Ketone Bodies in Diabetes Mellitus: Friend or Foe?
Abstract
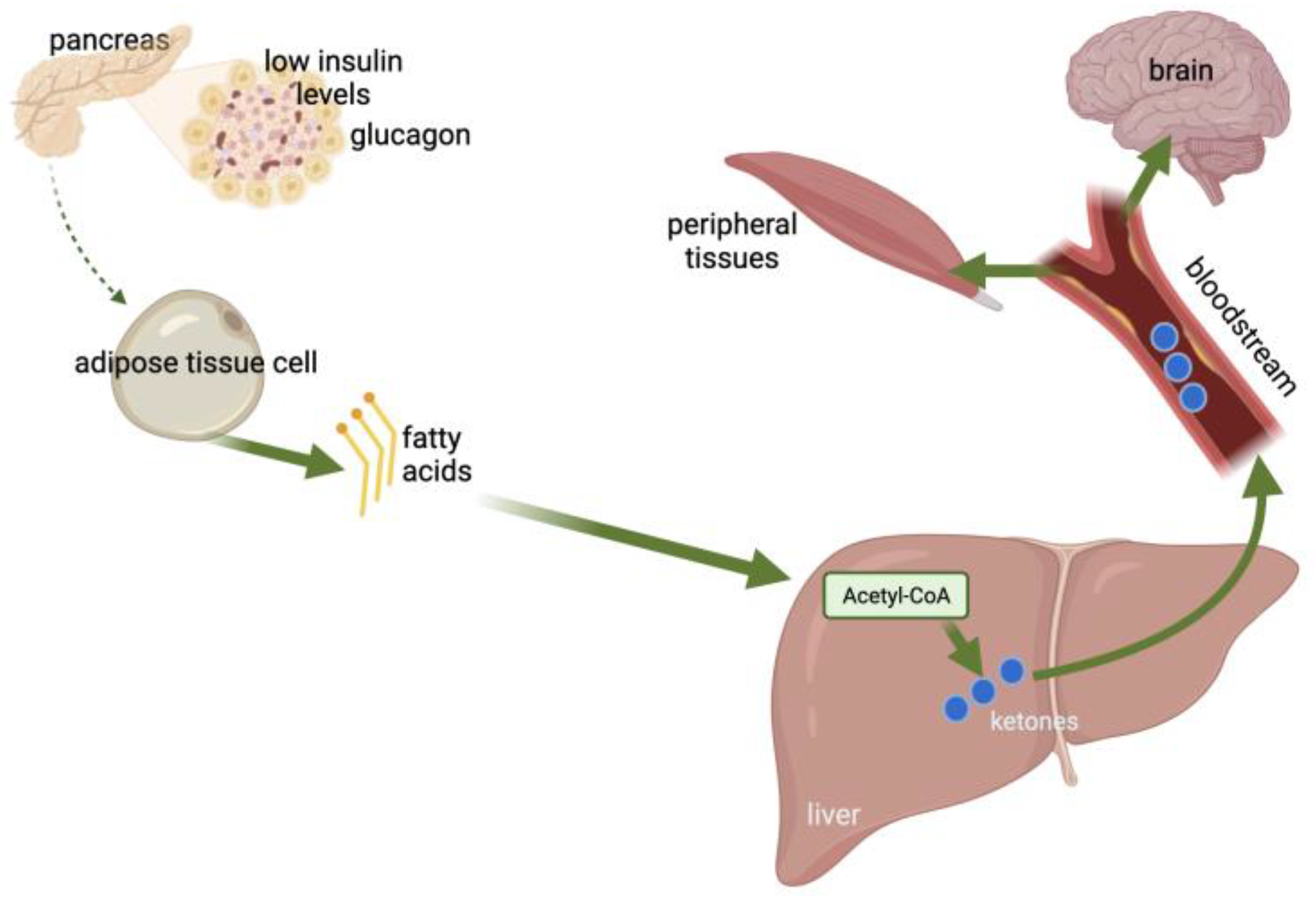
:1. Introduction
2. Ketones
2.1. DKA-Induced Effects
2.2. Elevated Ketone Concentrations during Pregnancy
2.3. Mild Ketonemia
3. Ketogenic Diets (KDs)
3.1. Types of KDs
3.2. Advantages of the KD
3.3. Disadvantages of the KD
4. KDs for DM
4.1. The KD for T1DM
4.2. The KD for T2DM
| First Author | Included Studies | Results |
|---|---|---|
| Choi [47] | 14 RCTs | The effects of KD on glycemic control were greater relative to those of LFD for patients with T2DM, indicated by lower HbA1c and HOMA, while comparable effects were observed for nondiabetic patients. KDs led to substantial BW loss, irrespective of patients’ DM status at baseline and improved lipid profiles in terms of lower TG and greater HDL for patients with DM. KDs were more effective in improving metabolic parameters associated with glycemic, BW and lipid control in patients with overweight/obesity, and especially preexisting DM, as compared to LFDs. |
| Goldenberg [93] | 23 RCTs | At 6 months, LCDs achieved higher rates of diabetes remission compared with control diets. Large clinically important improvements were seen in BW loss, TG, and insulin sensitivity at 6 months, though they were diminished at 12 months. VLCDs were less effective than less restrictive LCDs for BW loss at 6 months, but this was explained by diet adherence. |
| Luo [94] | 21 RCTs | LCD exerted a greater impact on CV risk factors in overweight/obese patients with T2DM, with lower FPG and HbA1c levels. LCD reduced BMI, BW, and WC in overweight/obese patients with T2DM. Also, adherence to KDs improved lipid profiles with TG concentrations being lowered and HDL noting an upward trend. |
| Parry-Strong [95] | 8 RCTs of ≥6 months duration | A VLCD/KD may cause reductions in HbA1c and TG levels in patients with pre-diabetes/T2DM, but evidence of an advantage over other strategies remains limited. |
| Rafiullah [91] | 8 RCTs | Compared with control diets, the VLCD resulted in a greater decrease in HbA1c and BW loss after 3 and 6 months. The VLCD was not better than a control diet after 12 months. It was superior in decreasing TG, increasing HDL and reducing the use of antidiabetic drugs for up to 12 months. |
| Snorgaard [75] | 10 RCTs | In the first year of intervention, LCD was followed reduced HbA1c more compared with HCD. The greater the CHO restriction, the greater the glucose-lowering effect. At 1 year or later however, HbA1c was similar between the 2 diet arms. The effect of the 2 diets on BMI/BW, LDL, QoL, and attrition rate was similar throughout interventions. |
| Tinguely [89] | 14 CTs | KD improves HbA1c at 3 weeks, and the effect persists for at least a year, a result associated with a reduction in glucose-lowering medications. Additionally, the short-term observed BW loss is maintained with a long-term diet. Adequate support (psychological counseling, enhancing positive affectivity, reinforcing mindful eating) is required to achieve benefits and ensure adherence. |
| Yuan [90] | 13 RCTs | Post-KD, the levels of fasting glucose, HbA1c, total cholesterol, LDL and TG decreased, but HDL increased. In addition, BW, WC, and BMI also decreased. |
| Zaki [92] | 15 RCTs and observational studies | An LCD induced a greater reduction in the HbA1c than other diets. A decrease in HbA1c and BW was recorded when the KD was consumed compared to the control diets. |
| Zhou [51] | 8 RCTs | The KD reduced BW, WC, HbA1c and TG, and increased HDL levels. The KD may be an effective dietary intervention for BW, glycemia and lipid profiles in overweight with T2DM. |
5. Assessing the Concentration of Ketone Bodies
5.1. Ketonemia and Ketonuria in T1DM
5.2. Ketonemia and Ketonuria in T2DM
5.3. Ketonemia and Ketonuria in Ketosis-Prone DM (KPDM)
5.4. Ketonemia and Ketonuria in GDM
5.5. Ketone Bodies and Antidiabetic Medication
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3BHB | 3-β-hydroxybutyrate |
| α-KIC | α-ketoisocaproate |
| AcAc | Acetoacetate |
| Acetyl-CoA | Acetyl-coenzyme A |
| ADA | American Diabetes Association |
| AGA | Appropriate-for-gestational age |
| BAT | Brown adipose tissue |
| BHB | β-hydroxybutyrate |
| BMI | Body mass index |
| BUN | Blood–urea–nitrogen |
| BW | Body weight |
| CAT1 | Carnitine acyltransferase 1 |
| CC | Case-control |
| CCK | Cholecystokinin |
| CDKL5 | Cyclin-dependent kinase-like 5 |
| CHO | Carbohydrate |
| CPT-1 | Carnitine palmitoyltransferase |
| CT | Clinical trial |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| DKA | Diabetic ketoacidosis |
| DM | Diabetes mellitus |
| FFAs | Free-fatty acids |
| FFM | Fat-free mass |
| FPG | Fasting plasma glucose |
| GC-MS | Gas chromatography-mass spectrometry |
| GDM | Gestational diabetes mellitus |
| GI | Glycemic index |
