Abstract
Evidence examining specific effects of a multidisciplinary team (MDT) on cardiometabolic risk factors (CMRFs) among multi-ethnic patients in real-world clinical settings is lacking. This one-year retrospective chart review (2018) analyzed 598 adults (African American 59%, Hispanic 35%, and Caucasian 6%) with mean age of 43.8 ± 14.0 years. Qualifying patients with primary inclusion criteria of having body mass indices and blood pressure (BP) measurements in the first and last quarter of the study period were treated under an MDT protocol and compared to those qualifying for MDT but treated solely by a primary care provider (PCP). MDT included endocrinologist-directed visits, lifestyle counseling, and shared medical appointments. MDT patients experienced a greater reduction (β; 95% CI) in weight (−4.29 kg; −7.62, −0.97), BMI (−1.43 kg/m2; −2.68, −0.18), systolic BP (−2.18 mmHg; −4.09, −0.26), and diastolic BP (−1.97 mmHg; −3.34, −0.60). Additionally, MDT patients had 77%, 83%, and 59% higher odds of reducing ≥5% of initial weight, 1 BMI point, and ≥2 mmHg DBP, respectively. Improvements in hemoglobin A1C measurements were observed in the MDT group (insufficient data to compare with the PCP group). Compared to PCP only, MDT co-management improves CMRF related to adiposity and hypertension in a multiethnic adult cohort in real-world clinical settings. Patient access to best practices in cardiometabolic care is a priority, including the incorporation of culturally adapted evidence-based recommendations translated within a multi-disciplinary infrastructure, where competing co-morbidities are better managed, and associated research and education programs can promote operational sustainability.
1. Introduction
The prevention and treatment of chronic cardiovascular disease (CVD) by focusing on metabolic drivers, such as abnormal adiposity, dysglycemia, hypertension (HTN), and dyslipidemia, require a complex cardiometabolic approach. This mandates the concurrent consideration of both biological and social determinants of health that impact patient care and outcomes [1]. Healthcare delivered by a team following a systematically designed set of multidisciplinary protocols can increase the effectiveness of interventions to mitigate cardiometabolic risk. This multidisciplinary team approach (MDT) has demonstrated effectiveness in controlling weight and associated complications within the controlled conditions of clinical trials. However, implementation in routine, real-world clinical settings with diverse populations based on ethnocultural factors has been limited. This is likely due to suboptimal chronic disease modeling (step 1 below), infrastructure building (step 2 below), sustainability, and effectiveness. Notably, adaptations of lifestyle medicine interventions for diabetes prevention based on the U.S. Diabetes Prevention Program and the Finnish Diabetes Prevention Study have been employed in a variety of clinical settings, but still, these results have been significantly less effective than the original report [2].
Chronic disease modeling is a critical first step to optimize and implement preventive care. To promote early intervention in patients and enhance sensitivity for detecting subjects affected by abnormal adiposity, the American Association of Clinical Endocrinology (AACE) proposed that obesity should be considered not solely by anthropometrics (e.g., ethnicity-adjusted body mass index (BMI) and waist circumference), but by prioritizing weight-related complications. Furthermore, to incorporate new scientific relationships among ectopic fat, inflammation, insulin resistance, and cardiometabolic health, AACE devised the Adiposity-Based Chronic Disease (ABCD) model (stage 1 = risk; stage 2 = predisease; stage 3 = disease; and stage 4 = complications), within which obesity is part of stage 3. Subsequently, to reconcile insulin resistance, prediabetes, type 2 diabetes (T2D), and vascular complications in the same four-stage preventive care strategy, AACE devised the Dysglycemia-Based Chronic Disease (DBCD) model, of which T2D is also stage 3 [3,4]. Unlike the BMI-centric approach to obesity, the complication-centric approach to intervention goals considers abnormal fat distribution and function, as well as ethnocultural, social determinants of health. In a more recent advancement, an umbrella three-dimensional (stage, driver, and social) Cardiometabolic-Based Chronic Disease (CMBCD) model was assembled to provide more breadth and robustness when deriving individual care plans [4,5]. In these driver-based chronic disease models, the consideration of primary drivers (genetics, environment, and behavior creating a unique personalized lifestyle), and well as the secondary/metabolic drivers mentioned above can fashion an approach that mitigates progression to CVD [4].
Infrastructure building is the second step to optimize and implement preventive care. Lifedoc Health (LDH) is a multi-disciplinary and data-driven healthcare organization committed to preventing two of the leading drivers of CVD in the CMBCD model—dysglycemia (specifically T2D) and abnormal adiposity (specifically obesity)—by increasing accessibility to care to minority populations that are underserved through an integrated and standardized outcome-oriented model. This broader approach is necessary for successful population health in the cardiometabolic space. The LDH programs have received state and National Committee for Quality Assurance recognition and accreditation. The LDH clinical model combines primary and specialty care, acute and chronic care, as well as care coordination, pharmacy, patient education, and lifestyle counseling into a unified dynamic approach. Providers undergo protocol training and reinforcement; primary care providers (PCPs) are coached for the early enrollment of patients with diverse ethnic backgrounds, with or without CMBCD risk factors for the MDT co-management of increased weight, high blood pressure, dyslipidemia, and dysglycemia.
