Gastrointestinal Fermentable Polysaccharide Is Beneficial in Alleviating Loperamide-Induced Constipation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Polysaccharide Fractions from SDF-S
2.3. Characterization of SSPs
2.3.1. Chemical Composition
2.3.2. Monosaccharide Composition
2.3.3. Molecular Weight
2.3.4. Fourier Transform Infrared Spectroscopy
2.4. Animals and Experimental Design
2.4.1. GI Transit Test
2.4.2. Defecation Test
2.5. Histological Observation
2.6. Assessment of Serum GI Hormones
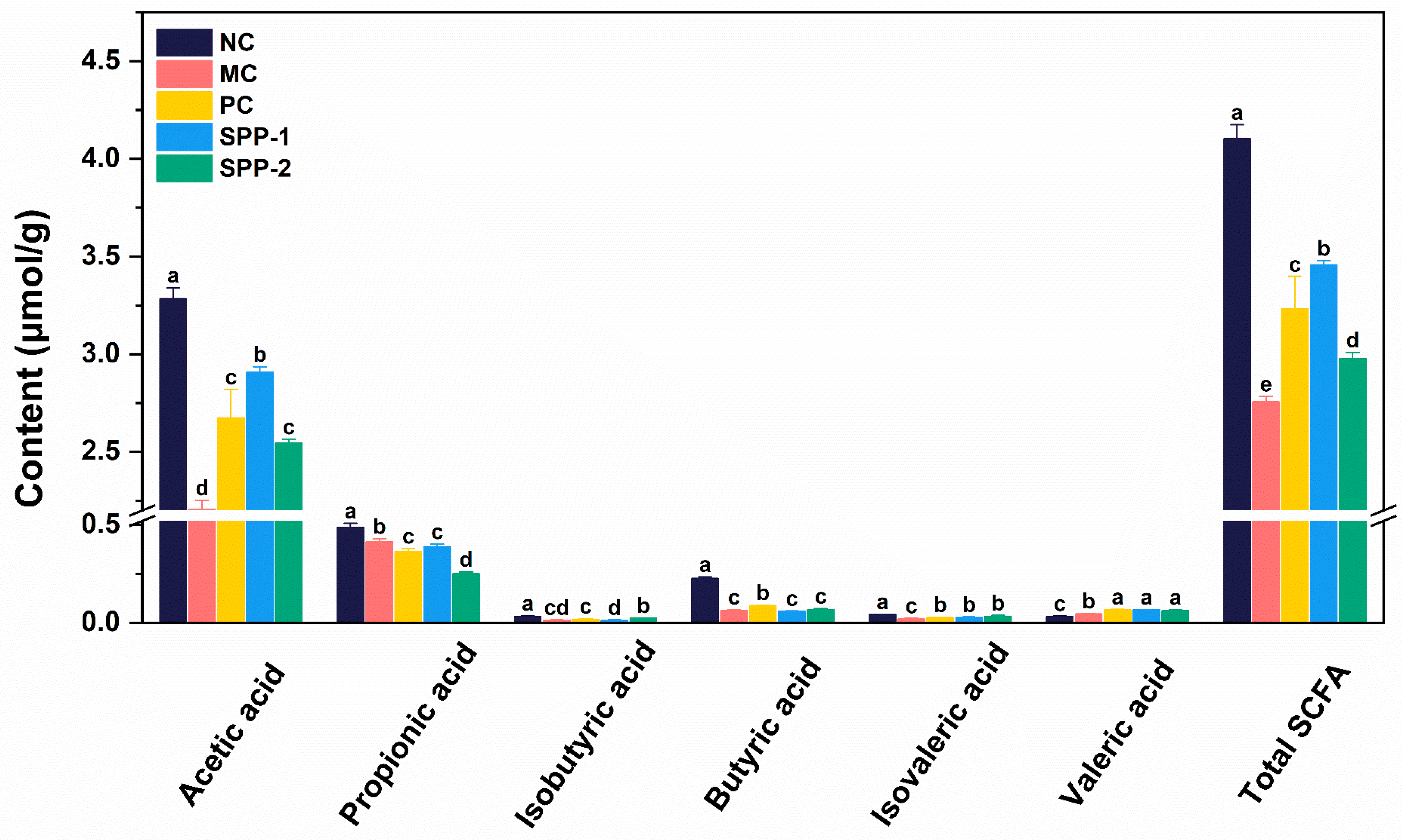
2.7. Assessment of Fecal SCFA profile
2.8. Bioinformatic Analysis Based on 16S rDNA Sequencing
2.9. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characterization of SSPs
3.2. Effects of SSPs on GI Transit and Defecation Function
3.3. Effects of SSPs on Intestinal Histological Morphology
3.4. Effects of SSPs on GI Hormone Levels in Serum
3.5. Effects of SSPs on Fecal SCFA Profile
3.6. Effects of SSPs on the Gut Microbiota
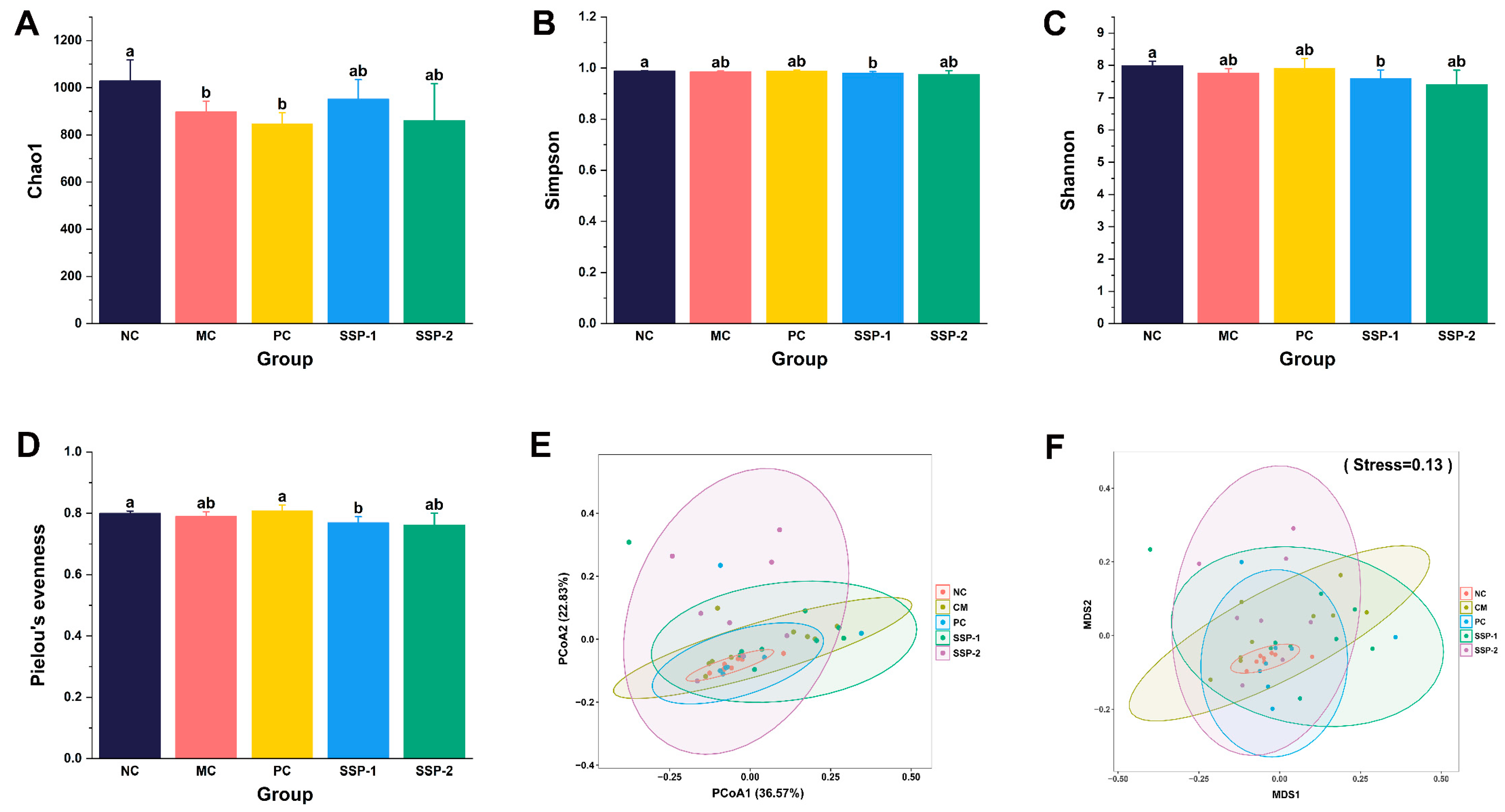
3.6.1. Effects of SSPs on the Gut Microbial Diversity
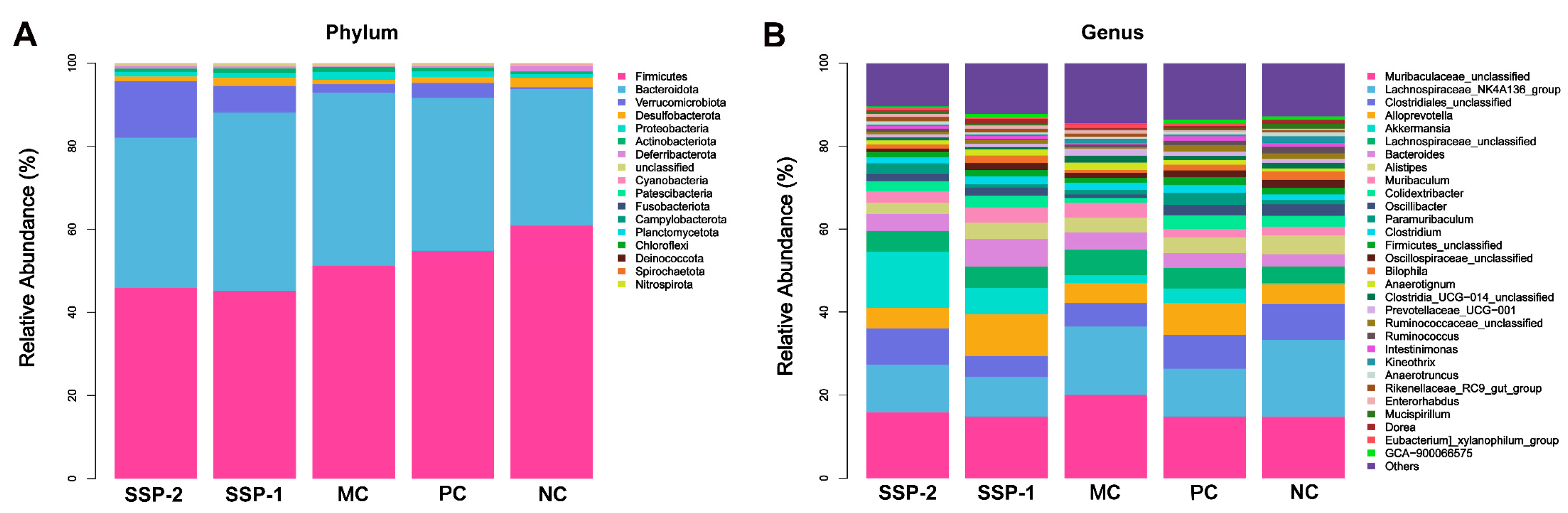
3.6.2. Effects of SSPs on Gut Microbiota Community Structure
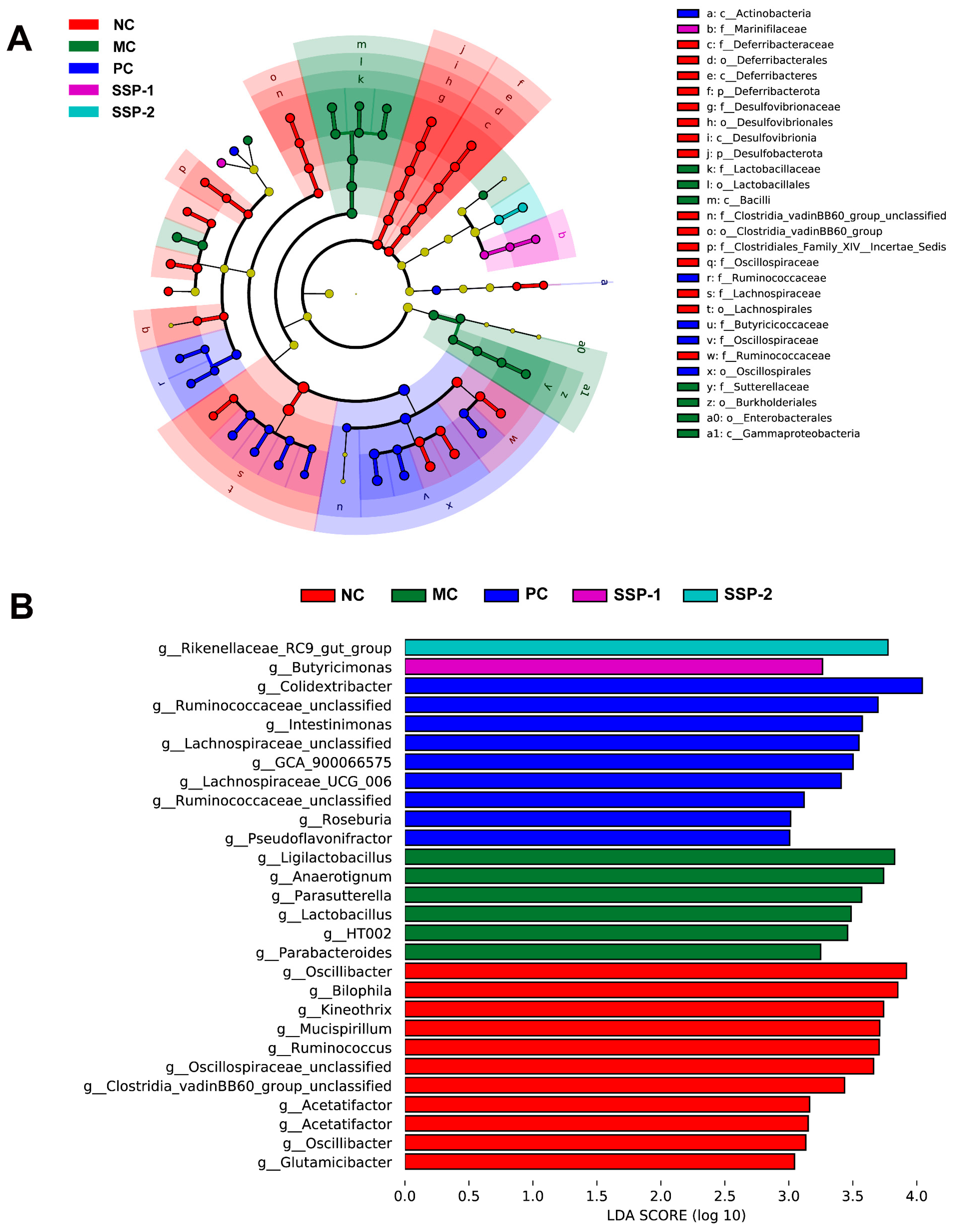
3.6.3. Change in Key Phylotypes of the Gut Microbiota
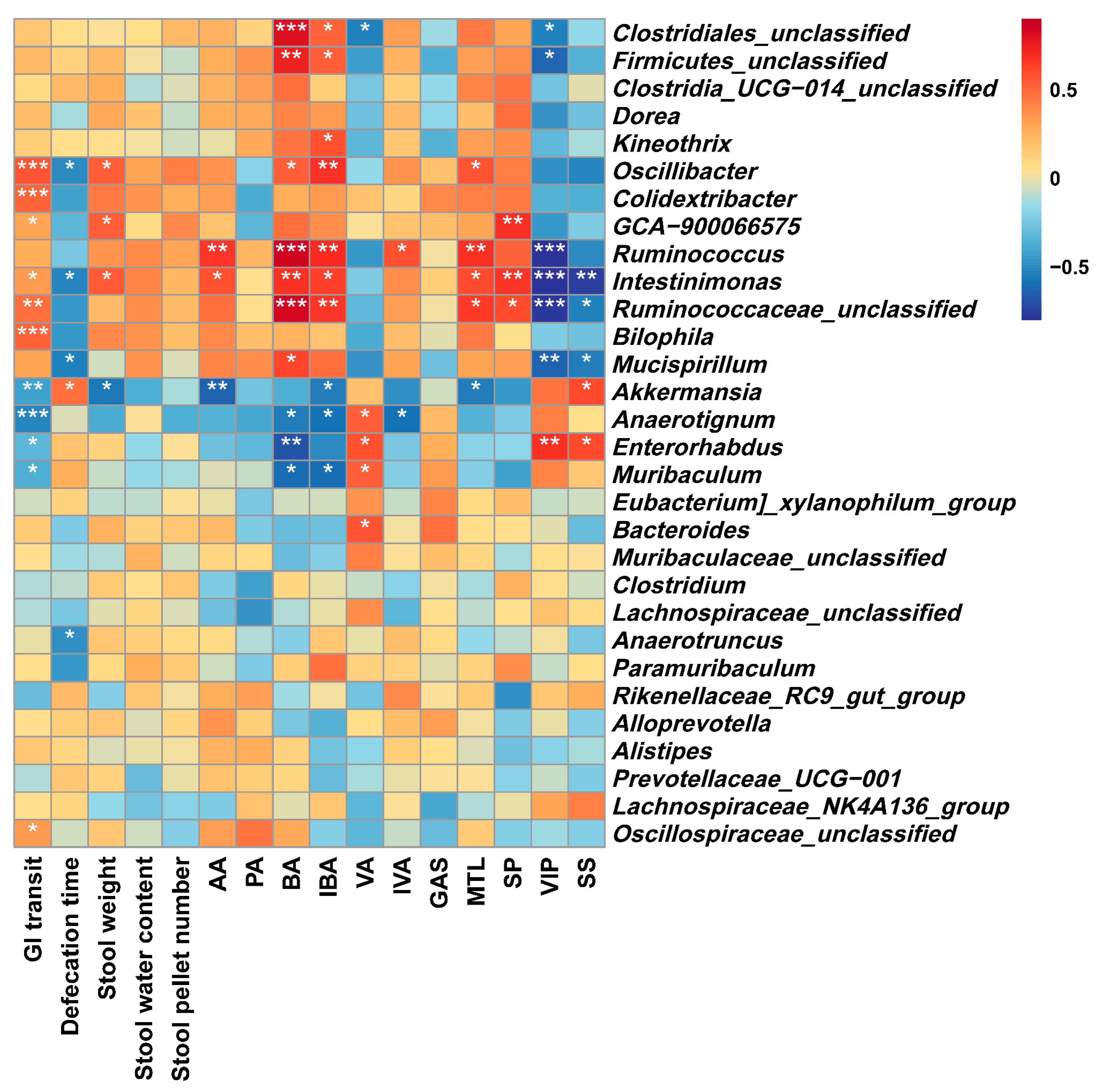