| GWG | Gestational weight gain |
| HbA1c | Glycosylated haemoglobin |
| HCD | High-carbohydrate diet |
| HDL | High-density lipoprotein |
| HOMA | Homeostatic model assessment index |
| HSL | Hormone-sensitive lipase |
| HS-SPME | Headspace solid-phase microextraction |
| IP | Insulin pump |
| IR | Insulin resistance |
| KD | Ketogenic diet |
| KPDM | Ketosis-prone diabetes mellitus |
| LADA | Latent autoimmune diabetes of adults |
| LCD | Low-carbohydrate diet |
| LCT | Long-chain triglycerides |
| LDL | Low-density lipoprotein |
| LED | Low-energy diet |
| LFD | low-fat diet |
| LGA | Large-for-gestational age |
| MAPK | Mitogen-activated protein kinase |
| MCT | Medium-chain triglycerides |
| MEM | Manual enzymatic method |
| MNT | Medical nutrition therapy |
| MODY | Mature-onset diabetes of the young |
| MUAC | Middle-upper arm circumference |
| N/A | Not applicable |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NICE | National Institute of Healthcare and Excellence |
| NF-κB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| NTD | Neural tube defects |
| NR | Not reported |
| OL | Open label |
| ONS | Oral nutrient supplements |
| POC | Point of care |
| PPP | pentose phosphate pathway |
| Pyr | Pyruvate |
| QoL | Quality of life |
| RCT | Randomized controlled trial |
| RQ | Respiratory quotient |
| SD | Standard diet |
| SGLT-2 | Sodium-glucose transport protein 2 |
| T1DM | Type 1 diabetes mellitus |
| T2DM | Type 2 diabetes mellitus |
| TEI | Total energy intake |
| TG | Triglycerides |
| VLCD | Very low carbohydrate diet |
| VLED | Very low-energy diet |
| WC | Waist circumference |
References
- Raghavan, S.; Vassy, J.L.; Ho, Y.; Song, R.J.; Gagnon, D.R.; Cho, K.; Wilson, P.W.F.; Phillips, L.S. Diabetes Mellitus–Related All-Cause and Cardiovascular Mortality in a National Cohort of Adults. J. Am. Heart Assoc. 2019, 8, e011295. [Google Scholar] [CrossRef]
- Safiri, S.; Karamzad, N.; Kaufman, J.S.; Bell, A.W.; Nejadghaderi, S.A.; Sullman, M.J.M.; Moradi-Lakeh, M.; Collins, G.; Kolahi, A.-A. Prevalence, Deaths and Disability-Adjusted-Life-Years (DALYs) Due to Type 2 Diabetes and Its Attributable Risk Factors in 204 Countries and Territories, 1990–2019: Results From the Global Burden of Disease Study 2019. Front. Endocrinol. 2022, 13, 838027. [Google Scholar] [CrossRef]
- Zhu, R.; Zhou, S.; Xia, L.; Bao, X. Incidence, Morbidity and years Lived With Disability due to Type 2 Diabetes Mellitus in 204 Countries and Territories: Trends From 1990 to 2019. Front. Endocrinol. 2022, 13, 905538. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Kahanovitz, L.; Sluss, P.M.; Russell, S.J. Type 1 Diabetes—A Clinical Perspective. Point Care 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Agha-Jaffar, R.; Johnston, D.G.; Robinson, S. Pathophysiology. In Comprehensive Clinical Approach to Diabetes During Pregnancy; Goulis, D.G., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 73–91. ISBN 9783030892432. [Google Scholar]
- Anastasiou, E.; Farmakidis, G.; Gerede, A.; Goulis, D.G.; Koukkou, E.; Kourtis, A.; Mamopoulos, A.; Papadimitriou, K.; Papadopoulos, V.; Stefos, T. Clinical practice guidelines on diabetes mellitus and pregnancy: ΙI. Gestational diabetes mellitus. Hormones 2020, 19, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Vounzoulaki, E.; Tan, B.K. Epidemiology. In Comprehensive Clinical Approach to Diabetes During Pregnancy; Goulis, D.G., Ed.; Springer: Cham, Switzerland, 2022; pp. 13–28. [Google Scholar]
- Vince, K.; Perković, P.; Matijević, R. What is known and what remains unresolved regarding gestational diabetes mellitus (GDM). J. Perinat. Med. 2020, 48, 757–763. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Mezhnina, V.; Ebeigbe, O.P.; Velingkaar, N.; Poe, A.; Sandlers, Y.; Kondratov, R.V. Circadian clock controls rhythms in ketogenesis by interfering with PPARα transcriptional network. Proc. Natl. Acad. Sci. USA 2022, 119, e2205755119. [Google Scholar] [CrossRef]
- George, F.C. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar]
- Chen, Q.; Du, J.; Cui, K.; Fang, W.; Zhao, Z.; Chen, Q.; Mai, K.; Ai, Q. Acetyl-CoA derived from hepatic mitochondrial fatty acid β-oxidation aggravates inflammation by enhancing p65 acetylation. iScience 2021, 24, 103244. [Google Scholar] [CrossRef]
- Wang, T.; Yao, W.; Li, J.; He, Q.; Shao, Y.; Huang, F. Role of gut microbiota, gut-brain and gut liver axes in physiological regulation of inflammation, energy balance, and metabolism: Acetyl-CoA from inflammation-induced fatty acids oxidation promotes hepatic malate-aspartate shuttle activity and glycolysis. Am. J. Physiol.—Endocrinol. Metab. 2018, 315, E496–E510. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- BioRender BioRender. Available online: https://www.biorender.com/ (accessed on 17 September 2023).
