Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications
Abstract
:1. Introduction
1.1. Dietary Polyphenols
1.2. Oral Microbiota, Polyphenols, and Oral Health
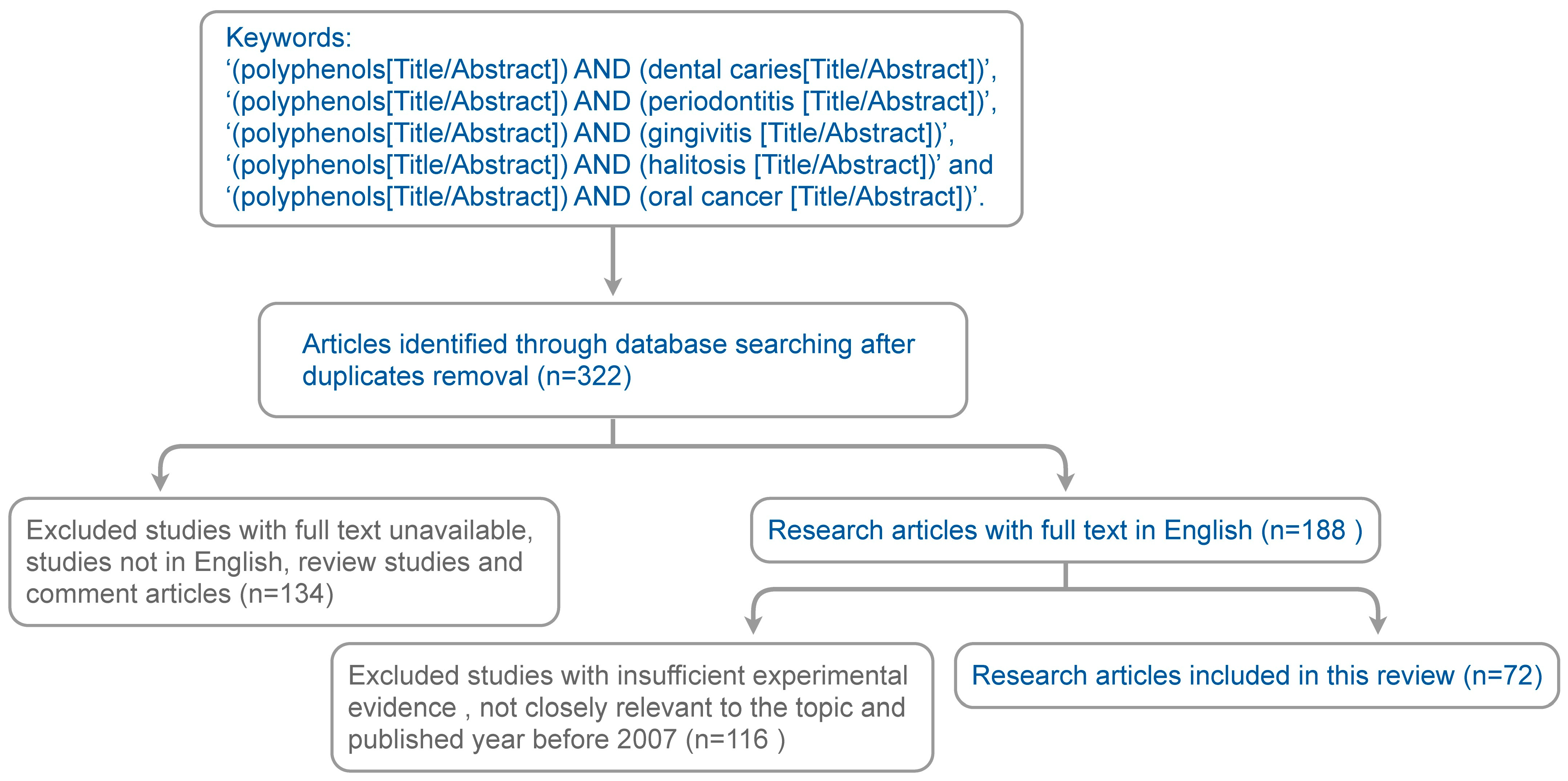
2. Method for Literature Search
3. Polyphenols and Oral Health
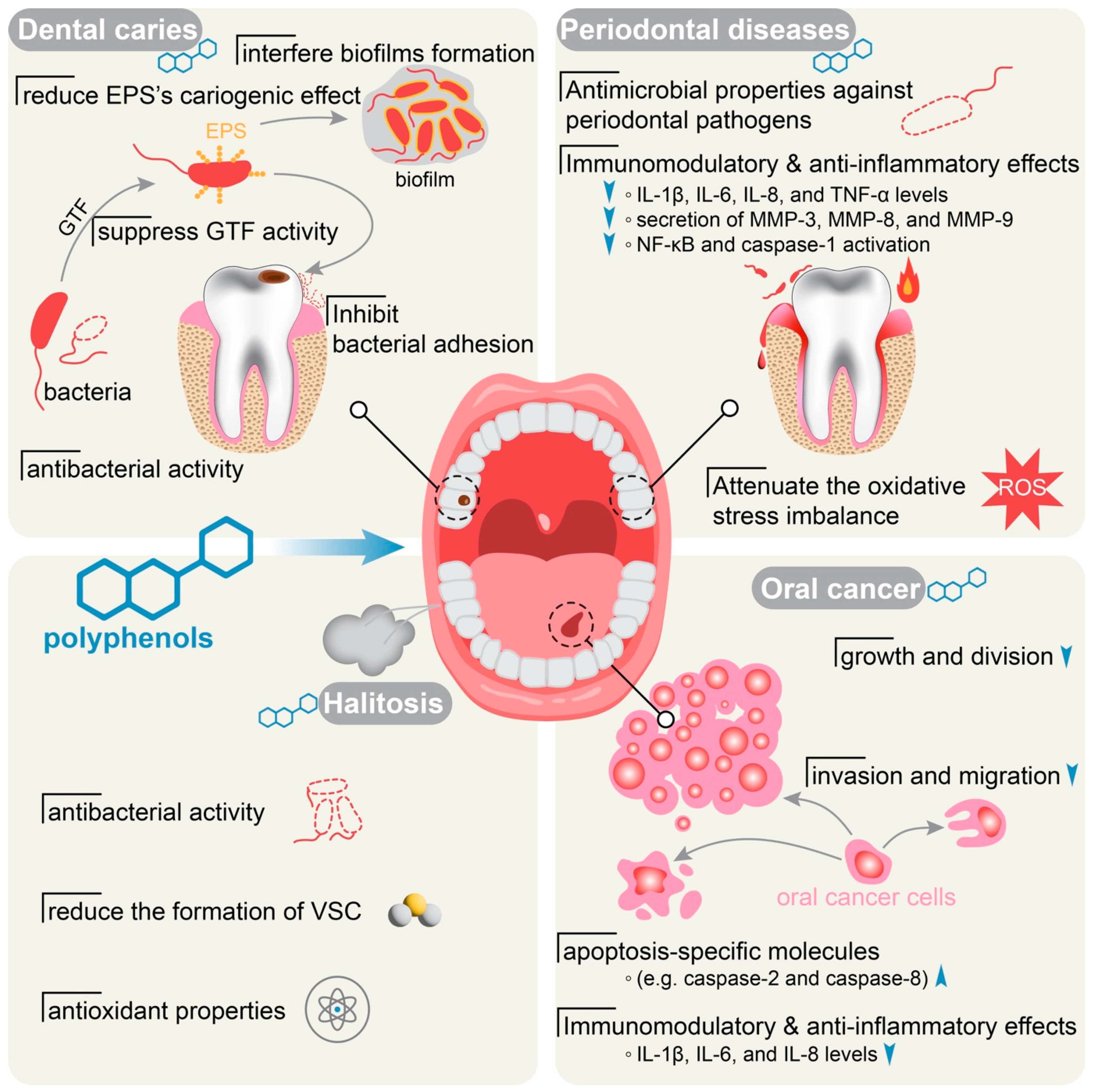
3.1. Polyphenols and Dental Caries
| Study Group | Active Components | Study Design | Pathogens | Cells/Tissues/Animals | Results |
|---|---|---|---|---|---|
| Ren. et al., 2023 [45] | Lonicera caerulea fruit polyphenols | In vitro | L. rhamnosus (RYX-01) | N/A | Inhibition of RYX-01 growth Reduction of EPS and biofilm formation Inhibition of quorum sensing and biofilm formation-related gene expression |
| Pärnänen et al., 2023 [59] | Fermented Lingonberry Juice | A One Year Prospective Human Intervention Study (25 patients) | S. mutans Candida Lactobacilli | N/A | Reduction of S. mutans and Candida counts Increased Lactobacilli counts significantly Reduction in decayed surfaces (DS) index, bleeding on probing (BOP), and visible plaque index (VPI) No effect on probing pocket depths(DDPs) |
| Goto et al., 2023 [53] | Roasted Green Tea (RGT)-specific polyphenols | In vitro | S. mutans | N/A | Inhibition of S. mutans biofilm formation and GTF activity |
| Chhaliyil et al., 2022 [58] | Polyphenolic mouthwash | in-vivo study was performed using saliva and dental biofilm samples collected from 75 healthy subjects. | N/A | N/A | Reduction in bacterial taxa associated with oral diseases in refined sugar group and unrefined sugar group |
| Nomura et al., 2021 [43] | Flavedo, albedo, fruits, and leaves of Citrus unshiu extracts | In vitro | S. mutans | N/A | Inhibition of S. mutans |
| Yabuta et al., 2021 [57] | Backhousia citriodora (lemon myrtle) extract | In vitro | S. mutans | N/A | Reduction of the glycolytic pH drop Inhibition of lactate production No effect on lactate dehydrogenase activity |
| Xu et al., 2021 [61] | EGCG–phospholipid complex | In vitro | S. mutans | N/A | Strong antibacterial activity on S.mutans Reduction of acid production and tooth surface adhesion Inhibition of glucan and biofilm formation by suppressing the GTF activity |
| Schneider-Rayman et al., 2021 [52] | Green tea polyphenol, epigallocatechin gallate (EGCG) | In vitro | S. mutans | N/A | Inhibition of the planktonic growth and the biofilm formation Reduction of S. mutans EPS production Reduction in gtfB, gtfC, and ftf genes involved in EPS production, and the nox and sodA genes involved in the protection against oxidative stress |
| Magacz et al., 2021 [42] | Acetone extracts of Reynoutria. japonica, R. sachalinensis, and R. x bohemica | In vitro | S. mutans | N/A | Modulated the activity of the lactoperoxidase system |
| Goyal et al., 2021 [54] | Polyphenols gallic acid and tannic acid | In vitro | S. mutans | N/A | Inhibition of dextransucrase activity |
| Babaeekhou et al., 2021 [44] | N-hexane, ethyl acetate, methanol, and aqueous extracts of Ginger | In vitro | S. mutans S. sobrinus | N/A | Inhibition of S. mutans and S. sobrinus |
| Selvaraj et al., 2020 [62] | Toothpaste containing probiotics and Neem | In vivo (60 patients) | S. mutans | N/A | Reduction of bacterial count |
| Kim et al., 2020 [50] | Green or black tea extracts | In vitro | S. mutans S. sobrinus | N/A | Inhibition of biofilm formation, cell viability, and GTF activity Maintained the pH |
| Ben Lagha et al., 2020 [46] | Tart cherry (Prunus cerasus L.) extract | In vitro | C. albicans S. mutans F. nucleatum | Oral epithelial cell line GMSM-K, human oral epithelial cell line B11 | Inhibition of biofilm formation Attenuated the adherence of C. albicans and S. mutans to a hydroxylapatite surface as well as the adherence of F. nucleatum to oral epithelial cells. |
| Veloz et al., 2019 [36] | Polyphenolic compounds in Chilean Propolis | In vitro | S. mutans | N/A | Inhibition of bacterial growth and biofilm formation |
| Philip et al., 2019 [51] | Extracts of cranberry, blueberry, and strawberry, and a combination of the three berry extracts (Orophenol) | In vitro | S. mutans | N/A | Reduction in biofilm metabolic activity, acid production, and EPS biovolumes No bactericidal on S. mutans |
| Farkash et al., 2019 [47] | Padma hepaten and a polyphenol extraction from green tea | In vitro | S. mutans C. albicans | N/A | Inhibition of biofilm formation without affecting the planktonic growth Reduction in EPS secretion |
| Yabuta et al., 2018 [48] | Extract from Lemon myrtle (Backhousia citriodora) | In vitro | S. mutans | N/A | Inhibition of S. mutans biofilm |
| Damiano et al., 2017 [49] | Ziziphus jujuba Mill fresh leaves | In vitro | S. mutans | N/A | Inhibition of biofilm bioactivity |
| Hambire et al., 2015 [60] | 0.5% Camellia sinensis extract | A randomized blinded controlled trial with 60 healthy children of age 9–14 years | N/A | N/A | More effective compared to 0.05% sodium fluoride and 0.2% chlorhexidine gluconate mouth rinses |
| Koo et al., 2010 [56] | Cranberry PAC fraction | In vivo | S. mutans | Sprague-Dawley rats | Reduction of biofilm formation and smooth-surface caries Diminished the synthesis of insoluble glucans by GtfB adsorbed on a saliva-coated hydroxyapatite surface |
3.2. Polyphenols and Periodontal Diseases
| Study Group | Plants/Active Components | Study Design | Pathogens | Cells/Tissues/Animals | Results |
|---|---|---|---|---|---|
| Ullah et al., 2023 [69] | Cistus × incanus L., Scutellaria lateriflora L. | In vitro | P. gingivalis | Human keratinocyte epithelial cells HaCaT | Inhibition of P. gingivalis growth Reduction in P. gingivalis HaCaT invasiveness and biofilm |
| Pärnänen et al., 2023 [59] | Fermented lingonberry juice (FLJ) | One-year prospective clinical intervention study (25 patients) | S. mutans Candida Lactobacilli | N/A | Reduction of S. mutans and Candida counts Increased Lactobacilli counts Reduction in decayed surfaces (DS) index, bleeding on probing (BOP), and visible plaque index (VPI) No effect on probing pocket depths(DDPs) |
| Alkimavičienė et al., 2023 [94] | Proanthocyanidins (PACs) | Clinical study in 46 patients with periodontitis | N/A | N/A | Inhibition of S. mutans biofilm formation and GTF activity Better clinical outcomes for moderate pockets Improved MMP-3 concentration in saliva |
| Vaillancourt et al., 2022 [81] | A berry polyphenolic fraction (Orophenol®) composed of extracts from cranberry, wild blueberry, and strawberry | In vitro | P. gingivalis | Human oral keratinocyte cell line B11 | Inhibition of P. gingivalis growth Decreased P. gingivalis hemolytic activity, its adherence to a basement membrane matrix model, and its proteinase activities Reduction in production of ROS by oral keratinocytes stimulated with P. gingivalis |