3.6.4. Correlations between the Gut Microbiota and Constipation-Related Biological Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. The Pre-Experimental Design of the Effective Dose of SDF-S in Alleviating Constipation in Mice
Appendix A.1. Methods
Appendix A.2. Results
| Group | Body Weight-0 d (g) | Body Weight-14 d (g) | Daily Feed Intake (g) | Gastrointestinal Transit Rate (%) |
|---|---|---|---|---|
| NC | 30.98 ± 1.09 a | 34.11 ± 1.49 a | 4.27 ± 0.31 a | 75.06 ± 5.32 a |
| MC | 30.75 ± 1.47 a | 33.45 ± 2.42 ab | 3.64 ± 0.35 bc | 33.87 ± 8.46 d |
| PC | 30.64 ± 1.89 a | 33.28 ± 1.79 abc | 4.05 ± 0.33 a | 49.36 ± 9.45 b |
| LD | 30.04 ± 2.74 a | 31.28 ± 2.29 c | 3.44 ± 0.34 c | 37.02 ± 11.05 d |
| MD | 29.60 ± 1.28 a | 31.39 ± 1.79 bc | 3.76 ± 0.26 b | 40.58 ± 8.13 cd |
| HD | 30.49 ± 1.88 a | 32.00 ± 2.36 bc | 4.08 ± 0.31 a | 47.71 ± 8.65 bc |
Appendix B. Characterization of SDF-S
| Item | SDF-S |
|---|---|
| Neutral carbohydrate (%) | 59.31 ± 0.73 |
| Uronic acid (%) | 25.36 ± 0.48 |
| Total carbohydrate (%) | 84.68 ± 0.64 |
| Iodine reactions | Negative |
| Mw (kDa) | 5.32 |
| Mw/Mn | 1.605 |
| Monosaccharide composition (mol%) | |
| Glc | 91.86 |
| Gal | 3.15 |
| Ara | 1.07 |
| Rha | 0.65 |
| Man | 0.42 |
| Rib | 0.11 |
| Fuc | 0.13 |
| GlcA | 0.14 |
| GalA | 2.48 |
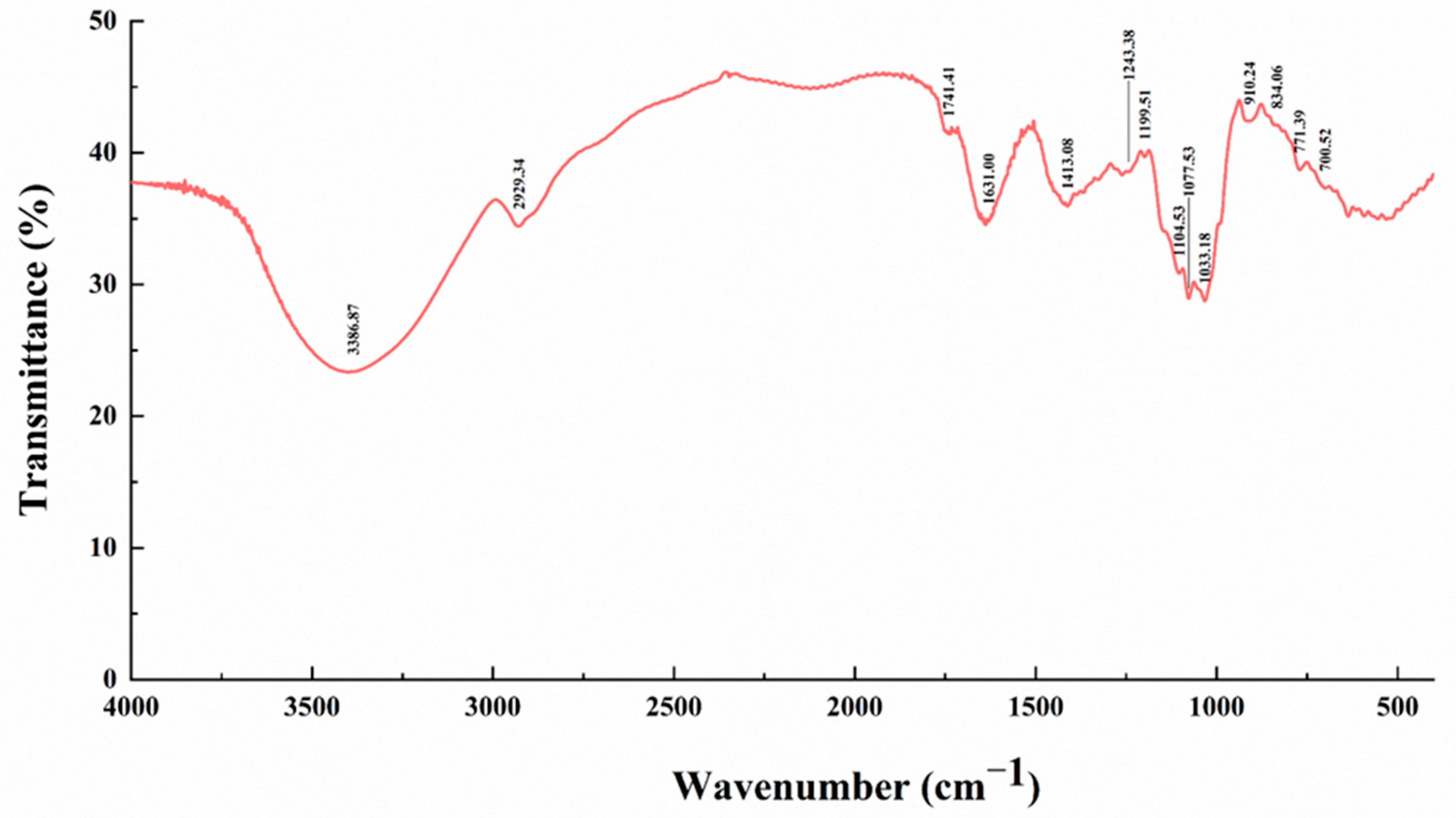
References
- Lacy, B.E.; Mearin, F.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.e5. [Google Scholar] [CrossRef]