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262. [Google Scholar] [CrossRef]
- Qian, M.; Wu, N.; Li, L.; Yu, W.; Ouyang, H.; Liu, X.; He, Y.; Al-Mureish, A. Effect of Elevated Ketone Body on Maternal and Infant Outcome of Pregnant Women with Abnormal Glucose Metabolism During Pregnancy. Diabetes. Metab. Syndr. Obes. 2020, 13, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia (acetoacetate) upregulates NADPH oxidase 4 and elevates oxidative stress, ICAM-1, and monocyte adhesivity in endothelial cells. Cell. Physiol. Biochem. 2015, 35, 364–373. [Google Scholar] [CrossRef]
- Sussman, D.; van Eede, M.; Wong, M.D.; Adamson, S.L.; Henkelman, M. Effects of a ketogenic diet during pregnancy on embryonic growth in the mouse. BMC Pregnancy Childbirth 2013, 13, 109. [Google Scholar] [CrossRef]
- Horton, W.E.; Sadler, T.W. Effects of maternal diabetes on early embryogenesis. Alterations in morphogenesis produced by the ketone body, B-hydroxybutyrate. Diabetes 1983, 32, 610–616. [Google Scholar] [CrossRef]
- Horton, W.E.; Sadler, T.W. Mitochondrial alterations in embryos exposed to B-hydroxybutyrate in whole embryo culture. Anat. Rec. 1985, 213, 94–101. [Google Scholar] [CrossRef]
- Shum, L.; Sadler, T.W. Recovery by mouse embryos following teratogenic exposure to ketosis. Diabetologia 1991, 34, 289–295. [Google Scholar] [CrossRef]
- Shum, L.; Sadler, T.W. Embryonic catch-up growth after exposure to the ketone body D,L,-beta-hydroxybutyrate in vitro. Teratology 1988, 38, 369–379. [Google Scholar] [CrossRef]
- Hunter, E.S.; Sadler, T.W. D-(-)-beta-hydroxybutyrate-induced effects on mouse embryos in vitro. Teratology 1987, 36, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Håkan Borg, L.A.; Eriksson, U.F.J. Altered mitochondrial morphology of rat embryos in diabetic pregnancy. Anat. Rec. 1995, 241, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.C.P.; Stanisstreet, M.; Clarke, C.A. Morphological and physiological effects of beta-hydroxybutyrate on rat embryos grown in vitro at different stages. Teratology 1989, 40, 237–251. [Google Scholar] [CrossRef]
- Hwang, C.Y.; Choe, W.; Yoon, K.S.; Ha, J.; Kim, S.S.; Yeo, E.J.; Kang, I. Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer. Nutrients 2022, 14, 4932. [Google Scholar] [CrossRef]
- Bailey, E.E.; Pfeifer, H.H.; Thiele, E.A. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005, 6, 4–8. [Google Scholar] [CrossRef]
- Rho, J.M.; Anderson, G.D.; Donevan, S.D.; White, H.S. Acetoacetate, acetone, and dibenzylamine (a contaminant in l-(+)-beta-hydroxybutyrate) exhibit direct anticonvulsant actions in vivo. Epilepsia 2002, 43, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.W. History of the ketogenic diet. Epilepsia 2008, 49, 3–5. [Google Scholar] [CrossRef]
- Wilder, R.M. The effects of ketonemia on the course of epilepsy. Mayo Clin. Proc. 1921, 2, 307–308. [Google Scholar]
- Woodyatt, R.T. Objects and method of diet adjustment in diabetes. Arch. Intern. Med. 1921, 28, 125–141. [Google Scholar] [CrossRef]
- Martin-McGill, K.J.; Bresnahan, R.; Levy, R.G.; Cooper, P.N. Ketogenic diets for drug-resistant epilepsy. Cochrane Database Syst. Rev. 2020, 2020. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. National Institute for Health and Care Excellence Draft for Consultation Epilepsies in Children, Young People and Adults: Diagnosis and Management [12] Evidence Review: Ketogenic Diets for Drug-Resistant Epilepsy NICE Guideline <number> Evidence Review Underpinning Recommendation 8.1.1 and a Research Recommendation in the NICE Guideline DRAFT FOR CONSULTATION Contents; NICE: London, UK, 2021. [Google Scholar]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Theodoridis, X.; Gkouskou, K.K.; Evangeliou, A.; Dardiotis, E.; Bogdanos, D.P. To Keto or Not to Keto? A Systematic Review of Randomized Controlled Trials Assessing the Effects of Ketogenic Therapy on Alzheimer Disease. Adv. Nutr. 2020, 11, 1583–1602. [Google Scholar] [CrossRef]
- Pav, S.; Azaro, E.L.; Mart Inez, O.; Amayra, I.; L Opez-Paz, J.F.; Caballero, P.; Al-Rashaida, M.; Luna, P.M.; Garc, M.; Erez, M.P.; et al. Nutrition in Clinical Care Ketogenic diet and cognition in neurological diseases: A systematic review. Nutr. Rev. 2020, 79, 802–813. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, J.; Chang, X.; Wu, P.; Li, S.; Wu, Y. Efficacy of ketogenic diet in CDKL5-related epilepsy: A single arm meta-analysis. Orphanet J. Rare Dis. 2022, 17, 385. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Tousinas, G.; Balodimou, C.; Anastasilakis, D.A.; Gkiouras, K.; Dardiotis, E.; Evangeliou, A.E.; Bogdanos, D.P.; Goulis, D.G. Ketogenic therapy for Parkinson’s disease: A systematic review and synthesis without meta-analysis of animal and human trials. Maturitas 2022, 163, 46–61. [Google Scholar] [CrossRef]
- Ogawa, E.; Hishiki, T.; Hayakawa, N.; Suzuki, H.; Kosaki, K.; Suematsu, M.; Takenouchi, T. Ketogenic diet in action: Metabolic profiling of pyruvate dehydrogenase deficiency. Mol. Genet. Metab. Reports 2023, 35, 100968. [Google Scholar] [CrossRef] [PubMed]
- Armeno, M.; Caraballo, R.; Vaccarezza, M.; Alberti, M.J.; Ríos, V.; Galicchio, S.; de Grandis, E.S.; Mestre, G.; Escobal, N.; Matarrese, P.; et al. National consensus on the ketogenic diet. Rev. Neurol. 2014, 59, 213–223. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Wilbourn, A.J.; Signore, J.M. Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 1971, 21, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International Tables of Glycemic Index and Glycemic Load Values: 2008. Diabetes Care 2008, 31, 2281. [Google Scholar] [CrossRef]
- Pfeifer, H.H.; Thiele, E.A. Low-glycemic-index treatment: A liberalized ketogenic diet for treatment of intractable epilepsy. Neurology 2005, 65, 1810–1812. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Jeon, S.M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Patikorn, C.; Saidoung, P.; Pham, T.; Phisalprapa, P.; Lee, Y.Y.; Varady, K.A.; Veettil, S.K.; Chaiyakunapruk, N. Effects of ketogenic diet on health outcomes: An umbrella review of meta-analyses of randomized clinical trials. BMC Med. 2023, 21, 196. [Google Scholar] [CrossRef]
- Malhotra, V.; Sawal, A.; Malhotra, V.; Sawal, A. Metabolic Effects of Ketogenic Diets and Their Utilization in Obesity Management: A Systematic Review. Cureus 2023, 15, e36720. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Gkouskou, K.K.; Gkiouras, K.; Bogdanos, D.P.; Eliopoulos, A.G.; Goulis, D.G. The Niche of n-of-1 Trials in Precision Medicine for Weight Loss and Obesity Treatment: Back to the Future. Curr. Nutr. Rep. 2022, 11, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 792846. [Google Scholar] [CrossRef]
- Srivastava, S.; Baxa, U.; Niu, G.; Chen, X.; Veech, R.L. A Ketogenic Diet Increases Brown Adipose Tissue Mitochondrial Proteins and UCP1 Levels in Mice. IUBMB Life 2013, 65, 58–66. [Google Scholar] [CrossRef]