| Qi et al., 2022 [96] | Turkish Gall’s effective constituent was prepared into nanoparticles (T-NPs) by the principle of oxidative self-polymerization. | In vitro | P. gingivalis | N/A | Stronger antibacterial activity on oral pathogens T-NPs induced bacteria lysis by promoting the excessive production of ROS without periodontal tissue damage |
| He et al., 2022 [97] | Tea polyphenols (TP) and AdipoRon (APR) | In vitro and in vivo | N/A | Bone marrow stromal cells BMSCs and RAW 264.7 cells Sixty 8-week-old male C57BL/6 mice | Programmed core-shell nanofibers for sequential and controlled release of tea polyphenols and AdipoRon Reduction of proinflammatory cytokines levels in vitro Promoted osteogenic differentiation in an inflammatory microenvironment in vitro Alleviated periodontal tissue inflammation and enhanced the regeneration of alveolar bone in vivo |
| Iviglia et al., 2021 [80] | A polyphenolic mixture extracted from the pomace of the Croatian grape variety | In vitro | N/A | Human osteoblast-like SAOS2 cells | Anti-inflammatory and antioxidant properties Reduction of acid production and tooth surface adhesion |
| Dal-Fabbro et al., 2021 [93] | Red wine consumption or its polyphenols | In vivo | N/A | 3-month Wistar rats with apical periodontitis | Reduction of the inflammatory process in apical periodontitis and periapical bone resorption |
| Torre et al., 2020 [98] | Polyphenol-rich grape pomace extracts | In vitro | N/A | Human bone marrow stromal cells hMSC | Decreased receptor activator of nuclear factor κ-Β ligand Enhanced expression of genes involved in osteoblast differentiation |
| Galarraga-Vinueza ME, et al., 2020 [87] | Cranberry concentrates at 25, 50, and 100 µg/mL | In vitro | N/A | THP-1 cells (monocytic line, Human gingival fibroblasts (HFIB-G cell line) osteosarcoma-derived osteoblasts SAOS-2 cell line | Downregulated the expression of IL-8 and IL-6 in LPS-stimulated macrophages with cranberry concentrates at 50 and 100 µg/mL Upregulated the expression of IL-10 in LPS-stimulated macrophages by cranberry concentrates at 100 µg/mL |
| Ben Lagha, et al., 2020 [70] | Highbush blueberry proanthocyanidins | In vitro | P. gingivalis | Gingival keratinocyte cell line B11 In vitro gingival keratinocyte barrier model | Reduction in bacterial growth |
| Tsou et al., 2019 [75] | Coffee extract and its primary phenolic acid, chlorogenic acid | In vitro | P. gingivalis | N/A | Inhibition of P. gingivalis viability Reduction of associated protease activity. |
| Ben Lagha et al., 2019 [83] | Green and black tea extracts in distilled water 10 mg/mL EGCG, theaflavin fraction in 95% ethanol | In vitro | N/A | U937 human monocytes, human monoblastic leukemia cell line U937-3xκ B-LUC, gingival keratinocyte cell line B11 | Inhibited the activation of NF-κB and caspase-1 as well as IL-1β secretion by monocytes/macrophages Protected keratinocytes against the TNF-α-mediated breakdown of barrier integrity. |
| Jekabsone et al., 2019 [71] | Pelargonium sidoides DC root extract (PSRE), proanthocyanidin fraction from PSRE (PAC) | In vitro | P. gingivalis, S. salivarius, S. aureus, S. epidermidis, A. actinomycetemcomitans and E. coli. | Human primary gingival fibroblasts HGF, Human peripheral blood mononuclear cells PBMCs | Strong antibacterial, anti-inflammatory, and gingival tissue-protecting properties under periodontitis-mimicking conditions |
| Farzanegan et al., 2019 [84] | Silymarin or resveratrol (100 μg/mL) and a combination of these two polyphenols | In vitro | N/A | Human gingival fibroblast cell line HGF-3 | Inhibited inflammatory effects of histamine on cultured HGFs by reduction of IL-6, IL-8, TPA-1, and TNF-α |
| Ben Lagha, et al., 2019 [99] | Cranberry Proanthocyanidins (PAC) | In vitro | A. actinomycetemcomitans | U937 human monocytes | Reduction of leukotoxin (LtxA) gene expression Neutralized the cytolytic and pro-inflammatory responses of human macrophages |
| Khalil et al., 2019 [72] | Methanolic extract of Salvadora persica | In vitro | S. aureus and Streptococcus sp. | N/A | Inhibition of bacterial growth |
| Kariu et al., 2017 [76] | Prenylated flavonoids isolated from Epimedium species plant | In vitro | P. gingivalis | N/A | Inhibition of gingipains activity in a non-competitive manner Inhibition of P. gingivalis growth and biofilm formation |
| Díaz Sánchez et al., 2017 [95] | New nutritional supplement made of oligomeric proanthocyanidins (PAC) | A prospective, double-blind, randomized, controlled clinical trial in 20 patients | N/A | N/A | Oligomeric PAC affects periodontal tissue health but has no effect on the accumulation of plaque on the tooth surface |
| Ben Lagha et al., 2017 [100] | EGCG from green tea and theaflavins from black tea | In vitro | F. nucleatum | N/A | Inhibited the bacterial adhesion and F. nucleatum-induced hemolysis No effects on bacterial growth at antiadhesive concentrations |
| Ben Lagha et al., 2017 [101] | Theaflavins from black tea | In vitro | P. gingivalis | U937-3xκB-LUC monocyte cell line | Inhibition of Arg- and Lys-gingipain and bacterial adhesion Enhanced tight junction integrity of gingival keratinocytes |
| Tipton et al., 2016 [88] | Cranberry high molecular weight non-dialyzable material (NDM) | In vivo | N/A | Normal human gingival fibroblasts from a healthy patient with noninflamed gingiva | Inhibition of IL-6 and MMP-3 production by human gingival fibroblasts |
| Inaba et al., 2016 [92] | Apple polyphenol (AP), Hop bract polyphenol (HBP), EGCG, KYT-1 (Arg-gingipain inhibitor); and KYT-36 (Lys-gingipain inhibitor) in combination | In vitro | P. gingivalis | OSCC cells | Inhibition of protease activated receptor 2 (PAR2) and PAR4 mRNA expressions, pMMP-9 activation, and cellular invasion Reduced activation of heat shock protein 27 and Ets1 and nuclear translocation of nuclear factor-kappa B (NFκ-B) |
| Widén et al., 2015 [73] | Blackcurrant and sea buckthorn juices | In vitro | S. mitis, S. mutans S. sanguinis, S. gordonii, S. aureus, S. epidermidis and P. aeruginosa. | N/A | Inhibition of bacterial growth |
| Shahzad et al., 2015 [74] | Forty-eight purified (HPLC grade) Polyphenol compounds | In vitro | S. mitis A. actinomycetemcomitans F. nucleatum P. gingivalis | N/A | Antibacterial activities against periodontopathic bacteria in both planktonic and biofilm modes of growth |
| Kong et al., 2015 [77] | Theaflavins | In vitro | P. gingivalis | Human gingival fibroblasts (HGFs) from healthy gingival tissue. | Antimicrobial effects against both planktonic culture and biofilm of P. gingivalis Inhibition of the proteinase activities of P. gingivalis collagenase and gingipains Reduction in the secretion and mRNA expression of MMP-1 & MMP-2 by HGFs stimulated with P. gingivalis |
| Ben Lagha et al., 2015 [89] | Wild Blueberry (Vaccinium angustifolium Ait.) Polyphenols | In vitro | F. nucleatum | U937-3xκB cells | Inhibition of F. nucleatum growth and biofilm formation Inhibited the activation of NF-κB induced by F. nucleatum Inhibited the secretion of IL-1β, TNF-α, IL-6, MMP-8 & MMP-9 |