- Talley, N.J.; Lasch, K.L.; Baum, C.L. A Gap in Our Understanding: Chronic Constipation and Its Comorbid Conditions. Clin. Gastroenterol. Hepatol. 2009, 7, 9–19. [Google Scholar] [CrossRef]
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global Prevalence of Functional Constipation According to the Rome Criteria: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 638–648. [Google Scholar] [CrossRef]
- Włodarczyk, J.; Waśniewska, A.; Fichna, J.; Dziki, A.; Dziki, Ł.; Włodarczyk, M. Current Overview on Clinical Management of Chronic Constipation. J. Clin. Med. 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Ford, A.C.; Mawe, G.M.; Dinning, P.G.; Rao, S.S.; Chey, W.D.; Simrén, M.; Lembo, A.; Young-Fadok, T.M.; Chang, L. Chronic Constipation. Nat. Rev. Dis. Primer 2017, 3, 17095. [Google Scholar] [CrossRef]
- Sharma, A.; Rao, S. Constipation: Pathophysiology and Current Therapeutic Approaches. In Gastrointestinal Pharmacology; Greenwood-Van Meerveld, B., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2017; pp. 59–74. ISBN 978-3-319-56360-2. [Google Scholar]
- Vriesman, M.H.; Koppen, I.J.N.; Camilleri, M.; Di Lorenzo, C.; Benninga, M.A. Management of Functional Constipation in Children and Adults. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 21–39. [Google Scholar] [CrossRef]