- Veech, R.L. Ketone esters increase brown fat in mice and overcome insulin resistance in other tissues in the rat. Ann. N. Y. Acad. Sci. 2013, 1302, 42–48. [Google Scholar] [CrossRef]
- Barberá, M.J.; Schlüter, A.; Pedraza, N.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome proliferator-activated receptor alpha activates transcription of the brown fat uncoupling protein-1 gene. A link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J. Biol. Chem. 2001, 276, 1486–1493. [Google Scholar] [CrossRef]
- Mercer, S.W.; Trayhurn, P. Effect of high fat diets on the thermogenic activity of brown adipose tissue in cold-acclimated mice. J. Nutr. 1984, 114, 1151–1158. [Google Scholar] [CrossRef]
- Roekenes, J.; Martins, C. Ketogenic diets and appetite regulation. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.A.; Seimon, R.V.; Lee, C.M.; Ayre, J.; Franklin, J.; Markovic, T.P.; Caterson, I.D.; Sainsbury, A. Do ketogenic diets really suppress appetite? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Bosco, G.; Camporesi, E.M.; Mangar, D. Ketosis, ketogenic diet and food intake control: A complex relationship. Front. Psychol. 2015, 6, 104339. [Google Scholar] [CrossRef]
- O’Hearn, L.A. The therapeutic properties of ketogenic diets, slow-wave sleep, and circadian synchrony. Curr. Opin. Endocrinol. Diabetes. Obes. 2021, 28, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef]
- Shemesh, E.; Chevli, P.A.; Islam, T.; German, C.A.; Otvos, J.; Yeboah, J.; Rodriguez, F.; Defilippi, C.; Lima, J.A.C.; Blaha, M.; et al. Circulating ketone bodies and cardiovascular outcomes: The MESA study. Eur. Heart J. 2023, 44, 1636–1646. [Google Scholar] [CrossRef]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Poulimeneas, D.; Grammatikopoulou, M.G.; Devetzi, P.; Petrocheilou, A.; Kaditis, A.G.; Papamitsou, T.; Doudounakis, S.E.; Vassilakou, T. Adherence to Dietary Recommendations, Nutrient Intake Adequacy and Diet Quality among Pediatric Cystic Fibrosis Patients: Results from the GreeCF Study. Nutrients 2020, 12, 3126. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Olsen, M.K.; Dudley, T.; Guyton, J.R. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int. J. Cardiol. 2006, 110, 212–216. [Google Scholar] [CrossRef]
- Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Joseph, J.J. Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance: A Scientific Statement From the American Heart Association. Circulation 2023, 147, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652. [Google Scholar] [CrossRef]
- Gerber, S.; Rogers, G.; Staffier, K.; Karlsen, M.; Folta, S.; Jacques, P.; Naumova, E.; McKeown, N. Adherence, Compliance, and Diet Quality Among Popular Diet Followers. Curr. Dev. Nutr. 2022, 6, 364. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Banting, W. Letter on Corpulence: Addressed to the Public; Mohun, Ebbs and Hough: New York, NY, USA, 1864. [Google Scholar]
- Teicholz, N. The scientific report guiding the US dietary guidelines: Is it scientific? BMJ 2015, 351, h4962. [Google Scholar] [CrossRef] [PubMed]
- Bero, L.A.; Norris, S.L.; Lawrence, M.A. Making nutrition guidelines fit for purpose. BMJ 2019, 365, l1579. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: A systematic review including GRADE assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-carb and ketogenic diets in type 1 and type 2 diabetes. Nutrients 2019, 11, 952. [Google Scholar] [CrossRef]
- Krebs, J.D.; Strong, A.P.; Cresswell, P.; Reynolds, A.N.; Hanna, A.; Haeusler, S. A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac. J. Clin. Nutr. 2016, 25, 78–84. [Google Scholar] [CrossRef]
- Leow, Z.Z.X.; Guelfi, K.J.; Davis, E.A.; Jones, T.W.; Fournier, P.A. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet. Med. 2018, 35, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Schmidt, S.; Damm-Frydenberg, C.; Holst, J.J.; Madsbad, S.; Nørgaard, K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: A randomized open-label crossover trial. Diabetes. Obes. Metab. 2017, 19, 1479–1484. [Google Scholar] [CrossRef]
- Schmidt, S.; Christensen, M.B.; Serifovski, N.; Damm-Frydenberg, C.; Jensen, J.E.B.; Fløyel, T.; Størling, J.; Ranjan, A.; Nørgaard, K. Low versus high carbohydrate diet in type 1 diabetes: A 12-week randomized open-label crossover study. Diabetes. Obes. Metab. 2019, 21, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.; Rush, A.; Kaye, J. Glycaemic stability of a cyclist with Type 1 diabetes: 4011 km in 20 days on a ketogenic diet. Diabet. Med. 2019, 36, 1503–1507. [Google Scholar] [CrossRef]
- Dressler, A.; Reithofer, E.; Trimmel-Schwahofer, P.; Klebermasz, K.; Prayer, D.; Kasprian, G.; Rami, B.; Schober, E.; Feucht, M. Type 1 diabetes and epilepsy: Efficacy and safety of the ketogenic diet. Epilepsia 2010, 51, 1086–1089. [Google Scholar] [CrossRef]
- de Bock, M.; Lobley, K.; Anderson, D.; Davis, E.; Donaghue, K.; Pappas, M.; Siafarikas, A.; Cho, Y.H.; Jones, T.; Smart, C. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: An illustrative case series. Pediatr. Diabetes 2018, 19, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef]
- Petta, I.; Fraussen, J.; Somers, V.; Kleinewietfeld, M. Interrelation of diet, gut microbiome, and autoantibody production. Front. Immunol. 2018, 9, 439. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The impact of diet and lifestyle on gut microbiota and human health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Sordi, V.; Bolla, A.M.; Saita, D.; Ferrarese, R.; Canducci, F.; Clementi, M.; Invernizzi, F.; Mariani, A.; Bonfanti, R.; et al. Duodenal mucosa of patients with type 1 diabetes shows distinctive inflammatory profile and microbiota. J. Clin. Endocrinol. Metab. 2017, 102, 1468–1477. [Google Scholar] [CrossRef]
- Tinguely, D.; Gross, J.; Kosinski, C. Efficacy of Ketogenic Diets on Type 2 Diabetes: A Systematic Review. Curr. Diab. Rep. 2021, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef]
- Rafiullah, M.; Musambil, M.; David, S.K. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: A meta-analysis. Nutr. Rev. 2022, 80, 488–502. [Google Scholar] [CrossRef]
- Zaki, H.A.; Iftikhar, H.; Bashir, K.; Gad, H.; Fahmy, A.S.; Elmoheen, A. A Comparative Study Evaluating the Effectiveness Between Ketogenic and Low-Carbohydrate Diets on Glycemic and Weight Control in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e25528. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.Z.; Day, A.; Brinkworth, G.D.; Sato, J.; Yamada, S.; Jönsson, T.; Beardsley, J.; Johnson, J.A.; Thabane, L.; Johnson, B.C. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: Systematic review and meta-analysis of published and unpublished randomized trial data. BMJ 2021, 372, m4743. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Xu, D.; Zhou, Y.; Qu, Z.; Yang, Q.; Lv, Q. Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1092031. [Google Scholar] [CrossRef]