| Tipton et al., 2014 [90] | Cranberry high molecular weight non-dialyzable material (NDM) | In vitro | N/A | Human gingival epithelial cells [Smulow-Glickman (S-G)] | Decreased nuclear levels of IL-1b-activated NF-jB (p65) & AP-1 (phospho-c-Jun), inhibited IL-6 production. |
| Jang et al., 2014 [78] | Baicalein | In vitro | S. mitis S. mutans S. sanguinis S. sobrinus S. oralis Streptococcus ratti F. nucleatum A. actinomycetemcomitans P. gingivalis | N/A | Inhibition of bacterial growth |
| Tipton et al., 2013 [91] | Cranberry high molecular weight non-dialyzable material (NDM) | In vitro | N/A | Human gingival epithelial cells and human gingival fibroblasts | Inhibition of constitutive and IL-17-stimulated IL6 & IL-8 production by epithelial cell and gingival fibroblasts |
| Zdarilová et al., 2010 [82] | Polyphenolic fraction of L. caerulea berries | In vitro | P. gingivalis | Human gingival fibroblasts from healthy donors free of periodontal disease. | Reduction of ROS production, intracellular glutathione (GSH) depletion, and lipid peroxidation Inhibited LPS-induced up-regulation of IL-1β, IL-6 and TNF-α Suppressed expression of cyclooxygenase-2 (COX-2) |
3.3. Polyphenols and Halitosis
| Study Group | Active Components | Study Design | Pathogens | Cells/Tissues | Results |
|---|---|---|---|---|---|
| Liu. et al., 2021 [107] | Thinned young apple polyphenols (YAP) | in vitro | P. gingivalis, P. intermedius, F. nucleatum | N/A | Inhibition of halitosis-related bacteria growth Destroyed integrity and permeability of the cell membrane |
| Veloso et al., 2020 [103] | Crude extracts obtained from Jucá, Cinnamon, Mallow, Pomegranate, Rosemary, Macassá, Clove, and Tamarind | in vitro | P. gingivalis, P. intermedia, F. nucleatum, P. micra | N/A | Pomegranate extract was the only extract that inhibited all the evaluated microorganisms |
| Morin et al., 2015 [106] | EGCG from green tea | in vitro | S. moorei | N/A | Inhibited S. moorei growth and bacterial adherence Reduction of the biofilm formation Suppression of bacterial β-galactosidase activity |
| Rassameemasmaung et al., 2008 [109] | Green tea mouthwash | Double-blinded and placebo-controlled clinical trial in 60 gingivitis patients | N/A | N/A | Reduced VSC level in gingivitis subjects after rinsing for 4 weeks |
| Lodhia et al., 2008 [110] | Green tea powder | In vitro and in vivo studies; Analyze the concentration of both H2S and CH3SH gases | N/A | N/A | Green tea exhibited significant temporary Reduced oral malodor due to its disinfectant and deodorizing properties |
3.4. Polyphenols and Oral Cancer
| Study Group | Active Components | Study Design | Cells/Tissues/Animals | Results |
|---|---|---|---|---|
| Sharma et al., 2023 [120] | Defatted seeds of Azadirachta indica and Momordica charantia | in vitro | Human oral epidermal carcinoma KB cell line | Bioactive extracts had antiproliferative activity and antioxidant capacity Suppressed KB cells Binding efficacy against tumor suppressor gene regulatory function |
| Nimbalkar et al., 2022 [127] | Polymeric black tea polyphenols (PBPs)/thearubigins (TRs) | in vivo | Hamster model of oral carcinogenesis | Modulated EGFR pathway associated with cell proliferation, hypoxia, and angiogenesis. |
| Liu et al., 2022 [128] | Grape seed proanthocyanidins (PAC) | in vitro | Oral squamous cell carcinoma cell lines SCC-4, Human oral squamous cell carcinoma cell lines HSC-3 | Developed a complex coacervates-based delivery of PAC Inhibited cell proliferation, migration, and invasion of cancer cells Reduction of MMP-2, MMP-9, and MMP-13 Suppressed protein kinase B (Akt) pathway |
| Basak et al., 2020 [126] | APG-157 (a botanical drug containing multiple polyphenols, including curcumin) | Phase I clinical trial (n = 25) | N/A | Reduced IL-1β, IL-6, and IL-8 concentrations in the salivary supernatant fluid of patients with cancer Reduction in Bacteroidetes species in cancer subjects Up-regulation of genes associated with differentiation and T-cell recruitment to the tumor microenvironment. |
| Sheng et al., 2018 [129] | Resveratrol, epigallocatechin gallate (EGCG), and tannic acid | in vitro | Normal human oral keratinocytes NHOKs, Human oral squamous cell carcinoma cell lines HSC-2 | Resveratrol in combination with doxorubicin additively augmented doxorubicin cytotoxicity in both types of cells. EGCG and tannic acid alleviated the toxicity caused by doxorubicin in keratinocytes, primarily by reducing doxorubicin-induced necrosis in normal human oral keratinocytes |
| Huang et al., 2018 [130] | Hydrogels formed ellagic acid (EA) and EGCG | in vitro | Human oral cancer cell line CAL-27 | Long-term steady-state release of bioactive EA Reduced viability of CAL-27 human oral cancer cells |
| Fan et al., 2015 [123] | Anthocyanins from black rice (Oryza sativa L.) | in vitro | Human oral cancer cell line CAL-27 | Suppression of CAL 27 cell metastasis Reduction in MMP-2, MMP-9, and NF-κB p65 expression through the suppression of PI3K/Akt pathway Inhibition of NF-κB levels |
| Chang et al., 2012 [115] | Black tea polyphenol extracts (BTE) | in vitro and in vivo | Oral squamous cell carcinoma cell lines SCC-4 5-week-old immunodeficient nude mice | Up-regulation of epithelial markers such as E-cadherin Inhibition of mesenchymal markers such as snail-1 and vimentin Inhibition of the tumor growth of SCC-4 cells via cancer cell xenografted nude mice mode |
| Chen et al., 2011 [125] | Green tea polyphenol epigallocatechin-3 gallate (EGCG) | in vitro and in vivo | Oral squamous cell carcinoma cell lines SCC-9 5-week-old immunodeficient nude mice | Inhibition of p-focal adhesion kinase (p-FAK), p-Src, snail-1, and vimentin Inhibition on the tumor growth of SCC-9 cells in vivo |
| Chatelain et al., 2011 [116] | Cranberry and grape seed extracts | in vitro | Oral squamous cell carcinoma cell lines CAL-27 and SCC-25 | Inhibition of oral cancer proliferation Up-regulation of caspase-2 and caspase-8 levels |
| Kingsley et al., 2010 [122] | Proanthocyanidins (PAC) | in vitro | Oral squamous cell carcinoma cell lines CAL-27 and SCC-25 | Inhibition of oral cancer proliferation Up-regulation of caspase-2 and caspase-8 levels Down-regulation of specific cell-cycle regulators |
| Srinivasan et al., 2008 [119] | Green tea polyphenols | in vivo | Wistar strain male albino rats | Reduced the number of tumors, tumor volume, and oral squamous cell carcinoma |
| Letchoumy et al., 2008 [131] | Black tea polyphenols Polyphenon-B and BTF-35 | in vivo | Male Syrian hamsters aged 6–10 weeks weighing between 90–110 g | Decreased tumor incidence, oxidative DNA damage, phase I enzyme activities Reduction in CYP1A1 and CYP1B1 Enhanced phase II enzyme activities in the buccal pouch and liver |
