B.; et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA 2011, 108, 9613–9618. [Google Scholar] [CrossRef]
- Schissel, S.L.; Tweedie-Hardman, J.; Rapp, J.H.; Graham, G.; Williams, K.J.; Tabas, I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J. Clin. Investig. 1996, 98, 1455–1464. [Google Scholar] [CrossRef]
- Wong, C.M.; Wong, K.H.; Chen, X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008, 78, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, Z.; Ma, W.; Fang, C.; Pei, Y.; Jing, Y.; Huang, J.; Han, X.; Xiao, W. Untargeted metabolomic profiling of sepsis-induced cardiac dysfunction. Front. Endocrinol. 2023, 14, 1060470. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Cosentino, F.; Lütolf, R.B.; Fleisch, M.; Seiler, C.; Hess, O.M.; Meier, B.; Lüscher, T.F. Tetrahydrobiopterin Improves Endothelial Function in Patients with Coronary Artery Disease. J. Cardiovasc. Pharmacol. 2000, 35, 173–178. [Google Scholar] [CrossRef]
- Dias, P.; Pourová, J.; Vopršalová, M.; Nejmanová, I.; Mladěnka, P. 3-Hydroxyphenylacetic Acid: A Blood Pressure-Reducing Flavonoid Metabolite. Nutrients 2022, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.H.; Tatum, S.M.; Symons, J.D.; Summers, S.A.; Holland, W.L. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 701–711. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Park, T.-S.; Hu, Y.; Noh, H.-L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.-C.; Abel, E.D.; et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008, 49, 2101–2112. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Ye, J.; Sun, G.; Sun, X. Mechanism overview and target mining of atherosclerosis: Endothelial cell injury in atherosclerosis is regulated by glycolysis. Int. J. Mol. Med. 2021, 47, 65–76. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, N.; Hui, H.; Wang, Y.; Liu, F.; Xu, L.; Liu, M.; Rao, Z.; Yuan, Z.; Shang, Y.; et al. Endothelial cell-derived tetrahydrobiopterin prevents aortic valve calcification. Eur. Heart J. 2022, 43, 1652–1664. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Liu, C.; Lv, X.H.; Li, H.X.; Cao, X.; Zhang, F.; Wang, L.; Yu, M.; Yang, J.K. Angiotensin-(1-7) suppresses oxidative stress and improves glucose uptake via Mas receptor in adipocytes. Acta Diabetol. 2012, 49, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Toyama, H.; Furuya, N.; Saichana, I.; Ano, Y.; Adachi, O.; Matsushita, K. Membrane-Bound, 2-Keto- d -Gluconate-Yielding d -Gluconate Dehydrogenase from “Gluconobacter dioxyacetonicus” IFO 3271: Molecular Properties and Gene Disruption. Appl. Environ. Microbiol. 2007, 73, 6551–6556. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-H.; Qi, C.; Lin, J.; Huang, P. Catalytic chemistry of glucose oxidase in cancer diagnosis and treatment. Chem. Soc. Rev. 2018, 47, 6454–6472. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Wang, J.; Pan, Y.; Huang, J.; Zhu, K.; Tu, H.; Chen, M. Characteristics of Metabolites in the Development of Atherosclerosis in Tibetan Minipigs Determined Using Untargeted Metabolomics. Nutrients 2023, 15, 4425. https://doi.org/10.3390/nu15204425
Shen L, Wang J, Pan Y, Huang J, Zhu K, Tu H, Chen M. Characteristics of Metabolites in the Development of Atherosclerosis in Tibetan Minipigs Determined Using Untargeted Metabolomics. Nutrients. 2023; 15(20):4425. https://doi.org/10.3390/nu15204425
Chicago/Turabian StyleShen, Liye, Jinlong Wang, Yongming Pan, Junjie Huang, Keyan Zhu, Haiye Tu, and Minli Chen. 2023. "Characteristics of Metabolites in the Development of Atherosclerosis in Tibetan Minipigs Determined Using Untargeted Metabolomics" Nutrients 15, no. 20: 4425. https://doi.org/10.3390/nu15204425
APA StyleShen, L., Wang, J., Pan, Y., Huang, J., Zhu, K., Tu, H., & Chen, M. (2023). Characteristics of Metabolites in the Development of Atherosclerosis in Tibetan Minipigs Determined Using Untargeted Metabolomics. Nutrients, 15(20), 4425. https://doi.org/10.3390/nu15204425