- Parry-Strong, A.; Wright-McNaughton, M.; Weatherall, M.; Hall, R.M.; Coppell, K.J.; Barthow, C.; Krebs, J.D. Very low carbohydrate (ketogenic) diets in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 2431–2442. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Ambrozkiewicz, F.; Paziewska, A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients 2023, 15, 500. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Gibas, S.; Salisbury, M.; Gomer, J.; Gibas, K. Ketogenic diets potentially reverse Type II diabetes and ameliorate clinical depression: A case study. Diabetes Metab. Syndr. 2019, 13, 1475–1479. [Google Scholar] [CrossRef]
- Brooke, J.; Stiell, M.; Ojo, O. Evaluation of the Accuracy of Capillary Hydroxybutyrate Measurement Compared with Other Measurements in the Diagnosis of Diabetic Ketoacidosis: A Systematic Review. Int. J. Environ. Res. Public Health 2016, 13, 837. [Google Scholar] [CrossRef]
- Dhatariya, K. The use of point-of-care blood ketone monitors in the management of diabetic ketoacidosis in adults. Ann. Clin. Biochem. 2014, 51, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Akturk, H.K.; Snell-Bergeon, J.; Pyle, L.; Fivekiller, E.; Garg, S.; Cobry, E. Accuracy of a breath ketone analyzer to detect ketosis in adults and children with type 1 diabetes. J. Diabetes Complicat. 2021, 35, 108030. [Google Scholar] [CrossRef]
- Moonla, C.; Del Caño, R.; Sakdaphetsiri, K.; Saha, T.; De la Paz, E.; Düsterloh, A.; Wang, J. Disposable screen-printed electrochemical sensing strips for rapid decentralized measurements of salivary ketone bodies: Towards therapeutic and wellness applications. Biosens. Bioelectron. 2023, 220, 114891. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, A.; Hoffmann, M.; Ascott-Evans, B.H. The role of point-of-care blood testing for ketones in the diagnosis of diabetic ketoacidosis. S. Afr. Med. J. 2015, 105, 756–759. [Google Scholar] [CrossRef]
- Lohano, P.D.; Ibrahim, M.; Raza, S.J.; Gowa, M.; Baloch, S.H. Comparing Finger-stick Beta-eta-hydroxybutyrate with Dipstick Urine Tests in the Detection of Ketone Bodies in the Diagnosis of Children with Diabetic Ketoacidosis. J. Coll. Physicians Surg. Pak. 2022, 32, 483–486. [Google Scholar] [CrossRef]
- Voulgari, C.; Tentolouris, N. The performance of a glucose-ketone meter in the diagnosis of diabetic ketoacidosis in patients with type 2 diabetes in the emergency room. Diabetes Technol. Ther. 2010, 12, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Dhatariya, K. Blood ketones: Measurement, interpretation, limitations, and utility in the management of diabetic ketoacidosis. Rev. Diabet. Stud. 2016, 13, 217–225. [Google Scholar] [CrossRef]
- Misra, S.; Oliver, N.S. Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet. Med. 2015, 32, 14–23. [Google Scholar] [CrossRef]
- Saasa, V.; Beukes, M.; Lemmer, Y.; Mwakikunga, B. Blood Ketone Bodies and Breath Acetone Analysis and Their Correlations in Type 2 Diabetes Mellitus. Diagnostics 2019, 9, 224. [Google Scholar] [CrossRef]
- Huang, J.; Yeung, A.M.; Bergenstal, R.M.; Castorino, K.; Cengiz, E.; Dhatariya, K.; Niu, I.; Sherr, J.L.; Umpierrez, G.E.; Klonoff, D.C. Update on Measuring Ketones. J. Diabetes Sci. Technol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, R.S.; Hendler, R.G.; Felig, P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes 1976, 25, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.E.H.; Wastney, M.E.; Bolton, T.M.; Braaten, J.T.; Berman, M. Ketone body kinetics in humans: The effects of insulin-dependent diabetes, obesity, and starvation. J. Lipid Res. 1984, 25, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Foster, D.W.; McGarry, J.D. The regulation of ketogenesis. Ciba Found. Symp. 1982, 87, 120–131. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the regulation of metabolism: Mechanisms and consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Shahid, W.; Khan, F.; Makda, A.; Kumar, V.; Memon, S.; Rizwan, A. Diabetic Ketoacidosis: Clinical Characteristics and Precipitating Factors. Cureus 2020, 12, e10792. [Google Scholar] [CrossRef] [PubMed]
- Nyenwe, E.A.; Kitabchi, A.E. The evolution of diabetic ketoacidosis: An update of its etiology, pathogenesis and management. Metabolism. 2016, 65, 507–521. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. North Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Tokubuchi, I.; Wada, N.; Tsuruta, M.; Ohki, T.; Oshige, T.; Sasaki, Y.; Iwata, S.; Kato, N.; Ohtsuka, Y.; et al. Age-related changes in the diurnal variation of ketogenesis in patients with type 2 diabetes and relevance to hypoglycemic medications. Endocr. J. 2015, 62, 235–241. [Google Scholar] [CrossRef]
- Balasubramanyam, A.; Nalini, R.; Hampe, C.S.; Maldonado, M. Syndromes of ketosis-prone diabetes mellitus. Endocr. Rev. 2008, 29, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Makahleh, L.; Othman, A.; Vedantam, V.; Vedantam, N. Ketosis-Prone Type 2 Diabetes Mellitus: An Unusual Presentation. Cureus 2022, 14, e30031. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Oliver, N.S.; Dornhorst, A. Diabetic ketoacidosis: Not always due to type 1 diabetes. BMJ 2013, 346, f3501. [Google Scholar] [CrossRef]
- Gaba, R.; Mehta, P.; Balasubramanyam, A. Evaluation and management of ketosis-prone diabetes. Expert Rev. Endocrinol. Metab. 2019, 14, 43–48. [Google Scholar] [CrossRef]
- Balasubramanyam, A. Syndromes of Ketosis-Prone Diabetes. Trans. Am. Clin. Climatol. Assoc. 2019, 130, 145–155. [Google Scholar]
- Das Gupta, R.; Atri, A.; Mondal, S.; Bhattacharjee, A.; Garai, R.; Hazra, A.K.; Choudhury, B.; Dutta, D.S.; Lodh, M.; Ganguly, A. Characterizing progressive beta-cell recovery after new-onset DKA in COVID-19 provoked A-β+ KPD (ketosis-prone diabetes): A prospective study from Eastern India. J. Diabetes Complicat. 2022, 36, 108100. [Google Scholar] [CrossRef]
- Hampe, C.S.; Nalini, R.; Maldonado, M.R.; Hall, T.R.; Garza, G.; Iyer, D.; Balasubramanyam, A. Association of amino-terminal-specific antiglutamate decarboxylase (GAD65) autoantibodies with β-cell functional reserve and a milder clinical phenotype in patients with GAD65 antibodies and ketosis-prone diabetes mellitus. J. Clin. Endocrinol. Metab. 2007, 92, 462–467. [Google Scholar] [CrossRef]
- Kitabchi, A.E.; Umpierrez, G.E.; Fisher, J.N.; Murphy, M.B.; Stentz, F.B. Thirty years of personal experience in hyperglycemic crises: Diabetic ketoacidosis and hyperglycemic hyperosmolar state. J. Clin. Endocrinol. Metab. 2008, 93, 1541–1552. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Deal, C.L. Maternal-Fetal and Neonatal Endocrinology: Physiology, Pathophysiology, and Clinical Management; Academic Press: Cambridge, MA, USA, 2020; ISBN 9780128148235. Available online: https://www.sciencedirect.com/book/9780128148235/maternal-fetal-and-neonatal-endocrinology (accessed on 17 September 2023).