- Jones, J.M. CODEX-Aligned Dietary Fiber Definitions Help to Bridge the ‘Fiber Gap’. Nutr. J. 2014, 13, 34. [Google Scholar] [CrossRef]
- Huang, J.; Lin, B.; Zhang, Y.; Xie, Z.; Zheng, Y.; Wang, Q.; Xiao, H. Bamboo Shavings Derived O-Acetylated Xylan Alleviates Loperamide-Induced Constipation in Mice. Carbohydr. Polym. 2022, 276, 118761. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, Y.; Zeng, S.; Zheng, Y.; Wang, H.; Liao, H.; Song, J.; Zhang, X.; Cao, J.; Li, C. Polysaccharides from Holothuria Leucospilota Relieve Loperamide-Induced Constipation Symptoms in Mice. Int. J. Mol. Sci. 2023, 24, 2553. [Google Scholar] [CrossRef]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina Platensis Ameliorates Diphenoxylate-Induced Constipation Symptoms in Mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef]
- Yang, H.; Wu, C.; Chen, L.; Chang, X.; Luo, G.; Wu, K.; Tian, W.A. Macrocephala Polysaccharide Induces Alterations to Gut Microbiome and Serum Metabolome in Constipated Mice. Microb. Pathog. 2023, 178, 106084. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Song, J.; Zhai, X.; Cheng, N.; Xu, C.; Gui, S.; Chen, J. Improvement of Loperamide-Hydrochloride-Induced Intestinal Motility Disturbance by Platycodon Grandiflorum Polysaccharides through Effects on Gut Microbes and Colonic Serotonin. Front. Cell. Infect. Microbiol. 