1. Introduction
Given the growing importance of exposome factors, including nutrition, in disease prevention and treatment, there is an increasing demand to explore its relationship with the most common skin conditions such as acne vulgaris and rosacea [
1,
2]. These facial dermatoses continue to gain attention as patients seek guidance from dermatologists on optimal nutrition to complement their treatment regimens [
3]. However, assessing the impact of diet on disease severity remains challenging due to the subjective nature of self-reported data, insufficient contemporary data on patients’ dietary habits, and the lack of standardized disease-specific nutritional protocols. Large observational controlled studies are therefore necessary to report associations between certain foods and disease risk, allowing valuable insights for preventive and therapeutic strategies to complement prescription medication [
4].
While the relationship between diet and inflammatory facial skin conditions is not fully understood, recent studies have demonstrated that certain dietary factors may influence their development and severity [
5]. For acne, consumption of highly processed carbohydrates, milk, and dairy products have been identified as dietary triggers [
6]. Their intake should be limited because of their role in increasing insulin, a hormone produced by the pancreas that is important for regulating blood glucose levels, and insulin-like growth factor (IGF)-1, a growth factor produced primarily by the liver and involved in multiple anabolic pathways. Fluctuations in insulin and IGF-1 levels can affect seborrhea, one primary cause of acne [
7,
8]. In addition, these fluctuations can stimulate the release of pro-inflammatory molecules, which are known to contribute to the development of acne and rosacea [
9]. While studies have reported a positive correlation between plasma IGF-1 levels and the clinical severity of acne [
10], insulin resistance has recently been reported in rosacea patients [
9,
11]. Overall, the understanding of the impact of diet on rosacea remains limited compared to acne and despite ongoing research efforts. Capsaicin-, heat-, or alcohol-related nutritional triggers may be intertwined with the multifactorial pathophysiology of rosacea, including dysbiosis of the skin microbiome, vascular malformation, and immune dysregulation, yet their impact varies greatly between individuals [
12,
13]. Overall, comprehensive dietary recommendations for rosacea are still deficient [
14,
15].
The present study aims to provide a comprehensive analysis of the dietary habits of patients with acne and rosacea. Its objective is to establish more precise dietary recommendations specifically tailored for each facial dermatosis and propose standardized dietary scores that clinicians can utilize to evaluate the risk for acne and rosacea attributable to patients’ dietary habits. By adopting this systematic approach, personalized treatment strategies can be devised, ultimately resulting in enhanced patient outcomes.
4. Discussion
This cross-sectional, controlled study involved a large cohort of acne and rosacea patients to provide valuable insights for healthcare professionals and patients into the relationship between specific foods and the presence and severity of these conditions. The study used a three-step approach to dietary analysis. First, a subjective assessment was conducted in which patients reported the effect of various foods on the clinical severity of their disease. Second, patients’ dietary intake was assessed using a standardized FFS and compared to a control group. Finally, the FFS reports were translated into clinical scores that served as tools to predict the risk for acne and rosacea based on dietary habits.