- White, S.L.; Pasupathy, D.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; Briley, A.L.; Seed, P.T.; Welsh, P.; Poston, L. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia 2017, 60, 1903–1912. [Google Scholar] [CrossRef]
- Baz, B.; Riveline, J.P.; Gautier, J.F. Endocrinology of pregnancy: Gestational diabetes mellitus: Definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016, 174, R43–R51. [Google Scholar] [CrossRef]
- Bronisz, A.; Ozorowski, M.; Hagner-Derengowska, M. Pregnancy ketonemia and development of the fetal central nervous system. Int. J. Endocrinol. 2018, 2018, 1242901. [Google Scholar] [CrossRef] [PubMed]
- Tsirou, E.; Grammatikopoulou, M.G.; Theodoridis, X.; Gkiouras, K.; Petalidou, A.; Taousani, E.; Savvaki, D.; Tsapas, A.; Goulis, D.G. Guidelines for Medical Nutrition Therapy in Gestational Diabetes Mellitus: Systematic Review and Critical Appraisal. J. Acad. Nutr. Diet. 2019, 119, 1320–1339. [Google Scholar] [CrossRef]
- Tanner, H.L.; Dekker Nitert, M.; Callaway, L.K.; Barrett, H.L. Ketones in Pregnancy: Why Is It Considered Necessary to Avoid Them and What Is the Evidence Behind Their Perceived Risk? Diabetes Care 2021, 44, 280–289. [Google Scholar] [CrossRef]
- Major, C.A.; Henry, M.J.; De Veciana, M.; Morgan, M.A. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet. Gynecol. 1998, 91, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Mijatovic, J.; Louie, J.C.Y.; Buso, M.E.C.; Atkinson, F.S.; Ross, G.P.; Markovic, T.P.; Brand-Miller, J.C. Effects of a modestly lower carbohydrate diet in gestational diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 112, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.M.; Reckless, J.P.D.; Cullen, D.R. Diurnal variations in blood intermediary metabolites in mild gestational diabetic patients and the effect of a carbohydrate-restricted diet. Diabetologia 1982, 22, 68–72. [Google Scholar] [CrossRef]
- Tsirou, E.; Grammatikopoulou, M.G.; Nigdelis, M.P.; Taousani, E.; Savvaki, D.; Assimakopoulos, E.; Tsapas, A.; Goulis, D.G. TIMER: A Clinical Study of Energy Restriction in Women with Gestational Diabetes Mellitus. Nutrients 2021, 13, 2457. [Google Scholar] [CrossRef]
- Duarte-Gardea, M.O.; Gonzales-Pacheco, D.M.; Reader, D.M.; Thomas, A.M.; Wang, S.R.; Gregory, R.P.; Piemonte, T.A.; Thompson, K.L.; Moloney, L. Academy of Nutrition and Dietetics Gestational Diabetes Evidence-Based Nutrition Practice Guideline. J. Acad. Nutr. Diet. 2018, 118, 1719–1742. [Google Scholar] [CrossRef]
- Sweeting, A.; Mijatovic, J.; Brinkworth, G.D.; Markovic, T.P.; Ross, G.P.; Brand-Miller, J.; Hernandez, T.L. The Carbohydrate Threshold in Pregnancy and Gestational Diabetes: How Low Can We Go? Nutrients 2021, 13, 2599. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.L.; Rozance, P.J. Re-examination of the estimated average requirement for carbohydrate intake during pregnancy: Addition of placental glucose consumption. Am. J. Clin. Nutr. 2023, 117, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Toohill, J.; Soong, B.; Flenady, V. Interventions for ketosis during labour. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- Seccombe, D.W.; Harding, P.G.R.; Possmayer, F. Fetal utilization of maternally derived ketone bodies for lipogenesis in the rat. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1977, 488, 402–416. [Google Scholar] [CrossRef]
- Spanou, L.; Dalakleidi, K.; Zarkogianni, K.; Papadimitriou, A.; Nikita, K.; Vasileiou, V.; Alevizaki, M.; Anastasiou, E. Ketonemia and ketonuria in gestational diabetes mellitus. Hormones 2015, 14, 644–650. [Google Scholar] [CrossRef]
- Pelletier, A.; Coderre, L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1325–E1332. [Google Scholar] [CrossRef]
- Sussman, D.; Ellegood, J.; Henkelman, M. A gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse. BMC Pregnancy Childbirth 2013, 13, 198. [Google Scholar] [CrossRef] [PubMed]
- Guh, J.Y.; Der Chuang, T.; Chen, H.C.; Hung, W.C.; Lai, Y.H.; Shin, S.J.; Chuang, L.Y. Beta-hydroxybutyrate-induced growth inhibition and collagen production in HK-2 cells are dependent on TGF-beta and Smad3. Kidney Int. 2003, 64, 2041–2051. [Google Scholar] [CrossRef]
- Stehbens, J.A.; Baker, G.L.; Kitchell, M. Outcome at ages 1, 3, and 5 years of children born to diabetic women. Am. J. Obstet. Gynecol. 1977, 127, 408–413. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Magkos, F. Medical Nutrition Therapy. In Comprehensive Clinical Approach to Diabetes During Pregnancy; Goulis, D.G., Ed.; Springer: Cham, Switzerland, 2022; pp. 205–229. [Google Scholar]
- Somagutta, M.R.; Agadi, K.; Hange, N.; Jain, M.S.; Batti, E.; Emuze, B.O.; Amos-Arowoshegbe, E.O.; Popescu, S.; Hanan, S.; Kumar, V.R.; et al. Euglycemic Diabetic Ketoacidosis and Sodium-Glucose Cotransporter-2 Inhibitors: A Focused Review of Pathophysiology, Risk Factors, and Triggers. Cureus 2021, 13, e13665. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Euglycemic Ketoacidosis as a Complication of SGLT2 Inhibitor Therapy. Clin. J. Am. Soc. Nephrol. 2021, 16, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Lupsa, B.C.; Kibbey, R.G.; Inzucchi, S.E. Ketones: The double-edged sword of SGLT2 inhibitors? Diabetologia 2023, 66, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Hung, S.Y.; Chen, I.W. Relationship of concomitant anti-diabetic drug administration with sodium-glucose co-transporter 2 inhibitor-related ketosis. J. Int. Med. Res. 2022, 50, 03000605221090095. [Google Scholar] [CrossRef]
- Garg, M.; Ghanim, H.; Kuhadiya, N.D.; Green, K.; Hejna, J.; Abuaysheh, S.; Torre, B.; Batra, M.; Makdissi, A.; Chaudhuri, A.; et al. Liraglutide acutely suppresses glucagon, lipolysis and ketogenesis in type 1 diabetes. Diabetes. Obes. Metab. 2017, 19, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lu, X.; Arbab, A.A.I.; Wu, X.; Mao, Y.; Loor, J.J.; Yang, Z. Metformin acts to suppress β-hydroxybutyric acid-mediated inflammatory responses through activation of AMPK signaling in bovine hepatocytes. J. Anim. Sci. 2021, 99, skab153. [Google Scholar] [CrossRef]