| Mohan et al., 2007 [117] | Green and black tea polyphenols alone and in combination with bovine milk lactoferrin (bLF) | in vitro | Human tongue squamous carcinoma CAL-27 and normal human gingival fibroblast (HGF) cells | Inhibition of CAL-27 cell growth Transduced the apoptosis signal via the generation of reactive oxygen species and decrease in the Bcl-2/Bax ratio Activation of caspase-3 |
| Letchoumy et al., 2007 [118] | Black tea polyphenols, Polyphenon-B, and BTF-35 | in vivo | Male Syrian hamsters aged 6–10 weeks weighing between 90–110 g | Reduced the incidence of DMBA-induced hamster buccal pouch carcinomas by modulating markers of cell proliferation, cell survival, tumor infiltration, angiogenesis, and apoptosis |
| King et al., 2007 [121] | Proanthocyanidin (PAC) | in vitro | Human oral squamous cell carcinoma CAL 27, human cervical carcinoma Ca Ski, human cervical adenocarcinoma GH354, and human foreskin fibroblasts Hs27 cell lines | Suppression of cellular proliferation of OSCC Induced apoptosis in cervical and oral cancer cell lines |
| Ho et al., 2007 [124] | Epigallocatechin-3-gallate (EGCG) | in vitro | OC2 cells | Inhibited invasion and migration of OC2 cells Decreased expressions of MMP-2, MMP-9, and uPA in a dose-dependent manner |
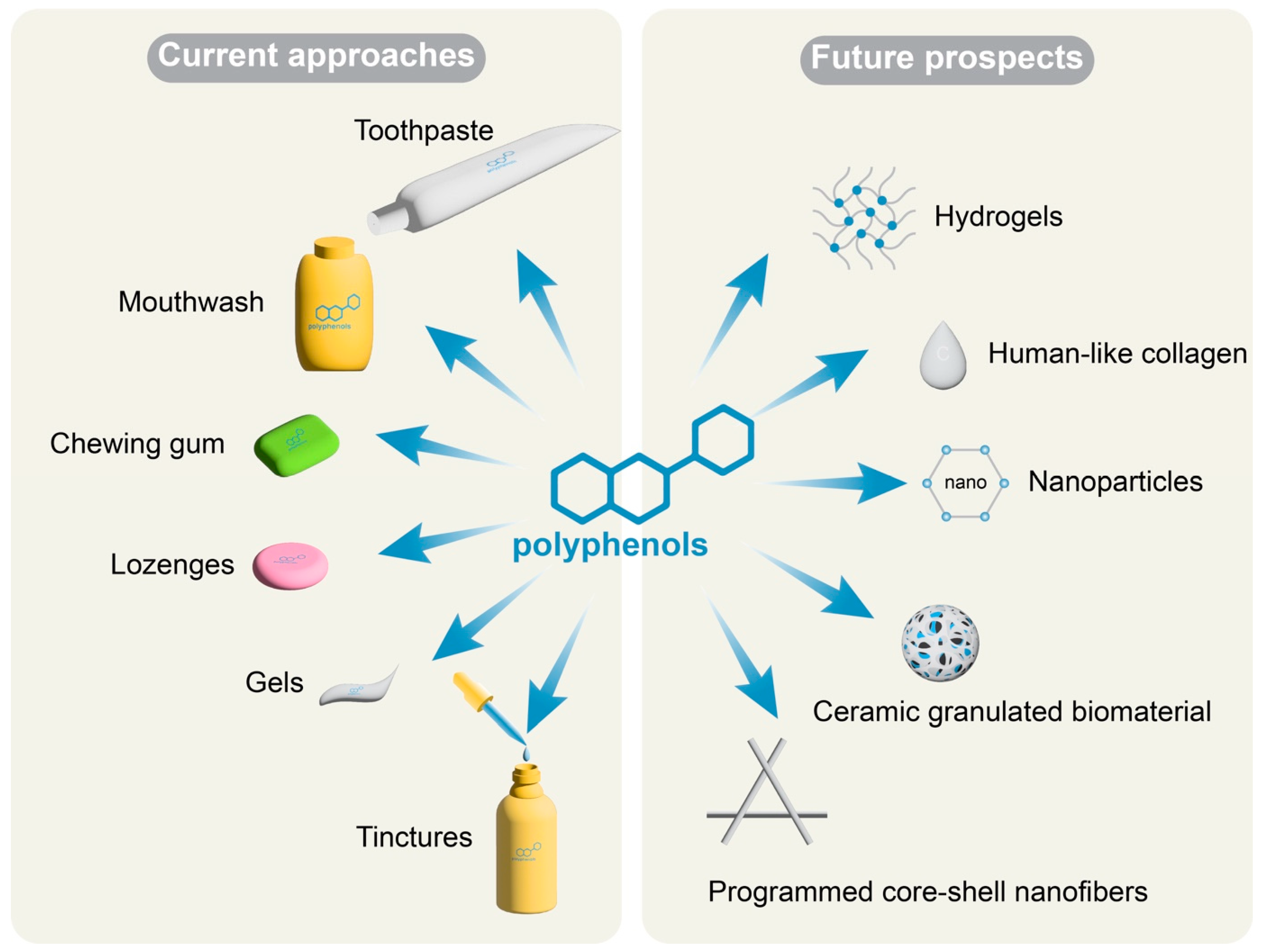
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isola, G. The impact of diet, nutrition and nutraceuticals on oral and periodontal health. Nutrients 2020, 12, 2724. [Google Scholar] [CrossRef]
- Petersen, P. The world oral health report 2003: Continuous improvement of oral health in the 21st century-the approach of the who global oral health programme. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.N.; Singh, P.R.; Shah, K.; Chauhan, N.S. Chapter 1 Natural Oral Care in Dental Therapy: Current and Future Prospects; John Wiley & Sons: Hobken, NJ, USA, 2020; pp. 1–29. [Google Scholar]
- Dye, B.A. The global burden of oral disease: Research and public health significance. J. Dent. Res. 2017, 96, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Cascaes, A.M.; Silva, N.; Fernandez, M.D.S.; Bomfim, R.A.; Vaz, J.D.S. Ultra-processed food consumption and dental caries in children and adolescents: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Medori, M.C.; Dhuli, K.; Donato, K.; Connelly, S.T.; Bellinato, F.; Gisondi, P.; Bertelli, M. Polyphenols and lactobacillus reuteri in oral health. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E246–E254. [Google Scholar] [CrossRef]
- Bunte, K.; Hensel, A.; Beikler, T. Polyphenols in the prevention and treatment of periodontal disease: A systematic review of in vivo, ex vivo and in vitro studies. Fitoterapia 2019, 132, 30–39. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Kovac, J.; Slobodnikova, L.; Trajcikova, E.; Rendekova, K.; Mucaji, P.; Sychrova, A.; Bittner Fialova, S. Therapeutic potential of flavonoids and tannins in management of oral infectious diseases—A review. Molecules 2022, 28, 158. [Google Scholar] [CrossRef]
- Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive applications of polyphenols in dentistry—A review. Int. J. Mol. Sci. 2021, 22, 4892. [Google Scholar] [CrossRef]
- Ding, Y.; Yao, H.; Yao, Y.; Fai, L.Y.; Zhang, Z. Protection of dietary polyphenols against oral cancer. Nutrients 2013, 5, 2173–2191. [Google Scholar] [CrossRef]
- Trisha, A.T.; Shakil, M.H.; Talukdar, S.; Rovina, K.; Huda, N.; Zzaman, W. Tea polyphenols and their preventive measures against cancer: Current trends and directions. Foods 2022, 11, 3349. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and anticancer role of resveratrol against oral squamous cell carcinoma. Pharmaceutics 2023, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Gawande; Vidya, V.; Kalikar, S.V. Flavonoids: An overview. J. Pharm. Res. 2012, 18, 641–649. [Google Scholar]
- Khanbabaee, K.; Ree, T.V. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Encinar, J.A.; Rodríguez-Díaz, J.C.; Micol, V. Antimicrobial capacity of plant polyphenols against gram-positive bacteria: A comprehensive review. Curr. Med. Chem. 2020, 27, 2576–2606. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002, 957, 57–69. [Google Scholar] [CrossRef]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural polyphenols for prevention and treatment of cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Sheen, J.M.; Hu, W.L.; Hung, Y.C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxidative Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhang, Y.; Liu, X.; Zhou, Q.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Xie, T.; Shen, Y. Natural polyphenols-platinum nanocomplexes stimulate immune system for combination cancer therapy. Nano Lett. 2022, 22, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, T.; Huang, G.; Jiang, S.; Gu, Y.; Chen, F. Oral microbiomes: More and more importance in oral cavity and whole body. Protein Cell 2018, 9, 488–500. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Takeshita, T. The oral microbiome and human health. J. Oral Sci. 2017, 59, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Moye, Z.D.; Zeng, L.; Burne, R.A. Fueling the caries process: Carbohydrate metabolism and gene regulation by Streptococcus mutans. J. Oral Microbiol. 2014, 6, 24878. [Google Scholar] [CrossRef]
- García-Manríquez, N.; Lozano, C.; Muñoz, A.; Morales, M.F.; Giacaman, R.A. Anticaries properties of natural berries: Systematic literature review. Nutr. Rev. 2023, 2023, nuad063. [Google Scholar] [CrossRef] [PubMed]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and antibiofilm activity against Streptococcus mutans of individual and mixtures of the main polyphenolic compounds found in chilean propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Buhlin, K.; Dufrêne, Y.F.; Gomelsky, M.; Moroni, A.; Ramstedt, M.; Rumbaugh, K.P.; Schulte, T.; Sun, L.; Åkerlund, B.; et al. Biofilm formation—What we can learn from recent developments. J. Intern. Med. 2018, 284, 332–345. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Giacaman, R.A. Sugars and beyond. The role of sugars and the other nutrients and their potential impact on caries. Oral Dis. 2018, 24, 1185–1197. [Google Scholar] [CrossRef]
- Niaz, T.; Shabbir, S.; Noor, T.; Abbasi, R.; Imran, M. Alginate-caseinate based ph-responsive nano-coacervates to combat resistant bacterial biofilms in oral cavity. Int. J. Biol. Macromol. 2020, 156, 1366–1380. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Ramos, P.; Kłósek, M.; Bobela, E.; Czuba, Z.P.; Balwierz, R.; Olczyk, P. Propolis as a cariostatic agent in lozenges and impact of storage conditions on the stability of propolis. Pharmaceutics 2023, 15, 1768. [Google Scholar] [CrossRef]