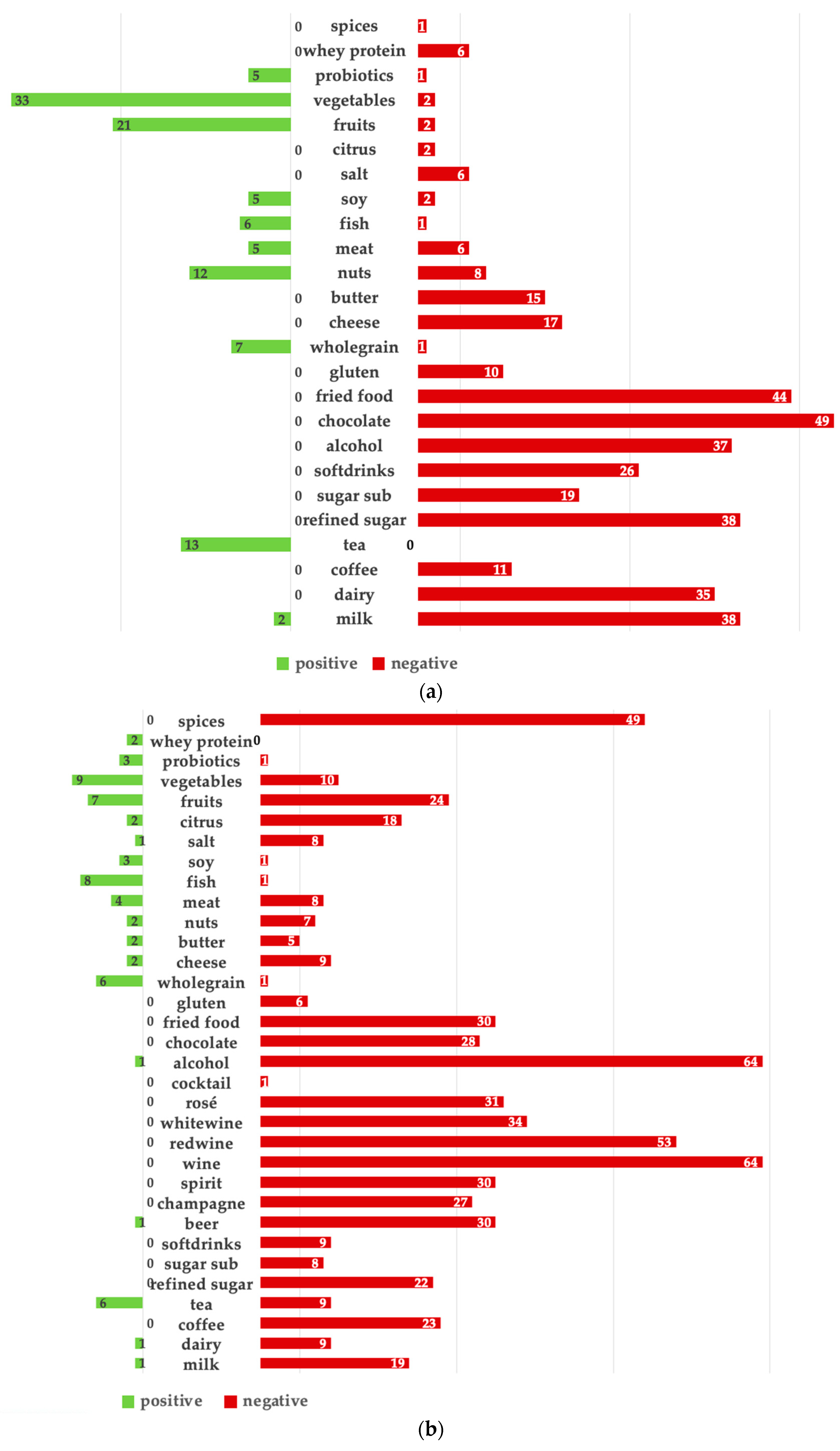
The subjective assessment revealed that most acne and rosacea patients recognized an impact of diet on their skin condition, emphasizing the importance of addressing nutrition in any treatment plan [
29,
30]. Overall, triggers were more clearly identified than beneficial foods, as evidenced by the number of responses. Reported acne triggers were consistent with existing findings in the literature, suggesting that the overall consumption of chocolate, fried foods, refined sugar, milk, alcohol, and dairy products should be individually limited [
8,
31,
32]. Alcohol, specifically wine, was the leading self-reported rosacea trigger, reinforcing previous data showing that alcohol consumption is a known risk factor for rosacea [
33]. Interestingly, several seemingly unrelated foods, including fruits, spices, chocolate, and citrus fruits, were also reported as rosacea triggers. A possible pathogenic mechanism underlying the exacerbation potential of these foods may be explained by the presence of cinnamaldehyde, a compound that triggers transient receptor potential (TRP) ion channels found on sensory nerves and keratinocytes. The activation of these channels causes the release of substance P, resulting in an inflammatory response and dilation of arterioles in rosacea skin [
12,
34]. Coffee was also a self-reported rosacea trigger. However, recent studies have shown that coffee drinkers actually have a lower likelihood of developing rosacea, possibly due to the vasoconstrictive effects of caffeine [
35,
36,
37]. It should be considered that the triggering effect in the present cohort may have been influenced by the vasodilatory effects of hot beverages in general [
38].
The food items reported to be beneficial for both the AG and RG can be classified as a Mediterranean diet (MD), characterized by a high consumption of vegetables, legumes, oily fish, olive oil, nuts, and only moderate intake of meat, cheese, and alcohol [
39,
40,
41]. While the MD is generally known to be beneficial for health and conditions such as diabetes, cancer, and cardiovascular disease [
42,
43], the data from this study suggest additional advantageous effects on facial inflammatory dermatoses. This supports recent findings of a negative correlation between acne and the adherence to a MD [
44], as well as a reduced incidence of rosacea with adherence to a MD [
39]. Thus, adhering to a MD appears to be beneficial for both acne and rosacea. Omega-3 fatty acids, available in oily fish, algae, nuts, and seeds, along with probiotics found in fermented vegetables, are currently being studied as potential food items to reduce inflammatory skin conditions. Several mechanisms have been proposed to elucidate their potentials, with acne exhibiting more data than rosacea [
45,
46,
47]. Omega-3 fatty may modulate sebum production, reduce inflammatory cytokines, inhibit Cutibacterium acnes growth, enhance skin barrier function, and provide antioxidant properties [
5,
45,
48,
49]. Oral probiotics could restore an imbalanced gut microbiome, leading to favorable effects on distant sites, including the epidermal barrier function of the skin [
50].