2023, 13, 1105272. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Yang, S.; Ma, Y.; Gong, Z.; Cui, W.; Wang, Y.; Wang, W. Extraction Optimization and Constipation-Relieving Activity of Dietary Fiber from Auricularia polytricha. Food Biosci. 2020, 33, 100506. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic Acid and Butyric Acid Released in Large Intestine Play Different Roles in the Alleviation of Constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Alhabeeb, H.; AlFaiz, A.; Kutbi, E.; AlShahrani, D.; Alsuhail, A.; AlRajhi, S.; Alotaibi, N.; Alotaibi, K.; AlAmri, S.; Alghamdi, S.; et al. Gut Hormones in Health and Obesity: The Upcoming Role of Short Chain Fatty Acids. Nutrients 2021, 13, 481. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Goff, H.D.; Zhang, W.; Zhong, F. The Effect of Viscous Soluble Dietary Fiber on Nutrient Digestion and Metabolic Responses II: In Vivo Digestion Process. Food Hydrocoll. 2020, 107, 105908. [Google Scholar] [CrossRef]
- Lan, J.; Wang, K.; Chen, G.; Cao, G.; Yang, C. Effects of Inulin and Isomalto-Oligosaccharide on Diphenoxylate-Induced Constipation, Gastrointestinal Motility-Related Hormones, Short-Chain Fatty Acids, and the Intestinal Flora in Rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture Food Data Central. Available online: https://fdc.nal.usda.gov/ (accessed on 26 June 2023).