Several obstacles may limit the effectiveness of MDT co-management in these patients, including (a) patient and provider perceptions or stigmatization of obesity [6,7], (b) time constraints of providers and limited training to manage obesity and related complications, (c) competing priorities for referral of those with multiple chronic conditions, (d) the presence of numerous social determinants of health (e.g., limited access to preventive care, and job, transportation, and housing insecurity), and (e) patient inertia (e.g., due limited health literacy about their family’s health). The purpose of this pilot study is to demonstrate the effectiveness of implementing the LDH model. This will be accomplished by evaluating the specific impact of a critical aspect of LHD: the effect of MDT on measured markers of CMBCD progression in a multi-racial population with routine, real-world patient encounters.
2. Materials and Methods
2.1. Study Design and Population
This is a retrospective chart review based on a 1-year analysis (2018) of longitudinal structured data. Data were collected in an electronic health record system from a cohort of 43,079 patients evaluated at LDH over a 15-year period and comprised 83% minorities, including 58% Hispanic (H) and 25% African American (AA). Patients were 56.6% female, with 75% of healthcare coverage coming via government insurance, 10% from commercial insurance, and 15% cash/uninsured. From this, a total of 3596 patients ≥ 18 years of age were evaluated at LDH in 2018 (±6 weeks). Of these, a total of 1112 patients (30.9%) meeting preliminary inclusion criteria, namely BMI and blood pressure averages documented in both the first quarter (Q1) and last quarter (Q4) of the period, were randomly selected. Additional inclusion criteria were included to further refine the patient sample by having the following diagnoses: (1) overweight (OW)/obesity (OB) (via ICD-10 or BMI ≥ 25), (2) HTN stage 1 or 2 (via ICD-10, systolic blood pressure (SBP) between 130–139 or ≥140 mmHg, respectively and/or diastolic blood pressure (DBP) between 80–89 or ≥90 mmHg, respectively), and/or (3) prediabetes/T2D (via ICD-10 or hemoglobin A1c (A1C) ≥ 5.7% (38 mmol/mol) and 6.5% (48 mmol/mol), respectively). Dyslipidemia was not included as an activation diagnosis because a fasting lipid profile was not available for all patients. From this subgroup, 598 patients (53.8%) were ultimately included in the analysis. The exclusion criterion was an inadequate visit profile for either intervention group (Figure 1). The demography of the sample selected was 65% government insurance, 18% commercial insurance, and 17% cash/uninsured, and AA 58.9%, H 35.3%, and Caucasian (C) 5.9%.
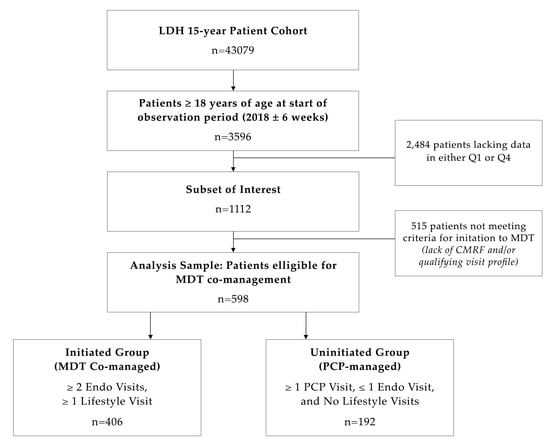
Figure 1.
Study sample selection. Abbreviations: CMRF: Cardiometabolic risk factors, Endo: Endocrinology, LDH: Lifedoc Health, MDT: Multidisciplinary team, PCP: Primary Care Provider, Q1: First quarter, Q4: Last quarter.
2.2. Study Variables
The primary exposure in this pilot study was type of care (MDT vs. PCP only). Primary outcomes were 1-year changes in weight and BMI between Q1 and Q4, while secondary outcomes included 1-year changes in SBP, DBP, and number of cardiometabolic risk factors (CMRFs) in the same period. Weight was measured with light clothing and without shoes, using a calibrated scale (Digital Platform Scale Pro Plus 2101KL, Health-o-meter®, McCook, IL, USA). Height was measured using a 264 Digital Stationary Stadiometer (Seca®, Chino, CA, USA). Blood pressure was measured in the right arm, using the appropriate cuff size, in a sitting position, with a validated Lumeon aneroid sphygmomanometer (McKesson®, Irving, TX, USA). The A1C was measured using both in-house rapid testing (Alere Afinion 2 Point of Care Analyzer, Abbott®, Lake Forest, IL, USA) and laboratory test by immunoassay in a certified laboratory [8].