- Magacz, M.; Oszajca, M.; Nawrot-Hadzik, I.; Drożdż, R.; Jurczak, A.; Hadzik, J.; Smakosz, A.; Krzyściak, W. Phenolic compounds of reynoutria sp. As modulators of oral cavity lactoperoxidase system. Antioxidants 2021, 10, 676. [Google Scholar] [CrossRef]
- Nomura, R.; Ohata, J.; Otsugu, M.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory effects of flavedo, albedo, fruits, and leaves of citrus unshiu extracts on Streptococcus mutans. Arch. Oral Biol. 2021, 124, 105056. [Google Scholar] [CrossRef]
- Babaeekhou, L.; Ghane, M. Antimicrobial activity of ginger on cariogenic bacteria: Molecular networking and molecular docking analyses. J. Biomol. Struct. Dyn. 2021, 39, 2164–2175. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Pei, F.; Cao, X.; Zhang, W.; Du, R.; Ge, J.; Ping, W. Purification of exopolysaccharides from Lactobacillus rhamnosus and changes in their characteristics by regulating quorum sensing genes via polyphenols. Int. J. Biol. Macromol. 2023, 240, 124414. [Google Scholar] [CrossRef]
- Ben Lagha, A.; LeBel, G.; Grenier, D. Tart cherry (Prunus cerasus L.) fractions inhibit biofilm formation and adherence properties of oral pathogens and enhance oral epithelial barrier function. Phytother. Res. PTR 2020, 34, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Farkash, Y.; Feldman, M.; Ginsburg, I.; Steinberg, D.; Shalish, M. Polyphenols inhibit Candida albicans and Streptococcus mutans biofilm formation. Dent. J. 2019, 7, 42. [Google Scholar] [CrossRef]
- Yabuta, Y.; Mukoyama, H.; Kaneda, Y.; Kimura, N.; Bito, T.; Ichiyanagi, T.; Ishihara, A.; Watanabe, F. A lemon myrtle extract inhibits glucosyltransferases activity of Streptococcus mutans. Biosci. Biotechnol. Biochem. 2018, 82, 1584–1590. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Forino, M.; De, A.; Vitali, L.A.; Lupidi, G.; Taglialatela-Scafati, O. Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujuba leaves used for infusion preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Kim, J.H.; Nam, O.H. Tea extracts differentially inhibit Streptococcus mutans and Streptococcus sobrinus biofilm colonization depending on the steeping temperature. Biofouling 2020, 36, 256–265. [Google Scholar] [CrossRef]
- Philip, N.; Bandara, H.; Leishman, S.J.; Walsh, L.J. Inhibitory effects of fruit berry extracts on Streptococcus mutans biofilms. Eur. J. Oral Sci. 2019, 127, 122–129. [Google Scholar] [CrossRef]
- Schneider-Rayman, M.; Steinberg, D.; Sionov, R.V.; Friedman, M.; Shalish, M. Effect of epigallocatechin gallate on dental biofilm of Streptococcus mutans: An in vitro study. BMC Oral Health 2021, 21, 447. [Google Scholar] [CrossRef] [PubMed]
- Goto, I.; Saga, S.; Ichitani, M.; Kimijima, M.; Narisawa, N. Investigation of components in roasted green tea that inhibit Streptococcus mutans biofilm formation. Foods 2023, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ahmad, S.; Mahmood, A.; Chander Sharma, S. Interactions of dextransucrase purified from Streptococcus mutans 890 with plant polyphenols. Biochem. Biophys. Rep. 2021, 26, 100980. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Sharma, S.; Mahmood, A. Inhibition of dextransucrase activity in Streptococcus mutans by plant phenolics. Indian J. Biochem. Biophys. 2013, 50, 48–53. [Google Scholar]
- Koo, H.; Duarte, S.; Murata, R.M.; Scott-Anne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of cranberry proanthocyanidins on formation of biofilms by Streptococcus mutans on saliva-coated apatitic surface and on dental caries development in vivo. Br. Dent. J. 2010, 44, 116–126. [Google Scholar] [CrossRef]
- Yabuta, Y.; Sato, Y.; Miki, A.; Nagata, R.; Bito, T.; Ishihara, A.; Watanabe, F. Lemon myrtle extract inhibits lactate production by Streptococcus mutans. Biosci. Biotechnol. Biochem. 2021, 85, 2185–2190. [Google Scholar] [CrossRef] [PubMed]
- Chhaliyil, P.; Fischer, K.F.; Schoel, B.; Chhalliyil, P. Impact of refined and unrefined sugar and starch on the microbiota in dental biofilm. J. Int. Soc. Prev. Community Dent. 2022, 12, 554–563. [Google Scholar] [CrossRef]
- Pärnänen, P.; Lomu, S.; Räisänen, I.T.; Tervahartiala, T.; Sorsa, T. Antimicrobial and anti-inflammatory oral effects of fermented lingonberry juice-a one-year prospective human intervention study. Eur. J. Dent. 2023. [Google Scholar] [CrossRef]
- Hambire, C.U.; Jawade, R.; Patil, A.; Wani, V.R.; Kulkarni, A.A.; Nehete, P.B. Comparing the antiplaque efficacy of 0.5% Camellia sinensis extract, 0.05% sodium fluoride, and 0.2% chlorhexidine gluconate mouthwash in children. J. Int. Soc. Prev. Community Dent. 2015, 5, 218–226. [Google Scholar] [CrossRef]
- Xu, X.; Dai, Z.; Zhang, Z.; Kou, X.; You, X.; Sun, H.; Guo, H.; Liu, M.; Zhu, H. Fabrication of oral nanovesicle in-situ gel based on epigallocatechin gallate phospholipid complex: Application in dental anti-caries. Eur. J. Pharmacol. 2021, 897, 173951. [Google Scholar] [CrossRef]
- Selvaraj, K.; Bharath, N.; Natarajan, R.; Dinesh, S.; Murugesan, S.; Selvaraj, S. Comparative evaluation of antimicrobial efficacy of toothpastes containing probiotic and neem as primary ingredient on salivary Streptococcus mutans in melmaruvathur population: An in vivo study. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. S1), S595–S600. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, E.; Alreshaid, R.; Araujo de Godoi, M. Anti-inflammatory benefits of food ingredients in periodontal diseases. Pathogens 2023, 12, 520. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Reviews. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Host-mediated resolution of inflammation in periodontal diseases. Periodontology 2000 2006, 40, 144–163. [Google Scholar] [CrossRef]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host modulation and treatment of periodontal disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef]
- Ullah, H.; Minno, A.D.; Filippis, A.; Sommella, E.; Buccato, D.G.; Lellis, L.F.; El-Seedi, H.R.; Khalifa, S.A.M.; Piccinocchi, R.; Galdiero, M.; et al. In vitro antimicrobial and antibiofilm properties and bioaccessibility after oral digestion of chemically characterized extracts obtained from Cistus × incanus L., Scutellaria lateriflora L., and their combination. Foods 2023, 12, 1826. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Howell, A.; Grenier, D. Highbush blueberry proanthocyanidins alleviate Porphyromonas gingivalis-induced deleterious effects on oral mucosal cells. Anaerobe 2020, 65, 102266. [Google Scholar] [CrossRef]
- Jekabsone, A.; Sile, I.; Cochis, A.; Makrecka-Kuka, M.; Laucaityte, G.; Makarova, E.; Rimondini, L.; Bernotiene, R.; Raudone, L.; Vedlugaite, E.; et al. Investigation of antibacterial and antiinflammatory activities of proanthocyanidins from pelargonium sidoides dc root extract. Nutrients 2019, 11, 2829. [Google Scholar] [CrossRef]