- Qin, Y.; Naumovski, N.; Ranadheera, C.S.; D’Cunha, N.M. Nutrition-Related Health Outcomes of Sweet Potato (Ipomoea batatas) Consumption: A Systematic Review. Food Biosci. 2022, 50, 102208. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Liu, X.; Hu, W.; Zhang, C.; Guo, Y.; Wu, W. Effects of Steaming on Sweet Potato Soluble Dietary Fiber: Content, Structure, and Lactobacillus Proliferation In Vitro. Foods 2023, 12, 1620. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, M.C.C.; Carpita, C. Measurement of Uranic Acids without Interference from Neutral Sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Qu, C.; Lin, G.; Zhang, Z.; Xie, J.; Chen, H.; Liang, J.; Li, C.; Wang, H.; Su, Z. Character and Laxative Activity of Polysaccharides Isolated from Dendrobium Officinale. J. Funct. Foods 2017, 34, 106–117. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Xu, Q.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria Exert Species-Specific Effects on Constipation in BALB/c Mice. Food Funct. 2017, 8, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sui, D.; Fu, W.; Yu, X.; Li, Y.; Wu, X.; Hou, Y.; Guo, M.; Xu, H. Laxative Effects of Yangyin Tongmi Capsule on a Model of Diphenoxylate-Induced Constipation in Mice. Evid. Based Complement. Alternat. Med. 2020, 2020, 1471824. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology; Day, C.E., Ed.; Methods in Molecular Biology; Springer New York: New York, NY, USA, 2014; Volume 1180, pp. 31–43. ISBN 978-1-4939-1049-6. [Google Scholar]
- Zhao, Z.; Liu, W.; Pi, X. In Vitro Effects of Stachyose on the Human Gut Microbiota. Starch-Stärke 2021, 73, 2100029. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Chen, M.; Gu, J.; Zhou, J.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant Activities of Sagittaria sagittifolia L. Polysaccharides with Subcritical Water Extraction. Int. J. Biol. Macromol. 2019, 134, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.-J.; Hong, T.; Shi, H.-F.; Yin, J.-Y.; Koev, T.; Nie, S.-P.; Gilbert, R.G.; Xie, M.-Y. Probiotic Fermentation Modifies the Structures of Pectic Polysaccharides from Carrot Pulp. Carbohydr. Polym. 2021, 251, 117116. [Google Scholar] [CrossRef]
- Mu, T.; Sun, H.; Zhang, M.; Wang, C. (Eds.) Chapter 4—Sweet Potato Pectin. In Sweet Potato Processing Technology; Academic Press: Cambridge, MA, USA, 2017; pp. 183–261. ISBN 978-0-12-812871-8. [Google Scholar]
- He, Y.; Liu, G.; Xia, C.; Chen, J.; Zhao, J.; Li, X.; Deng, J.; Wang, X.; Xiang, Z.; Zeng, P. Laxative Effect of Mulberry Ferment on Two Models of Constipated Mice. J. Funct. Foods 2022, 90, 104971. [Google Scholar] [CrossRef]