2.3. Definitions
Ethnocultural classifiers were self-reported as either AA, H, or C. Obesity was defined as a BMI ≥ 30 kg/m2 and overweight as BMI 25–29.9 kg/m2 in this multiethnic population without Asian representation (in whom BMI cutoffs are lower) [9]. HTN was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or personal history of HTN/use of antihypertensive medication [10]. Prediabetes was defined as either fasting blood glucose between 100–125 mg/dL, 2 h post-load blood glucose level between 140–199 mg/dL or A1C between 5.7% (38 mmol/mol) and 6.4% (46 mmol/mol), inclusive. T2D was defined as either a fasting blood glucose ≥ 126 mg/dL, 2 h post-load blood glucose level ≥ 200 mg/dL, A1C ≥ 6.5% (48 mmol/mol), or personal history of T2D [11].
2.4. Study Groups and Interventions
Intervention groups were defined as: (a) MDT—patients with ≥2 endocrinology visits and at least 1 visit with a lifestyle intervention provider in the same period, and (b) PCP—patients evaluated at least one time by PCP with ≤1 endocrinology visit and no visits with any lifestyle intervention provider (nutrition, wellness, or education) within the observation period. MDT included primarily endocrinology-oriented visits coordinated by an endocrinologist and provided by a nurse practitioner, lifestyle counseling provided by an exercise physiologist including physical activity and nutritional counselling, and care coordination. Although cardiac function evaluations and eye exams increased the detection of risk factors/complications, these were not considered as grouping variables in this pilot study design. The number of CMRFs for T2D, HTN, and obesity, as well as the time of exposure to LDH interventions were recorded. All patients included in the analysis were evaluated at an LDH site. To account for variability in time of exposure to the intervention, study patients were classified as either (a) “established patients”, namely those receiving care at LDH between 1 January 2008, and 15 November 2017, or (b) “new patients”, describing attending the center only between 15 November 2017 and 31 March 2018 (end of Q1). To account for any selection bias within the analyzed sample, sociodemographic proportions, insurance coverage, and disease prevalence were compared between included patients (n = 598) and those excluded from the analysis (n = 515). The results show no significant differences among ethnocultural classifiers, gender, or age between the groups. Additionally, all disease prevalence (except for obesity, 67.0% in included vs. 48.1% in total LDH populations, p ≤ 0.001) as well as the proportion of established patients was also similar.
Per LDH’s outcome-oriented protocol (Figure 2), a patient began MDT when ≥1 cardiometabolic conditions (overweight/obesity (OW/OB), prediabetes/T2D, HTN, or A1C > 5.6 (38 mmol/mol)) were detected. The present analysis compared the group of patients co-managed by MDT to a group of patients that should have started MDT but were not and continued only with standard PCP care. Providers in both groups had full access to UpToDate as a protocol to guide their approach. Once MDT co-management was initiated, blood samples were collected following a ≥8 h overnight fast and 75 g oral glucose tolerance test with serum glucose and insulin samples at 0, 30, 60, 90, and 120 min drawn [12]. Patients underwent treatment based on a previously established protocolized drug curriculum used at LDH, arrived at based on not only individual clinical conditions but also with a large experience with insurance coverage specifications for the LDH patient population. Specifically, those with OW/OB were treated with topiramate (25 mg po BID) and a low dose of phentermine (18.5 mg po QD), unless contraindicated or otherwise not tolerated. Those with insulin resistance were treated with metformin (1000 mg/day initial dose and then up titrated to 2000 mg/day). Patients with T2D were treated with anti-diabetic drugs clinically proven to promote weight loss, primarily metformin and either incretin-based therapies, such as glucagon-like peptide 1-receptor agonists and dipeptidyl-peptidase 4 inhibitors, or sodium-glucose cotransporter-2 inhibitors. LDH protocols are influenced by reimbursement experiences. For example, Qsymia® (Phentermine/Topiramate ER) is not covered by the insurances that serve our population. An alternative was to prescribe them separately; phentermine is paid for by the patient at a low cost and topiramate is covered by insurers. Lifestyle recommendations were provided for nutrition and wellness, with educational visits and nutritional counseling based on caloric/carbohydrate restriction and accompanying physical activity recommendations. In the MDT group, most visits were coordinated, implemented, and performed along with regularly scheduled medical and lifestyle counseling visits. Patients’ individual needs were considered in the implementation of follow-up and engagement protocols in both groups.
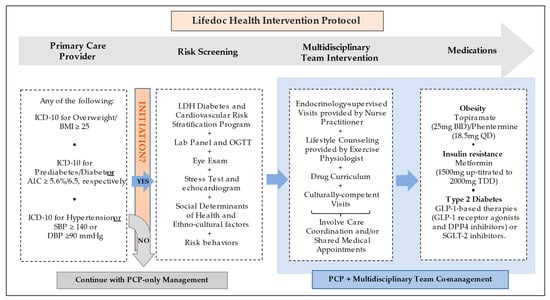
Figure 2.