- Tokubuchi, I.; Tajiri, Y.; Iwata, S.; Hara, K.; Wada, N.; Hashinaga, T.; Nakayama, H.; Mifune, H.; Yamada, K. Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats. PLoS ONE 2017, 12, e0171293. [Google Scholar] [CrossRef]
- Lucidi, P.; Porcellati, F.; Cioli, P.; Candeloro, P.; Marinelli Andreoli, A.; Bolli, G.B.; Fanelli, C.G. Greater Suppression of Glucagon, Lipolysis, and Ketogenesis with Insulin Glargine U300 as Compared with Glargine U100 in Type 1 Diabetes Mellitus. Diabetes Technol. Ther. 2020, 22, 57–61. [Google Scholar] [CrossRef]
- Castellanos, L.; Tuffaha, M.; Koren, D.; Levitsky, L.L. Management of Diabetic Ketoacidosis in Children and Adolescents with Type 1 Diabetes Mellitus. Paediatr. Drugs 2020, 22, 357–367. [Google Scholar] [CrossRef]
| Sample | Intervention(s)/ Exposure(s) | Results | First Author |
|---|---|---|---|
| Mouse embryos of two distinct stages (3–4 and 5–6 somites) | Racemic mixture of DL-BHB at levels of 8, 16, or 32 mM/L (24 h) | Growth reduction and inhibition/delay of neural tube closure were noted in the cranial and/or caudal regions of exposed embryos. The effects were dose- and age-dependent, with younger embryos being more affected, and higher doses producing greater malformations. Cytoplasmic vacuoles in the neuroepithelium, mesenchyme and ectoderm were noted, involving mitochondria undergone high-amplitude swelling with matrix density loss and cristae. | Horton [23] |
| Early mouse somite embryos | Culture for 4, 8, or 24 h in the presence of 32 mM DL-BHB and then culture (24 h) in control serum | Treated embryos showed progressive mitochondrial alterations, starting at 4 h with loss of matrix density, culminating at 24 h with high-amplitude swelling, complete matrix density loss and cristae disappearance. These changes were reversible following removal from BHB and culturing for 24 h in control serum. The early somite embryos showed a limited capacity to oxidatively metabolize BHB. | Horton [24] |
| Whole embryo cultures | 24 h culture in: (i) Δ-BHB (48 mM), or (ii) control medium | All embryos exhibited NTD and lower rates of glucose metabolism by the PPP and Krebs cycle, compared to controls. The effect of the Δ-isomer on the Krebs cycle may result from glucose intermediates replacement generated from D-BHB metabolism. | Hunter [27] |
| Rat embryos at 9.5 days of gestation | Cultured in vitro for 24/48 h, with/without 4 × 10(-2) M BHB for all, or part of the culture period | Embryos exposed to BHB for a complete 48 h culture were more affected than those exposed for part of the culture and embryos were more vulnerable to BHB during the first ½ of a 48 h culture than during the second ½. Embryos cultured with BHB from 9.5 days of gestation for 24 h revealed some BHB effects after 24 h in culture. Many abnormalities were embryonic retardations, with embryos showing characteristics of normal, yet younger embryos. | Moore [29] |
| Early somite stage mouse embryos | Culture in: (i) control serum (60 h) (ii) serum with 32 mmol/L DL-BHB (24 h), followed by control serum (36 h, recovery) (iii) 32 mmol/L DL-BHB serum (60 h) | Although neural tube closure occurred in the recovery arm, complete recovery was limited to the ventral regions of the forebrain. The remainder of the prosencephalon and the rhombencephalon failed to catch-up growth completely. In these areas, cell numbers were approximately 70% of control values. Although the gross anatomical disturbances produced by high ketone levels may be compensated for, several histological alterations remain. | Shum [25] |
| Early somite stage mouse embryos | 32 mM DL-BHB (24 h, Period I), and then transfer in control medium (36 h maximum, Period II) | At the end of Period I, all D,L,-BHB-exposed embryos were growth-retarded with NTD regarding closure. At 36 h of Period II, cranial and caudal NTD of embryos were reduced. These embryos also exhibited an excess in growth velocity during recovery thus, at the end of Period II, total protein content was comparable to control. Embryos who did not enter the control serum remained growth-retarded and showed more cranial and caudal NTD. | Shum [26] |
| CD-1 mouse embryos whose mothers were fed either an SD or a KD | SD or KD, 30 days prior to, as well as during gestation | At E13.5 the average KD embryo was volumetrically larger, with a larger heart but smaller brain, pharynx, hypothalamus, midbrain, cervical spinal cord and pons, compared with the SD embryo. At E17.5, KD embryos were smaller, with smaller hearts and thymuses, but with enlarged cervical spines, midbrains, thalamus, and pons. | Sussman [22] |
| in vivo embryos of control or streptozotocin-diabetic rats at gestational days 9–11 | Cultured in a whole-embryo culture system for 48 h with high concentration of DM-related substrates and metabolites | Cytoplasmic vacuoles were observed in the ectoderm of day-9 embryos and in the neuroepithelium and blood cells of days-10–11 embryos of diabetic rats. These were mitochondria undergoing large-amplitude swelling with matrix density loss and disturbed cristae. No differences were noted in the brain, heart, or liver of day-15 fetuses from normal and diabetic rats. Day-9 embryos cultured in high concentrations of D-glucose, Pyr, BHB, and α-KIC for 48 h also showed high-amplitude mitochondrial swelling in the neuroepithelium. | Yang [28] |
| KD Type | Fat (% TEI) | Protein (% TEI) | CHO (% TEI) |
|---|---|---|---|
| Classical | 90% | 10% (protein + CHO) | |
| MCT | 75% (21–25% from LCT and 45–55% from MCT) | 25% (protein + CHO) | |
| Modified Atkins | nearly 75% | liberal intake | 10–20 g/day |
| Low GI | 60% | 30% | ≈10% (40–60 g/day with a low GI <50) |
| First Author | Design | Participants | Intervention(s) | Duration | Results |
|---|---|---|---|---|---|