- Deloose, E.; Verbeure, W.; Depoortere, I.; Tack, J. Motilin: From Gastric Motility Stimulation to Hunger Signalling. Nat. Rev. Endocrinol. 2019, 15, 238–250. [Google Scholar] [CrossRef]
- Guilloteau, P.; Le Meuth-Metzinger, V.; Morisset, J.; Zabielski, R. Gastrin, Cholecystokinin and Gastrointestinal Tract Functions in Mammals. Nutr. Res. Rev. 2006, 19, 254–283. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef] [PubMed]
- Yik, Y.I.; Farmer, P.J.; King, S.K.; Chow, C.W.; Hutson, J.M.; Southwell, B.R. Gender Differences in Reduced Substance P (SP) in Children with Slow-Transit Constipation. Pediatr. Surg. Int. 2011, 27, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Weckbecker, G.; Lewis, I.; Albert, R.; Schmid, H.A.; Hoyer, D.; Bruns, C. Opportunities in Somatostatin Research: Biological, Chemical and Therapeutic Aspects. Nat. Rev. Drug Discov. 2003, 2, 999–1017. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent Advances in Vasoactive Intestinal Peptide Physiology and Pathophysiology: Focus on the Gastrointestinal System. F1000Research 2019, 8, 1629. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.; Ma, Q.; Strappe, P.; Zhou, Z. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, 1801187. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Kim, C.C.; Kelly, W.J.; Patchett, M.L.; Tannock, G.W.; Jordens, Z.; Stoklosinski, H.M.; Taylor, J.W.; Sims, I.M.; Bell, T.J.; Rosendale, D.I. Monoglobus pectinilyticus Gen. Nov., sp. Nov., a Pectinolytic Bacterium Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2017, 67, 4992–4998. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, F.; Wang, Z.; Cao, J.; Li, C. Effect and Mechanism of Functional Compound Fruit Drink on Gut Microbiota in Constipation Mice. Food Chem. 2023, 401, 134210. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 6, 19. [Google Scholar] [CrossRef]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and Related Bacteria—From Metagenomic Species to Metabolic Features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sun, X.; Liu, X.; Yang, Y.; Li, Z. Chapter Seven—Autoimmune Diseases in China. In Advances in Immunology; Dong, C., Jiang, Z., Eds.; Advances in Immunology in China—Part A; Academic Press: Cambridge, MA, USA, 2019; Volume 144, pp. 173–216. [Google Scholar]
- Lu, W.; Li, J.; Gong, L.; Xu, X.; Han, T.; Ye, Y.; Che, T.; Luo, Y.; Li, J.; Zhan, R.; et al. H2S Modulates Duodenal Motility in Male Rats via Activating TRPV1 and KATP Channels. Br. J. Pharmacol. 2014, 171, 1534–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, X.; Deng, N.; Peng, X.; Tan, Z. Diarrhea with Deficiency Kidney-Yang Syndrome Caused by Adenine Combined with Folium senna Was Associated with Gut Mucosal Microbiota. Front. Microbiol. 