- Khalil, M.A.; El-Sabbagh, M.S.; El Naggar, E.B.; El-Erian, R.H. Antibacterial activity of salvadora persica against oral pathogenic bacterial isolates. Niger. J. Clin. Pract. 2019, 22, 1378–1387. [Google Scholar] [CrossRef]
- Widén, C.; Renvert, S.; Persson, G.R. Antibacterial activity of berry juices, an in vitro study. Acta Odontol. Scand. 2015, 73, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015, 6, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Tsou, S.H.; Hu, S.W.; Yang, J.J.; Yan, M.; Lin, Y.Y. Potential oral health care agent from coffee against virulence factor of periodontitis. Nutrients 2019, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Kariu, T.; Nakao, R.; Ikeda, T.; Nakashima, K.; Potempa, J.; Imamura, T. Inhibition of gingipains and Porphyromonas gingivalis growth and biofilm formation by prenyl flavonoids. J. Periodontal Res. 2017, 52, 89–96. [Google Scholar] [CrossRef]
- Kong, L.; Qi, X.; Huang, S.; Chen, S.; Wu, Y.; Zhao, L. Theaflavins inhibit pathogenic properties of P. gingivalis and mmps production in p. Gingivalis-stimulated human gingival fibroblasts. Arch. Oral Biol. 2015, 60, 12–22. [Google Scholar] [CrossRef]
- Jang, E.J.; Cha, S.M.; Choi, S.M.; Cha, J.D. Combination effects of baicalein with antibiotics against oral pathogens. Arch. Oral Biol. 2014, 59, 1233–1241. [Google Scholar] [CrossRef]
- Kwiecień, I.; Łukaszyk, A.; Miceli, N.; Taviano, M.F.; Davì, F.; Kędzia, E.; Ekiert, H. In Vitro Cultures of Scutellaria brevibracteata subsp. subvelutina as a Source of Bioactive Phenolic Metabolites. Molecules 2023, 28, 1785. [Google Scholar] [CrossRef]
- Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M. Functionalization with a polyphenol-rich pomace extract empowers a ceramic bone filler with in vitro antioxidant, anti-inflammatory, and pro-osteogenic properties. J. Funct. Biomater. 2021, 12, 31. [Google Scholar] [CrossRef]
- Vaillancourt, K.; Ben Lagha, A.; Grenier, D. Effects of a berry polyphenolic fraction on the pathogenic properties of Porphyromonas gingivalis. Front. Oral Health 2022, 3, 923663. [Google Scholar] [CrossRef]
- Zdarilová, A.; Rajnochová Svobodová, A.; Chytilová, K.; Simánek, V.; Ulrichová, J. Polyphenolic fraction of Lonicera caerulea L. Fruits reduces oxidative stress and inflammatory markers induced by lipopolysaccharide in gingival fibroblasts. Food Chem. Toxicol. 2010, 48, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Lagha, A.B.; Grenier, D. Tea polyphenols protect gingival keratinocytes against tnf-α-induced tight junction barrier dysfunction and attenuate the inflammatory response of monocytes/macrophages. Cytokine 2019, 115, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Farzanegan, A.; Shokuhian, M.; Jafari, S.; Shirazi, F.S.; Shahidi, M. Anti-histaminic effects of resveratrol and silymarin on human gingival fibroblasts. Inflammation 2019, 42, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Palaska, I.; Papathanasiou, E.; Theoharides, T.C. Use of polyphenols in periodontal inflammation. Eur. J. Pharmacol. 2013, 720, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Shoji, N.; Yoshida, A.; Yu, Z.; Endo, Y.; Sasano, T. Lipopolysaccharide stimulates histamine-forming enzyme (histidine decarboxylase) activity in murine dental pulp and gingiva. Arch. Oral Biol. 2006, 51, 856–860. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Dohle, E.; Ramanauskaite, A.; Al-Maawi, S.; Obreja, K.; Magini, R.; Sader, R.; Ghanaati, S.; Schwarz, F. Anti-inflammatory and macrophage polarization effects of cranberry proanthocyanidins (pacs) for periodontal and peri-implant disease therapy. J. Periodontal Res. 2020, 55, 821–829. [Google Scholar] [CrossRef]
- Tipton, D.A.; Hatten, A.A.; Babu, J.P.; Dabbous, M. Effect of glycated albumin and cranberry components on interleukin-6 and matrix metalloproteinase-3 production by human gingival fibroblasts. J. Periodontal Res. 2016, 51, 228–236. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Dudonné, S.; Desjardins, Y.; Grenier, D. Wild blueberry (Vaccinium angustifolium Ait.) polyphenols target Fusobacterium nucleatum and the host inflammatory response: Potential innovative molecules for treating periodontal diseases. J. Agric. Food Chem. 2015, 63, 6999–7008. [Google Scholar] [CrossRef]
- Tipton, D.A.; Carter, T.B.; Dabbous, M. Inhibition of interleukin 1β-stimulated interleukin-6 production by cranberry components in human gingival epithelial cells: Effects on nuclear factor κb and activator protein 1 activation pathways. J. Periodontal Res. 2014, 49, 437–447. [Google Scholar] [CrossRef]
- Tipton, D.A.; Cho, S.; Zacharia, N.; Dabbous, M.K. Inhibition of interleukin-17-stimulated interleukin-6 and -8 production by cranberry components in human gingival fibroblasts and epithelial cells. J. Periodontal Res. 2013, 48, 638–646. [Google Scholar] [CrossRef]
- Inaba, H.; Tagashira, M.; Kanda, T.; Murakami, Y.; Amano, A.; Matsumoto-Nakano, M. Apple- and hop-polyphenols inhibit Porphyromonas gingivalis-mediated precursor of matrix metalloproteinase-9 activation and invasion of oral squamous cell carcinoma cells. J. Periodontol. 2016, 87, 1103–1111. [Google Scholar] [CrossRef]
- Dal-Fabbro, R.; Cosme-Silva, L.; Rezende Silva Martins de Oliveira, F.; Capalbo, L.C.; Plazza, F.A.; Ervolino, E.; Cintra LT, A.; Gomes-Filho, J.E. Effect of red wine or its polyphenols on induced apical periodontitis in rats. Int. Endod. J. 2021, 54, 2276–2289. [Google Scholar] [CrossRef]
- Alkimavičienė, E.; Pušinskaitė, R.; Basevičienė, N.; Banienė, R.; Savickienė, N.; Pacauskienė, I.M. Efficacy of proanthocyanidins in nonsurgical periodontal therapy. Int. Dent. J. 2023, 73, 195–204. [Google Scholar] [CrossRef]
- Díaz Sánchez, R.M.; Castillo-Dalí, G.; Fernández-Olavarría, A.; Mosquera-Pérez, R.; Delgado-Muñoz, J.M.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. A prospective, double-blind, randomized, controlled clinical trial in the gingivitis prevention with an oligomeric proanthocyanidin nutritional supplement. Mediat. Inflamm. 2017, 2017, 7460780. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Yang, J.; Chi, Y.; Wen, P.; Wang, Z.; Yu, S.; Xue, R.; Fan, J.; Li, H.; Chen, W.; et al. Natural polyphenol self-assembled ph-responsive nanoparticles loaded into reversible hydrogel to inhibit oral bacterial activity. Mol. Biomed. 2022, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, S.; Li, Z.; Xu, J.; Liu, Y.; Luo, E. Coaxial tp/apr electrospun nanofibers for programmed controlling inflammation and promoting bone regeneration in periodontitis-related alveolar bone defect models. Mater. Today Bio. 2022, 16, 100438. [Google Scholar] [CrossRef]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M.; Russo, N. Polyphenols from grape pomace induce osteogenic differentiation in mesenchymal stem cells. Int. J. Mol. Med. 2020, 45, 1721–1734. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Howell, A.; Grenier, D. Cranberry proanthocyanidins neutralize the effects of aggregatibacter actinomycetemcomitans leukotoxin. Toxins 2019, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Haas, B.; Grenier, D. Tea polyphenols inhibit the growth and virulence properties of fusobacterium nucleatum. Sci. Rep. 2017, 7, 44815. [Google Scholar] [CrossRef] [PubMed]