Lifedoc Health intervention protocol. Abbreviations: A1C: hemoglobin A1c, BID: twice a day, BMI: body mass index, DBP: diastolic blood pressure, DPP-4: Dipeptidyl dipeptidase 4, GLP-1: Glucagon-like peptide-1, ICD-10: International Classification of Diseases 10, LDH: Lifedoc Health, OGTT: Oral glucose tolerance test, PCP: Primary Care Provider, QD: Once a day, SBP: systolic blood pressure, SGLT-2: Sodium-Glucose Cotransporter-2, TDD: Total daily dose.
2.5. Statistical Analysis
Data were analyzed using R (Version 3.6.2). Continuous variables were presented as mean ± SE and baseline differences among two exposure groups were evaluated using a t-test. Frequencies were presented as percentages and 95% confidence intervals (CI) and exposure groups compared using a Z-test. Differences in sociodemographic proportions and disease prevalence between those included and excluded from the analysis were evaluated using Pearson’s chi square. Multiple linear regression analysis was used to investigate the relationship between exposure groups and changes for each outcome from Q1 to Q4 for weight, BMI, SBP, DBP, CMRFs, as well as from first to subsequent A1C measurement. Data on pharmacotherapeutic differences were not included as a part of this analysis. Other confounding variables were included in the model and based on the 10% rule [12]. To account for the effect modification, a variable was also included if a significant interaction term was observed in a model consisting of that variable and the exposure group. To explore exposure effects on clinically significant changes in cardiometabolic outcomes, a binary logistic regression analysis (reduction in at least vs. no reduction in weight (5%), BMI (1 kg/m2), SBP (2 mmHg), or A1C (0.3%)) was performed. Confounders and effect modifiers were included, following the same rule as the multiple linear regression. A p-value of <0.05 was considered statistically significant. The patients consented to the use of the identified data for research purposes.
3. Results
3.1. Baseline Subject Characteristics
In total, 598 adults (58.9% AA, 35.3% H, and 5.9% C), with a mean age 43.8 ± 14.0 years were included in the analysis. At baseline, subjects co-managed by MDT were older and heavier than those in the PCP group. The between-group distribution of gender, ethnocultural backgrounds, SBP, DBP, type of patient (established vs. new), and time exposure to interventions were similar. The mean number (±SE) of CMRFs for the total sample was 1.43 ± 1.0, which was higher in the MDT group (1.76 ± 0.93) compared to PCP group (0.72 ± 0.77, p ≤ 0.001). Total sample mean A1C was 6.8 (51 mmol/mol) ± 2.0, and higher in MDT group (7.0 ± 2.1) than the PCP group (5.8 ± 1.4, p ≤ 0.001). The total sample average number of visits was 10.5, but it was two times higher in the MDT group (mean 12.3) compared to the PCP group (mean = 6.6). In MDT, 30.9% of visits were for lifestyle intervention (Table 1).

Table 1.
Baseline demographics, health care, and cardiometabolic risk factors by type of intervention.
3.2. Changes in CMRF during the Intervention
At the end of the period, a significant improvement in CMRF was observed across the total sample with changes in mean weight (−0.8 kg, p ≤ 0.001), BMI (−0.3 kg/m2, p ≤ 0.001), SBP (−1.4 mmHg, p = 0.01), and DBP (−0.9 mmHg, p = 0.01), though changes in the number of CMRF were not significant (−0.04, p = 0.16). The positive changes were present in the MDT group: weight (−1.4 kg, p ≤ 0.001), BMI (−0.5 kg/m2, p ≤ 0.001), SBP (−1.5 mmHg, p = 0.01), and DBP (−1.1 mmHg, p = 0.01), with a reduction in the number of CMRF (−0.1, p ≤ 0.001). In contrast, only an increase in the number of CMRF (+0.1, p = 0.02) was observed in the PCP group (Table 2). A total of 61 percent of patients (n = 364) had a baseline reading for A1C in Q1; among them, 137 (37.6%) had a baseline A1C ≥ 6.5% (48 mmol/mol), and MDT co-management was initiated in 97% of these patients (n = 133 of 137). Due to the small number of patients in the PCP group with A1C ≥ 6.5%, between-group changes in A1C were not assessed. As compared with baseline A1C, the MDT group experienced sustained and significant reduction in A1C values, (ΔA1C reading #2 vs. #1 = −0.52% [95% CI −0.89], −0.15; p = 0.01; n = 132; ΔA1C reading #3 vs. #1 = −0.49% [95% CI −0.91, −0.06]; p = 0.03; n = 122). Changes between reading #4 and #1 maintained a similar trend, though the significance was lost (−0.32% [95% CI −0.80, −0.15; p = 0.18; n = 88]).

Table 2.