Interestingly, the analysis of patients’ actual dietary intake based on significant results in the FFS contradicted the self-reported beneficial and aggravating foods. For example, although perceived as beneficial, acne patients consumed significantly fewer fruits and vegetables than the control group. Remarkably, acne patients reported a lower consumption of milk compared to the ACG, suggesting that they were aware of milk and dairy products as dietary acne triggers and had adjusted their eating habits accordingly. Milk and dairy products can increase insulin and IGF-1 levels and activate the nutrient-sensitive kinase mammalian target of rapamycin complex-1 (mTORC1) [
51], which promotes anabolic pathways associated with increased seborrhea and follicular hyperkeratosis, both involved in acne development [
52,
53]. Laboratory analysis revealed associations between diet and these biomarkers, particularly in the AG. Significantly elevated IGF-1 levels were found in the AG compared to the ACG and RG. More frequent dairy intake in acne patients was associated with elevated IGF-1 levels compared to patients with normal IGF-1 levels. These findings suggest that diet may directly influence biological factors involved in the pathogenesis of these diseases. Future studies should investigate whether IGF-1 levels could be used as a screening tool to assess dairy intake in acne patients.
Patients with rosacea consumed significantly more animal products compared to controls, despite being self-reported as dietary triggers, and fewer legumes, despite being perceived as beneficial. Red and processed meat intake may have contributed to increased LDL, total cholesterol, and triglycerides, as seen in the laboratory analysis of the RG compared to the RCG and AG [
54]. Fried foods, processed meats such as ham and burgers, and aged cheeses are high in histamine, with isolated studies suggesting possible effects on rosacea skin [
12]. However, future research is needed to evaluate possible associations between meat intake and rosacea, as data are currently lacking. Similarly, the effects of soy on both acne and rosacea have not yet been investigated, with limited intake in the present acne and rosacea cohorts compared to individual control groups.
Dietary scores were derived from patient FFS responses. As seen in the subjective analysis of dietary beneficial and triggering food items, acne patients’ responses were more consistent, resulting in a more accurate score and higher p value compared to rosacea. The acne score included 13 items and resulted in a calculated odds ratio of 14.5, while the rosacea score included 7 items with a calculated odds ratio of 5.5. According to the ANS, daily intake of water, fresh fruit, and coffee; weekly intake of vegetables, unsweetened tea, bread, cheese, and pasta; monthly intake of oats, dairy products, cooked fruit, wine; and limited intake of ham were significantly associated with a decreased risk of acne. While these food items not only reflect a MD, further supporting that this dietary style may reduce the risk of acne, they also include the most frequent self-reported beneficial acne food items from the subjective assessment.
According to the RNS, regular coffee and nut consumption of more than three times per week was associated with a decreased risk of rosacea. Interestingly, the proposed score supports the suggestion in the previous literature that coffee does not increase the risk of rosacea and highlights possible benefits of nuts [
36]. As nuts, such as walnuts, are a valuable source of omega-3 fatty acids with anti-inflammatory properties, they may play a protective role, although further studies are needed to investigate the exact association with rosacea. Strictly limiting the intake of animal products, including processed meats and fried foods, has been shown to be associated with a reduced risk of rosacea. These findings have not been reported in the literature and, interestingly, are partially consistent with the subjective trigger assessment of the present cohort. Further validation is needed by evaluating the results in larger patient populations.
The use of dietary supplements was reported more frequently in the RG than in the AG. Although patients may take oral supplements with the intention of improving their health, there are currently no clinical recommendations suggesting that supplementation may be beneficial for acne and rosacea in the absence of existing deficiencies. As studies have even reported cases of acne and rosacea triggered by oral supplementation, including B 12 vitamin, clinicians should always critically evaluate their use alongside patients’ daily medications [
55,
56].
The impact of acne on patients’ quality of life was found to be more pronounced compared to rosacea, as indicated by the mean DLQI scores. However, no significant difference in the EQ-5D-EL scores was observed between the AG and RG. Both the AG and RG reported a significant decrease in quality of life and overall health compared to their respective control groups, and stress was a trigger for both acne and rosacea, consistent with the recent literature [
57].
To the best of our knowledge, the present study proposes a new way to approach the challenging topic of diet and its impact on disease risk. By proposing the first disease-specific risk scores for acne and rosacea attributable to patients’ dietary habits, the aim was to help clinicians to concretize clinical recommendations and provide clearer information for patients. Although further interventional studies are needed, assessing patients’ dietary habits in the presented manner may empower them to make informed lifestyle choices and promote long-term adherence to positive dietary changes.
It is important to note that this study has several limitations. Because dietary habits vary widely between cultures and countries, the results of the present German cohort may not be generalizable to a worldwide population. In addition, self-reported dietary habits always carry the risk of recall bias, and the cross-sectional design has shortcomings compared to placebo-controlled, interventional, randomized trials. Further research with larger sample sizes is warranted to validate and extend these findings.