- Ben Lagha, A.; Grenier, D. Black tea theaflavins attenuate porphyromonas gingivalis virulence properties, modulate gingival keratinocyte tight junction integrity and exert anti-inflammatory activity. J. Periodontal Res. 2017, 52, 458–470. [Google Scholar] [CrossRef]
- Bicak, D.A. A current approach to halitosis and oral malodor—A mini review. Open Dent. J. 2018, 12, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Veloso, D.J.; Abrão, F.; Martins, C.H.G.; Bronzato, J.D.; Gomes, B.; Higino, J.S.; Sampaio, F.C. Potential antibacterial and anti-halitosis activity of medicinal plants against oral bacteria. Arch. Oral Biol. 2020, 110, 104585. [Google Scholar] [CrossRef]
- Persson, S.; Edlund, M.B.; Claesson, R.; Carlsson, J. The formation of hydrogen sulfide and methyl mercaptan by oral bacteria. Oral Microbiol. Immunol. 1990, 5, 195–201. [Google Scholar] [CrossRef]
- Scully, C.; Greenman, J. Halitology (breath odour: Aetiopathogenesis and management). Oral Dis. 2012, 18, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Morin, M.P.; Bedran, T.B.; Fournier-Larente, J.; Haas, B.; Azelmat, J.; Grenier, D. Green tea extract and its major constituent epigallocatechin-3-gallate inhibit growth and halitosis-related properties of Solobacterium moorei. BMC Complement. Altern. Med. 2015, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shen, H.; Wang, F.; Zhou, X.; Zhao, P.; Yang, Y.; Guo, Y. Thinned-young apple polyphenols inhibit halitosis-related bacteria through damage to the cell membrane. Front. Microbiol. 2021, 12, 745100. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.; Hao, J.; Zhang, Y.; Huang, R. Tea polyphenols: Application in the control of oral microorganism infectious diseases. Arch. Oral Biol. 2019, 102, 74–82. [Google Scholar] [CrossRef]
- Rassameemasmaung, S.; Phusudsawang, P.; Sangalungkarn, V. Effect of green tea mouthwash on oral malodor. ISRN Prev. Med. 2013, 2013, 975148. [Google Scholar] [CrossRef]
- Lodhia, P.; Yaegaki, K.; Khakbaznejad, A.; Imai, T.; Sato, T.; Tanaka, T.; Murata, T.; Kamoda, T. Effect of green tea on volatile sulfur compounds in mouth air. J. Nutr. Sci. Vitaminol. 2008, 54, 89–94. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Chen, Y.J.; Lin, S.C.; Kao, T.; Chang, C.S.; Hong, P.S.; Shieh, T.M.; Chang, K.W. Genome-wide profiling of oral squamous cell carcinoma. J. Pathol. 2004, 204, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.; Wagner, V.P.; Sant’ana Filho, M.; Carrard, V.C.; Hugo, F.N.; Martins, M.D. A 10-year analysis of the oral squamous cell carcinoma profile in patients from public health centers in uruguay. Braz. Oral Res. 2015, 29, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jimi, E.; Furuta, H.; Matsuo, K.; Tominaga, K.; Takahashi, T.; Nakanishi, O. The cellular and molecular mechanisms of bone invasion by oral squamous cell carcinoma. Oral Dis. 2011, 17, 462–468. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, P.N.; Chu, S.C.; Lin, C.Y.; Kuo, W.H.; Hsieh, Y.S. Black tea polyphenols reverse epithelial-to-mesenchymal transition and suppress cancer invasion and proteases in human oral cancer cells. J. Agric. Food Chem. 2012, 60, 8395–8403. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, K.; Phippen, S.; McCabe, J.; Teeters, C.A.; O’Malley, S.; Kingsley, K. Cranberry and grape seed extracts inhibit the proliferative phenotype of oral squamous cell carcinomas. Evid.-Based Complement. Altern. Med. 2011, 2011, 467691. [Google Scholar] [CrossRef]
- Mohan, K.V.; Gunasekaran, P.; Varalakshmi, E.; Hara, Y.; Nagini, S. In vitro evaluation of the anticancer effect of lactoferrin and tea polyphenol combination on oral carcinoma cells. Cell Biol. Int. 2007, 31, 599–608. [Google Scholar] [CrossRef]
- Letchoumy, P.V.; Mohan, K.V.; Prathiba, D.; Hara, Y.; Nagini, S. Comparative evaluation of antiproliferative, antiangiogenic and apoptosis inducing potential of black tea polyphenols in the hamster buccal pouch carcinogenesis model. J. Carcinog. 2007, 6, 19. [Google Scholar] [CrossRef]
- Srinivasan, P.; Suchalatha, S.; Babu, P.V.; Devi, R.S.; Narayan, S.; Sabitha, K.E.; Shyamala Devi, C.S. Chemopreventive and therapeutic modulation of green tea polyphenols on drug metabolizing enzymes in 4-nitroquinoline 1-oxide induced oral cancer. Chem.-Biol. Interact. 2008, 172, 224–234. [Google Scholar] [CrossRef]
- Sharma, Y.; Kaur, A.; Mishra, R.; Kulkarni, S.; Bharadwaj, M.; Bala, K. Antiproliferative efficacy of the antioxidant bioactive compounds of defatted seeds of azadirachta indica and momordica charantia against the regulatory function of tumor suppressor gene inducing oral carcinoma. J. Biomol. Struct. Dyn. 2023, 41, 5246–5260. [Google Scholar] [CrossRef]
- King, M.; Chatelain, K.; Farris, D.; Jensen, D.; Pickup, J.; Swapp, A.; O’Malley, S.; Kingsley, K. Oral squamous cell carcinoma proliferative phenotype is modulated by proanthocyanidins: A potential prevention and treatment alternative for oral cancer. BMC Complement. Altern. Med. 2007, 7, 22. [Google Scholar] [CrossRef]
- Kingsley, K.; Jensen, D.; Toponce, R.; Dye, J.; Martin, D.; Phippen, S.; Ross, D.; Halthore, V.S.; O’Malley, S. Inhibition of oral cancer growth in vitro is modulated through differential signaling pathways by over-the-counter proanthocyanidin supplements. J. Diet. Suppl. 2010, 7, 130–144. [Google Scholar] [CrossRef]
- Fan, M.J.; Wang, I.C.; Hsiao, Y.T.; Lin, H.Y.; Tang, N.Y.; Hung, T.C.; Quan, C.; Lien, J.C.; Chung, J.G. Anthocyanins from black rice (Oryza sativa L.) demonstrate antimetastatic properties by reducing mmps and nf-κb expressions in human oral cancer cal 27 cells. Nutr. Cancer 2015, 67, 327–338. [Google Scholar] [CrossRef]
- Ho, Y.C.; Yang, S.F.; Peng, C.Y.; Chou, M.Y.; Chang, Y.C. Epigallocatechin-3-gallate inhibits the invasion of human oral cancer cells and decreases the productions of matrix metalloproteinases and urokinase-plasminogen activator. J. Oral Pathol. Med. 2007, 36, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.N.; Chu, S.C.; Kuo, W.H.; Chou, M.Y.; Lin, J.K.; Hsieh, Y.S. Epigallocatechin-3 gallate inhibits invasion, epithelial-mesenchymal transition, and tumor growth in oral cancer cells. J. Agric. Food Chem. 2011, 59, 3836–3844. [Google Scholar] [CrossRef]