Changes in weight, blood pressure, and number of cardiometabolic risk factors between quarter 1 and quarter 4 by the type of intervention during the 1 y observational period.
3.3. Factors Related to Changes in Cardiometabolic Risk
The MDT intervention was associated with a higher reduction in adiposity amount (by weight and BMI) and blood pressure. Compared to the PCP group, MDT intervention on average (β, 95% CI) was associated with greater reductions in weight (−4.29 kg, 95% CI −7.62, −0.97), BMI (−1.43 kg/m2, 95% CI −2.68, −0.18), SBP (−2.18 mmHg, 95% CI −4.09, −0.26), and DBP (−1.97 mmHg, 95% CI −3.34, −0.60) (Table 3). Binary analysis shows that those co-managed by MDT had 77% higher odds for reducing 5% or more of the initial weight, 83% higher odds of reducing 1 kg/m2 of BMI, and 59% higher odds of reducing ≥2 mmHg DBP compared to the PCP group (Table 4). On average, age was associated (β, 95% CI) with greater reductions in weight (−0.09 kg, 95% CI −0.15, −0.03), BMI (−0.03 kg/m2, 95% CI −0.06, −0.01), and no reduction in SBP (+0.07 mmHg, 95% CI 0.01, 0.13), while male gender was associated with a higher increase in SBP (3.46 mmHg, 95% CI 1.61, 5.31) and 42% lower odds of reducing ≥2 mmHg than women. Hispanic patients had a higher reduction in SBP (−2.49 mmHg, 95% CI −4.09, −0.26) compared to AA patients. Following the intervention, higher baseline values of weight (only in the continuous outcome analysis), BMI, SBP, DBP, and A1C were associated with a greater reduction in each variable in both continuous and binary outcome analyses.

Table 3.
Association between demographics and health care variables and changes in cardiometabolic factors (n = 598).

Table 4.
Association between demographics and health care variables and changes in cardiometabolic factors (n = 598).
4. Discussion
One of the primary challenges in routine clinical settings is the implementation and adaptation of protocolized interventions. At LDH, patients having at least one cardiometabolic condition (OW/OB, prediabetes/T2D, elevated A1C, or HTN) should begin MDT co-management, though this did not occur in 32% of the study sample. Enabling patients to access best clinical practice in a health system depends on several factors related to the patient, providers, and health system itself.
This study further explored the hypothesis that co-management with MDT improves the CMRF profile compared to management with PCP alone. This pilot study found that MDT care, compared with PCP only, was associated with improvements in adiposity and BP measurements, as well as the number of CMRFs. In addition, MDT patients having an A1C ≥ 6.5% (48 mmol/mol) achieved a sustained and significant improvement compared to baseline readings. In this sample composed largely of ethnic minority patients with OW/OB, increased risk for DBCD, and ≥1 CMRF, the presence of dysglycemia (by A1C) was actually evaluated in only 25% of the PCP patients. This finding is very significant since it suggests that the early screening of dysglycemia may not be as high a priority in the PCP setting, especially when competing with other acute or chronic conditions at the time of the encounter. MDT co-management, on the other hand, may prove timelier and more effective in monitoring and screening the evolution of dysglycemia in at-risk patients in a cardiometabolic multimorbidity model of disease [13]. The notion that T2D can be prevented or delayed if dysglycemia is identified in DBCD stage 2 (predisease) or earlier [14] should be emphasized.
Less weight loss has been reported with lifestyle intervention in AA women (−4.5%) compared to their C and H counterparts (−8.1% and −7.1%, respectively) in the Diabetes Prevention Program [15], as well as male and female AA patients with T2D in the Look AHEAD trial [16]. In the current pilot study, no ethnocultural disparities in weight loss were detected, suggesting a similar benefit across this demographic. A higher reduction in SBP (−2.49 mmHg) was found in H compared to AA, though this was likely not mediated by weight changes. Additionally, the number of CMRFs negatively affected SBP and DBP changes and was associated with an increase of 1.66 and 1.04 mmHg, respectively, though the clinical significance of these findings was not supported by the binary model.
Previous studies have showed the efficacy of an MDT approach. A systematic review and metanalysis including 16 studies compared multidisciplinary collaborative care (defined as care provision by ≥two different care providers) vs. usual care (defined as standard care provided solely by physicians) for patients with uncontrolled diabetes and showed that multidisciplinary collaborative care significantly improved the A1C (mean differences (MD) –0.55%; 95% CI –0.65%, –0.45%; p < 0.001) and SBP (MD –4.89 mmHg 95% CI –6.64, –3.13 mm Hg; p < 0.001) over 3–12 months [17]. In addition, the perspective of patients and providers on T2D management was reviewed. Although the MDT model improves diabetes treatment outcomes and contributes to complication reduction, trust and synergistic teamwork are important factors in promoting effective care. Patients commended the MDT for improved accessibility. However, a lack of human resources can constrain MDT efficiency, limiting interactions among health care providers, which hampers quality patient care [18].