| Krebs [77] | RCT | N = 10 patients | (i) typical CHO counting course (ii) same course + advice on following an LCD (75 g/day) | 12 weeks | The CHO-restricted arm showed reductions in HbA1c and insulin use and non-significant reductions in BW. No changes in BP, creatinine or lipid levels were noted, and all outcomes in the CHO-counting arm remained unchanged. No change was noted in the glycemic variability. |
| Leow [78] | Observational cohort | N = 11 patients | KD (<55 g CHO/day) | 2.6 ± 3.3 years | KD resulted in excellent HbA1c levels and low glycemic variability, but may also be associated with dyslipidemia and a high incidence of hypoglycemic episodes. |
| Ranjan [79] | OL cross-over RCT | N = 10 adults on IP | (i) isocaloric HCD (≥250 g/d) (ii) isocaloric LCD (≤50 g/d) | 1 week each intervention | Diet adherence was high and glucose levels were similar in both diets. The LCD resulted in more time with glucose between 3.9 and 10.0 mmol/L, less time with values ≤ 3.9 mmol/L, and less glucose variability than the HCD. CV markers were unaffected, but glucagon, FFA, and ketone concentrations were higher post-LCD. |
| Schmidt [80] | OL cross-over RCT | N = 14 patients with sensor-augmented IPs | (i) LCD < 100 g CHO/d) (ii) HCD > 250 g CHO/d) | 12 weeks each intervention | Time spent in the range 3.9–10.0 mmol/L did not differ, but time at <3.9 mmol/L and glycemic variability were lower at LCD. No severe hypoglycemia events were recorded. LCD induced a BW loss (2.0 ± 2.1 kg) and HCD a BW gain (2.6 ± 1.8 kg), but no other CV risk factors were affected. |
| Nolan [81] | Case study | N = 1 cyclist who successfully undertook a 4011 km cycle across Australia | VLCD | 20 days | Remarkable glycemic stability was noted, with 80.4% of time spent at 3.9–10 mmol/L. Interstitial glucose was <3 mmol/L for 2.1% of this time, and only one episode of hypoglycemia was recorded. |
| Assay | Ketones Assessed | Cut-Off Used (If Any) | Sensitivity (%) | Specificity (%) | Target Population | Reference | |
|---|---|---|---|---|---|---|---|
| Breath | HS-SPME/ GC-MS | Acetone | 3.9 ppm | 94.7 | 54.2 | Adults with T1DM | [100] |
| Saliva | Enzymatic sensor strip | BHB | NR | NR | NR | NR | [101] |
| Capillary blood | Dry chemistry | BHB | >3 mmol/L | 100 | 89 | Adults | [102] |
| 90.4 | 100 | Children with T1DM | [103] | ||||
| Urine | Semiquantitative assay | AcAc | ≥2+ | 89.9 | 52.7 | T2DM | [104] |
| 84.9 | 86.5 | Children with T1DM | [103] |
| (i) | A+β-KPDM: | Characterized by the presence of islet autoantibodies and absence of β cell function |
| (ii) | A+β+KPDM: | Characterized by the presence of islet autoantibodies with preserved β cell functional reserve |
| (iii) | A-β-KPDM: | Characterized by absence of islet autoantibodies with absence of β cell function |
| (iv) | A-β+KPDM: | Characterized by absence of islet autoantibodies with preserved β cell functional reserve |
| First Author | Participants | Design | Interventions | Results |
|---|---|---|---|---|
| Major [133] | n = 21 women with GDM on an LCD (CHO < 42% of TEI) n = 21 women with GDM on HCD (CHO > 45% of TEI) | CC | N/A | Reductions in postprandial glucose values were observed and fewer subjects required insulin for glycemia in the LCD arm. The incidence of LGA infants, cesarean deliveries for cephalopelvic disproportion and macrosomia were lower in the LCD arm. Urinary ketones were only identified in 2 women, both following an LCD, clearing their urinary ketones when CHO intake was increased. |
| Mijatovic [134] | N = 46 women with GDM | RCT | (i) modest LCD (∼135 g of CHO/day) for 6 weeks (ii) routine care (∼200 g of CHO/day) for 6 weeks | No detectable differences were apparent in blood ketones between LCD (mean intake of 165 g of CHO/day) and routine care arm, although CHO and TEI were lower in the intervention arm. No differences were noted regarding birth weight, rate of LGA infants, % fat mass, or FFM between groups. A modest LCD does not result in increased fasting BHB levels. |
| Potter [135] | n = 7 non-diabetic women (A) n = 7 women with mild GDM at diagnosis (B), and n = 7 women with mild GDM post-treatment with a 150 g CHO diet (C) | CC | N/A | Glucose levels were indifferent between groups. Ketone body levels were elevated in the GDM group prior to treatment (B) and rose higher after treatment with the 150 g CHO diet (C). Lactate levels were reduced when on the restricted CHO diet. |
| Tsirou [136] | N = 43 women with GDM | CT | (i) VLED (175 g of CHO/day) (ii) VLED + exercise (175 g of CHO/day) (iii) LED (175 g of CHO/day) (iv) LED + exercise (175 g of CHO/day) | GWG was lower in the VLED and higher in the LED arms. No differences were noted in the type of delivery, birth weight, composite score, prematurity, depression, RQ, Apgar score, MUAC, or insulin use. Most infants (88.4%) were AGA, born at a gestational age of 37–42 weeks (95.3%). Only 9.3% of the mothers experienced delivery complications, with the majority being at the VLED + exercise arm. The composite score was low (range 0–2.5) for all, indicating a “risk-free” pregnancy outcome. No differences were noted in the urine ketone levels between groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veneti, S.; Grammatikopoulou, M.G.; Kintiraki, E.; Mintziori, G.; Goulis, D.G. Ketone Bodies in Diabetes Mellitus: Friend or Foe? Nutrients 2023, 15, 4383. https://doi.org/10.3390/nu15204383
Veneti S, Grammatikopoulou MG, Kintiraki E, Mintziori G, Goulis DG. Ketone Bodies in Diabetes Mellitus: Friend or Foe? Nutrients. 2023; 15(20):4383. https://doi.org/10.3390/nu15204383
Chicago/Turabian StyleVeneti, Stavroula, Maria G. Grammatikopoulou, Evangelia Kintiraki, Gesthimani Mintziori, and Dimitrios G. Goulis. 2023. "Ketone Bodies in Diabetes Mellitus: Friend or Foe?" Nutrients 15, no. 20: 4383. https://doi.org/10.3390/nu15204383
APA StyleVeneti, S., Grammatikopoulou, M. G., Kintiraki, E., Mintziori, G., & Goulis, D. G. (2023). Ketone Bodies in Diabetes Mellitus: Friend or Foe? Nutrients, 15(20), 4383. https://doi.org/10.3390/nu15204383