- Basak, S.K.; Bera, A.; Yoon, A.J.; Morselli, M.; Jeong, C.; Tosevska, A.; Dong, T.S.; Eklund, M.; Russ, E.; Nasser, H.; et al. A randomized, phase 1, placebo-controlled trial of apg-157 in oral cancer demonstrates systemic absorption and an inhibitory effect on cytokines and tumor-associated microbes. Cancer 2020, 126, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, V.K.; Gangar, J.; Shai, S.; Rane, P.; Mohanta, S.K.; Kannan, S.; Ingle, A.; Mittal, N.; Rane, S.; Mahimkar, M.B. Prevention of carcinogen-induced oral cancers by polymeric black tea polyphenols via modulation of egfr-akt-mtor pathway. Sci. Rep. 2022, 12, 14516. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Wee, Y.; Luo, S.D.; Chang, S.F.; Jia, S.; Feng, S.W.; Huang, H.M.; Lin, J.H.; Wang, C.S. Proanthocyanidins-loaded complex coacervates-based drug delivery attenuates oral squamous cell carcinoma cells metastatic potential through down-regulating the akt signaling pathway. Front. Oncol. 2022, 12, 1001126. [Google Scholar] [CrossRef]
- Sheng, H.; Ogawa, T.; Niwano, Y.; Sasaki, K.; Tachibana, K. Effects of polyphenols on doxorubicin-induced oral keratinocyte cytotoxicity and anticancer potency against oral cancer cells. J. Oral Pathol. Med. 2018, 47, 368–374. [Google Scholar] [CrossRef]
- Huang, Z.; Delparastan, P.; Burch, P.; Cheng, J.; Cao, Y.; Messersmith, P.B. Injectable dynamic covalent hydrogels of boronic acid polymers cross-linked by bioactive plant-derived polyphenols. Biomater. Sci. 2018, 6, 2487–2495. [Google Scholar] [CrossRef]
- Vidjaya Letchoumy, P.; Chandra Mohan, K.V.; Stegeman, J.J.; Gelboin, H.V.; Hara, Y.; Nagini, S. Pretreatment with black tea polyphenols modulates xenobiotic-metabolizing enzymes in an experimental oral carcinogenesis model. Oncol. Res. 2008, 17, 75–85. [Google Scholar] [CrossRef]
- Huang, M.; Huang, Y.; Liu, H.; Tang, Z.; Chen, Y.; Huang, Z.; Xu, S.; Du, J.; Jia, B. Hydrogels for the treatment of oral and maxillofacial diseases: Current research, challenges, and future directions. Biomater. Sci. 2022, 10, 6413–6446. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.H.; Paliwal, S.; Muttigi, M.S.; Seetharam, R.N.; Prasad AS, B.; Nayak, Y.; Acharya, S.; Nayak, U.Y. Polyphenol-based targeted therapy for oral submucous fibrosis. Inflammopharmacology 2023, 31, 2349–2368. [Google Scholar] [CrossRef]
- Xing, M.; Fu, R.; Liu, Y.; Wang, P.; Ma, P.; Zhu, C.; Fan, D. Human-like collagen promotes the healing of acetic acid-induced gastric ulcers in rats by regulating nos and growth factors. Food Funct. 2020, 11, 4123–4137. [Google Scholar] [CrossRef]
- Mao, Y.; Hu, M.; Chen, L.; Chen, X.; Liu, M.; Zhang, M.; Nie, M.; Liu, X. Cgf-hlc-i repaired the bone defect repair of the rabbits mandible through tight junction pathway. Front. Bioeng. Biotechnol. 2022, 10, 976499. [Google Scholar] [CrossRef]
- Lombardo Bedran, T.B.; Feghali, K.; Zhao, L.; Palomari Spolidorio, D.M.; Grenier, D. Green tea extract and its major constituent, epigallocatechin-3-gallate, induce epithelial beta-defensin secretion and prevent beta-defensin degradation by porphyromonas gingivalis. J. Periodontal Res. 2014, 49, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Lombardo Bedran, T.B.; Morin, M.P.; Palomari Spolidorio, D.; Grenier, D. Black tea extract and its theaflavin derivatives inhibit the growth of periodontopathogens and modulate interleukin-8 and β-defensin secretion in oral epithelial cells. PLoS ONE 2015, 10, e0143158. [Google Scholar] [CrossRef]
- Ravagnan, G.; De Filippis, A.; Cartenì, M.; De Maria, S.; Cozza, V.; Petrazzuolo, M.; Tufano, M.A.; Donnarumma, G. Polydatin, a natural precursor of resveratrol, induces β-defensin production and reduces inflammatory response. Inflammation 2013, 36, 26–34. [Google Scholar] [CrossRef]
- Liu, J.K. Antiaging agents: Safe interventions to slow aging and healthy life span extension. Nat. Prod. Bioprospecting 2022, 12, 18. [Google Scholar] [CrossRef]
- Schestakow, A.; Meyer-Probst, C.T.; Hannig, C.; Hannig, M. Prevention of dental biofilm formation with polyphenols: A systematic review. Planta Med. 2023, 89, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
| Polyphenols | Subclasses and Examples | Food Sources |
|---|---|---|
| Lignans | e.g., secoisolariciresinol, matairesinol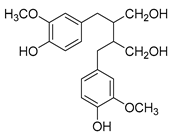 (Secoisolariciresino) | Linseed, lentils, garlic, asparagus, carrots, pears, and prunes. |
| Stibenes | e.g., resveratrol, trans-resveratrol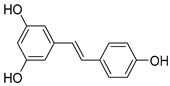 (Resveratrol) | Grapes, pomegranates, and groundnuts. |
| Phenolic acids | (A) Hydrobenzoic acids: protocatechuic acid, gallic acid, p-hydroxybenzoic acid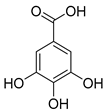 (gallic acid) | Blackberries, raspberries, strawberries, grapes, and black currants. |
(B) Hydroxycinic acids: caffeic acid, chlorogenic acid, coumaric acid, ferulic acid, sinapic acid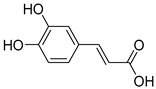 (caffeic acid) | Blueberries, kiwis, cherries, plums, apples, pears, peaches, chicories, artichokes, potatoes, and coffee. | |
| Flavonoids | Anthocyanins: malvidin, cyanidin, pelargonidin, peonidin, delphinidin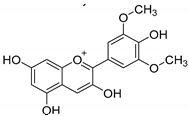 (malvidin) | Red, blue, and purple berries, red and purple grapes, aubergines, red cabbages, rhubarbs, red wine, and cherries. |
Flavonols: quercitin, kaempferol, mycricetin (quercitin) | Leeks, gingers, broccoli, red cabbages, yellow onions, cherries, tomatoes, blueberries, apricots, apples, black grapes, and teas. | |
Flavanones: hesperidin, naringenin, erioclictyol (Naringenin) | Citrus fruits (oranges, grapes, and lemons) and their juices. | |
Flavones: apigenin, luteolin (Apigenin) | Oregano, celery, thyme, parsley, capsicums, and pepper. | |
Isoflavonones: daidzein, genistein, glycitein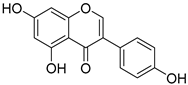 (Genistein) | Milk, tofu, soybeans, tempeh, miso, and legumes. | |
| Monomeric flavanols: catechin, epicatechin, epigallocatechin, epigallocatechin gallate Polymeric flavanols: theaflavins, thearubigins  (catechin) | Grapes, chocolate, red wine cocoa, berries, apples, apricots, black beans, green and black teas. | |
Proanthocyanidins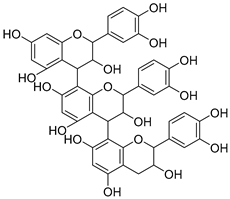 (Proanthocyanidins) | Chocolate, apples, berries, red grapes, and red wine. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Li, Z.; Chen, F.; Chai, Y. Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications. Nutrients 2023, 15, 4384. https://doi.org/10.3390/nu15204384
Guo Y, Li Z, Chen F, Chai Y. Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications. Nutrients. 2023; 15(20):4384. https://doi.org/10.3390/nu15204384
Chicago/Turabian StyleGuo, Yuanyuan, Zhiquan Li, Feng Chen, and Yujuan Chai. 2023. "Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications" Nutrients 15, no. 20: 4384. https://doi.org/10.3390/nu15204384
APA StyleGuo, Y., Li, Z., Chen, F., & Chai, Y. (2023). Polyphenols in Oral Health: Homeostasis Maintenance, Disease Prevention, and Therapeutic Applications. Nutrients, 15(20), 4384. https://doi.org/10.3390/nu15204384