At the time of this one-year analysis, there was no between-group difference in mean length-of-care, with a total sample average of 61.8 months (Table 1). Those co-managed with MDT averaged twice as many visits in the same period compared to PCP-only patients (12.3 vs. 6.6 visits/y, p ≤ 0.001). The findings of this paper support the sustained benefit of a higher number of total visits integrating regular clinical care, care coordination, and lifestyle counseling, which characterize the LDH model. This could explain lower patient attrition and higher levels of engagement, as it presents greater opportunity to reinforce lifestyle education and promote behavioral changes. While benefits of an MDT approach on various CMBCD risks have been reported in randomized, controlled trials, a specialist-based model for patients with severe obesity has been proposed (the “Weight Assessment and Management Clinic”) [19]. In another study, a high-intensity, lifestyle-based treatment program for obesity delivered in an underserved primary care population produced clinically significant weight loss at 2 years [20]. On the other hand, a review of treatment of obesity in primary care practices in the U.S. did not support the exclusive use of low-to-moderate intensity PCP counseling to achieve significant weight loss, though this can be achieved when used in combination with either pharmacotherapy or intensive counseling and meal replacements from a dietician or nurse [21].
The implementation of MDT interventions in clinical settings imposes certain challenges. In this study, MDT provided the opportunity for more interaction with providers, intensive pharmacological management, CMRF screening tools, and lifestyle counseling. At LDH, patients in the MDT group were co-managed by PCPs and most visits were scheduled simultaneously as shared medical appointments where possible to maximize the patient’s convenience. Shared medical appointments involved several care team members, including personnel trained in delivering patient education (lifestyle medicine provider), facilitating patient interaction (medical assistant), and a prescribing provider (endocrinologist or endocrinologist-directed nurse practitioner), initiating and sustaining a comprehensive care plan [22]. Shared medical appointments have been shown to improve A1C and SBP in patients with T2D [23], though there is less evidence of its efficacy in other chronic conditions [24].
The detection and management of CMBCD in a real-world clinical setting is complex as involves multiple drivers, morbidities, and genetic/environmental interactions [13,25]. Protocol initiation promotes the patient’s access to the best healthcare and is subject to both patient-imposed and provider-imposed barriers. In this study, some factors may have limited a patient’s initiation to MDT, such as ethnocultural disparities in weight perception, as well as other social determinants of health, including a lack of transportation or insurance, lower-income, low health literacy, or work-related constraints for appointments, each of which contributes to the proportion of no-shows. Understandably, both time constraints and multi-morbidity can impose barriers to better outcomes in PCP-driven interventions. This ultimately causes PCPs frustration in treating obesity, since limited time for simple lifestyle prescription does not lead to sustainable weight loss or improved management of comorbidities for the majority. Payer impact on patient outcomes should also be recognized, as their preferred fee-for-service reimbursement model contributes to the implementation of a volume-driven practices with more restricted visit durations, making it extremely difficult to cover all aspects of a patient with multiple competing chronic conditions. In this study, patients undergoing MDT co-management were older and heavier, suggesting that weight stigma could also contribute to a lack of provider’s initiation of younger and overweight patients. Clinical inertia—failure of providers to initiate or intensify treatment when indicated—and diagnosis inertia—unawareness/failure to diagnose a condition when present [26]—can also foster lack of protocol initiation. However, contrary to the general idea that providers are chiefly responsible for inertia, all participants in the care delivery experience, including patients, pharmacists, nurses, medical assistants, health authorities, payers, and policy makers, should be considered as playing a role [27]. Rather than an accusatory outlook towards reduced MDT initiation or provider inertia, a clear understanding of its multiple causes and determinants should be captured, and the development of specific integrated strategies in clinical practice should be promoted to overcome these barriers.
There are some limitations of this study. This is a retrospective study and, as such, is liable to residual confounders. Thus, causal inferences cannot be drawn. Since these data were drawn from routine clinical practice, not all were obtained at the same intervals, for which the averages of measurements from Q1 and Q4 were calculated. Similarly, laboratory tests were not available for all participants in Q1 and Q4, eliminating the possibility of analyzing changes in blood glucose and lipid profiles. Only the A1C readings of those patients with baseline measurements ≥ 6.5% were included in T2D range, as those tests are payer-reimbursable. Patients referred to MDT care by PCPs were older and heavier, which may suggest a referral bias, though MDTs remained more effective even after adjusting by baseline age and BMI. This study has the strength of accurately elucidating what occurs in daily clinical practice, including the challenges in analyzing outcomes, particularly in low-income ethnic minority populations, which are otherwise underrepresented in reference studies. The proportion of patients with obesity was higher among those included in the analysis. Considering that its primary outcome is the change in weight and BMI, this is likely associated with a higher level of participation and greater availability of data among obese patients. Therefore, the findings of this pilot study should be suitable to design a larger, more definitive clinical trial.
5. Conclusions
There is a need to simplify the translation of evidence-based interventions into daily clinical practice and to ease healthcare accessibility. LDH’s protocolized, integrated, outcome-based clinical MDT model may positively impact the progression of ABCD, DBCD, HTN, and CMBCD, to ultimately reduce the burden of CVD in a low-income, ethnic minority cohort of adults, compared to a PCP-only approach. The results from this analysis can be easily applied to optimize clinical practice, more positively improve health outcomes, and ultimately reduce healthcare system inertia and costs of care in real-world settings. Education and research are critical tools for comprehensive lifestyle medicine programs to optimize sustainability and performance. The significant improvement in the outcomes of the MDT group suggests that other associated factors should be further investigated and identified in large well-designed clinical trials to promote effective strategies during the current epidemic progression of cardiometabolic conditions.
Author Contributions
R.N.-M. contributed to develop the analysis plan, wrote the initial draft, and contributed to discussion and review. P.A.V.-M. conceived the paper, contributed to analysis plan, designed the clinical MDT protocol, coordinated its implementation and interventions, directed the data collection, and contributed to the drafting, discussion, and review. C.N. contributed to the design of the clinical MDT protocol, coordinated its implementation and interventions, and contributed to discussion and review. P.V.-R. directed the implementation of care coordination and patient engagement protocols and participated in data collection and review. A.N. implemented lifestyle counselling. X.M. contributed to the design of the analysis plan and performed the statistical analysis. M.L. contributed to the discussion and review. A.V.-R. managed data aggregation/quality, contributed to the drafting, analytical design, discussion, and review. G.G. coordinated the implementation of the protocols and contributed to data collection and formatting. J.I.M. contributed to manuscript review and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was self-funded by Lifedoc Health.
Institutional Review Board Statement
Based on federal regulation 45 CFR 46, no IEC/IRB review was sought as this does not meet the definition of human subject research as defined in 45 CFR 46.102. Specifically, this study was designed as a retrospective chart review study using historical datasets of proprietary, de-identified clinical data that have been previously collected.
Informed Consent Statement
Lifedoc Health was authorized by its patients for the use of their de-identified data for research and analysis.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, (P.A.V.-M.), upon reasonable request.
Acknowledgments
The authors are grateful to the team members.
Conflicts of Interest
J.I.M. reports receiving honoraria from Abbott Nutrition for lectures and is on the Advisory Board of Twin Health.
References
- Krause, J.; Van Lieshout, J.; Klomp, R.; Huntink, E.; Aakhus, E.; Flottorp, S.; Jaeger, C.; Steinhaeuser, J.; Godycki-Cwirko, M.; Kowalczyk, A.; et al. Identifying determinants of care for tailoring implementation in chronic diseases: An evaluation of different methods. Implement. Sci. 2014, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Morrell, M.; Rychetnik, L.; Morrell, S.L.; Espinel, P.T.; Bauman, A. Reduction of diabetes risk in routine clinical practice: Are physical activity and nutrition inter-ventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health 2010, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Mechanick, J.I.; Brett, E.M.; Garber, A.J.; Hurley, D.L.; Jastreboff, A.M.; Nadolsky, K.; Pessah-Pollack, R.; Plodkowski, R.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and american college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 2016, 22 (Suppl. S3), 1–203. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Farkouh, M.E.; Newman, J.D.; Garvey, W.T. Cardiometabolic-Based Chronic Disease, Adiposity and Dysglycemia Drivers: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Farkouh, M.E.; Newman, J.D.; Garvey, W.T. Cardiometabolic-Based Chronic Disease, Addressing Knowledge and Clinical Practice Gaps: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 539–555. [Google Scholar] [CrossRef]
- Kuchler, F.; Variyam, J.N. Mistakes were made: Misperception as a barrier to reducing overweight. Int. J. Obes. 2003, 27, 856–861. [Google Scholar] [CrossRef]
- Rubino, F.; Puhl, R.M.; Cummings, D.E.; Eckel, R.H.; Ryan, D.H.; Mechanick, J.I.; Nadglowski, J.; Ramos Salas, X.; Schauer, P.R.; Twenefour, D.; et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020, 26, 485–497. [Google Scholar] [CrossRef]
- LabCorp. Directory of Services and Interpretive Guide 2016–2019; Laboratory Corporation of America® Holdings and Wolters Kluver Clinical Drug Information, Inc., Burlington, NC, USA. Available online: https://www.labcorp.com/test-menu/search (accessed on 17 August 2022).
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S.
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Budtz–Jørgensen, E.; Keiding, N.; Grandjean, P.; Weihe, P. Confounder Selection in Environmental Epidemiology: Assessment of Health Effects of Prenatal Mercury Exposure. Ann. Epidemiol. 2007, 17, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Martínez, R.; González-Rivas, J.P.; Mechanick, J.I. Cardiometabolic risk: New chronic care models. J. Parenter. Enter. Nutr. 2021, 45, S85–S92. [Google Scholar] [CrossRef] [PubMed]
- López-Jaramillo, P.; Nieto-Martínez, R.E.; Aure-Fariñez, G.; Mendivil, C.O.; Lahsen, R.A.; Silva-Filho, R.L.; Andreotti, L.A.; Manrique, M.E.; Pasquel-Andrade, M.A.; Rangel, I.; et al. Identification and management of prediabetes: Results of the Latin America Strategic Prediabetes Meeting. Rev. Panam. Salud Publica 2017, 41, e172. [Google Scholar] [CrossRef] [PubMed]
- West, D.S.; Prewitt, T.E.; Bursac, Z.; Felix, H.C. Weight Loss of Black, White, and Hispanic Men and Women in the Diabetes Prevention Program. Obesity 2008, 16, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- West, D.S.; Dutton, G.; Delahanty, L.M.; Hazuda, H.P.; Rickman, A.D.; Knowler, W.C.; Vitolins, M.Z.; Neiberg, R.H.; Peters, A.; Gee, M.; et al. Weight Loss Experiences of African American, Hispanic, and Non-Hispanic White Men and Women with Type 2 Diabetes: The Look AHEAD Trial. Obesity 2019, 27, 1275–1284. [Google Scholar] [CrossRef]
- Siaw, M.Y.L.; Lee, J.Y.-C. Multidisciplinary collaborative care in the management of patients with uncontrolled diabetes: A systematic review and meta-analysis. Int. J. Clin. Pract. 2018, 73, e13288. [Google Scholar] [CrossRef]
- Tan HQ, M.; Chin, Y.H.; Ng, C.H.; Liow, Y.; Devi, M.K.; Khoo, C.M.; Goh, L.H. Multidisciplinary team approach to diabetes. An outlook on providers’ and patients’ perspectives. Prim. Care Diabetes 2020, 14, 545–551. [Google Scholar] [CrossRef]
- Welbourn, R.; on behalf of the Guidance Development Group; Dixon, J.; Barth, J.H.; Finer, N.; Hughes, C.A.; le Roux, C.W.; Wass, J. NICE-Accredited Commissioning Guidance for Weight Assessment and Management Clinics: A Model for a Specialist Multidisciplinary Team Approach for People with Severe Obesity. Obes. Surg. 2016, 26, 649–659. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Martin, C.K.; Newton, R.L.; Apolzan, J.W.; Arnold, C.L.; Davis, T.C.; Price-Haywood, E.G.; Denstel, K.D.; Mire, E.F.; Thethi, T.K.; et al. Weight Loss in Underserved Patients—A Cluster-Randomized Trial. N. Engl. J. Med. 2020, 383, 909–918. [Google Scholar] [CrossRef]
- Tsai, A.G.; Wadden, T.A. Treatment of Obesity in Primary Care Practice in the United States: A Systematic Review. J. Gen. Intern. Med. 2009, 24, 1073–1079. [Google Scholar] [CrossRef]
- Noffsinger, E.B. Operational challenges to implementing a successful physicals shared Medical Appointment Program. Part 1: Choosing the right type of shared medical appointment. Group Pract. J. 2002, 51, 24–34. [Google Scholar]
- Edelman, D.; Gierisch, J.; McDuffie, J.R.; Oddone, E.; Williams, J.W. Shared Medical Appointments for Patients with Diabetes Mellitus: A Systematic Review. J. Gen. Intern. Med. 2014, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Edelman, D.; McDuffie, J.R.; Oddone, E.; Gierisch, J.M.; Nagi, A.; Williams, J.W., Jr. VA Evidence-Based Synthesis Program Reports, in Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review; Department of Veterans Affairs (US): Washington, DC, USA, 2012.
- Nieto-Martinez, R.; Gonzalez-Rivas, J.; Mechanick, J. Cardiometabolic-Based Chronic Disease (CMBCD): A New Framework for Early and Sustainable Preventive Care. RSSDI Diabetes Update 2020; Jaypee Brothers Medical Publishers: Delhi, India, 2020. [Google Scholar]
- Phillips, L.S.; Branch Jr, W.T.; Cook, C.B.; Doyle, J.P.; El-Kebbi, I.M.; Gallina, D.L.; Miller, C.D.; Ziemer, D.C.; Barnes, C.S. Clinical inertia. Ann. Intern. Med. 2001, 135, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Pallarés-Carratalá, V.; Bonig-Trigueros, I.; Palazón-Bru, A.; Esteban-Giner, M.J.; Gil-Guillén, V.F.; Giner-Galvañ, V. Clinical inertia in hypertension: A new holistic and practical concept within the cardiovascular continuum and clinical care process. Blood Press. 2019, 28, 217–228. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).