2022, 13, 1007609. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, M.; Brown, S.; Chiarioni, G.; Dimidi, E.; Dudding, T.; Emmanuel, A.; Fox, M.; Ford, A.C.; Giordano, P.; Grossi, U.; et al. Chronic Constipation in Adults: Contemporary Perspectives and Clinical Challenges. 2: Conservative, Behavioural, Medical and Surgical Treatment. Neurogastroenterol. Motil. 2021, 33, e14070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan Oligosaccharides Attenuate Loperamide-Induced Constipation through Regulation of Gut Microbiota in Mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wei, X.; Deng, J.; Peng, C.; Ren, R.; Luo, Y.; Zhang, J.; Wei, X.; Hardiman, G.; Sun, Y.; et al. Integrating Omics and Network Pharmacology Reveals the Anti-Constipation Role of Chitosan with Different Molecular Weights in Constipated Mice. Int. J. Biol. Macromol. 2023, 235, 123930. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and Diet-Responsive Groups of Bacteria within the Human Colonic Microbiota. ISME J. 2011, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Zhu, H.; Qian, X.; Chen, Y.; Wang, Z.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Consumption of Butylated Starch Alleviates the Chronic Restraint Stress-Induced Neurobehavioral and Gut Barrier Deficits Through Reshaping the Gut Microbiota. Front. Immunol. 2021, 12, 755481. [Google Scholar] [CrossRef]
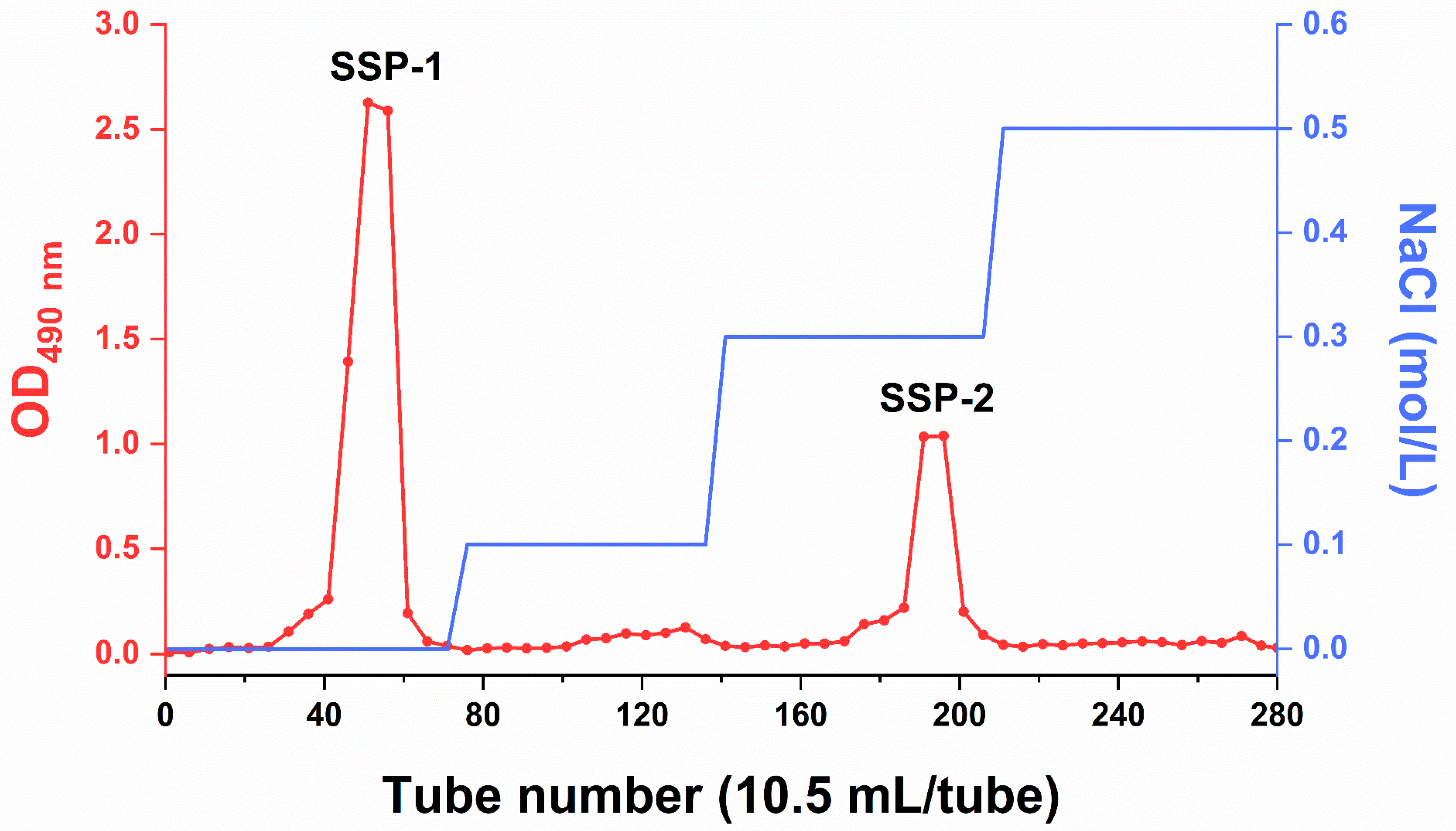
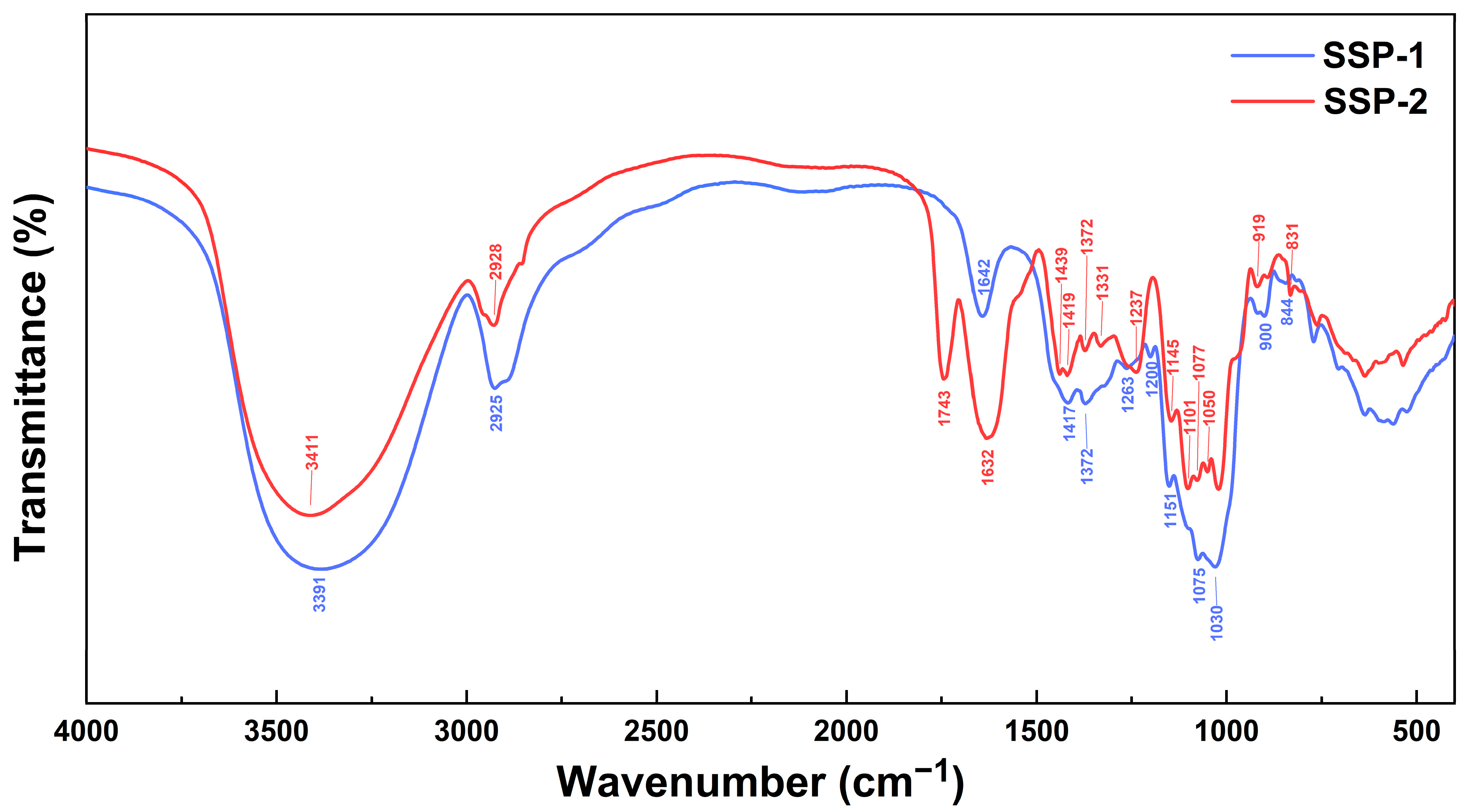



| Items | SSP-1 | SSP-2 |
|---|---|---|
| Yield (%) | 61.02 ± 4.62 a | 12.88 ± 0.74 b |
| Neutral carbohydrate (%) | 81.79 ± 0.87 a | 34.63 ± 1.29 b |
| Uronic acid (%) | 3.75 ± 0.32 b | 63.85 ± 0.42 a |
| Monosaccharide (mol%) | ||
| D-Glucose | 99.29 | 11.34 |
| D-Galactose | 0.42 | 26.25 |
| D-Mannose | 0.19 | 4.25 |
| L-Arabinose | 0.08 | 8.57 |
| L-Rhamnose | 0.02 | 9.70 |
| D-Ribose | 0.01 | 0.29 |
| L-Fucose | N.D. | 0.66 |
| D-Galacturonic acid | N.D. | 35.92 |
| D-Glucuronic acid | N.D. | 3.02 |
| Mw (kDa) | 2.04 | 41.66 |
| Mw/Mn | 1.24 | 1.09 |
| Index | Group | ||||
|---|---|---|---|---|---|
| NC | MC | PC | SSP-1 | SSP-2 | |
| Gastrointestinal transit rate (%) | 80.19 ± 11.44 a | 37.93 ± 4.67 c | 75.85 ± 16.53 a | 59.20 ± 9.23 b | 48.50 ± 7.79 bc |
| First black stool defecation time (min) | 73.50 ± 3.11 b | 156.00 ± 10.15 a | 79.75 ± 10.14 b | 81.25 ± 14.86 b | 82.50 ± 9.18 b |
| Stool water content (%) | 67.07 ± 6.36 a | 49.57 ± 7.28 b | 60.50 ± 8.87 a | 64.63 ± 2.65 a | 64.79 ± 2.77 a |
| Stool pellet number (ea) | 18.67 ± 8.39 ab | 4.00 ± 2.65 c | 15.33 ± 4.51 b | 17.67 ± 3.06 ab | 25.00 ± 3.74 a |
| Stool weight (mg) | 428.00 ± 35.64 a | 109.53 ± 88.65 d | 284.33 ± 62.88 bc | 226.23 ± 42.32 cd | 383.60 ± 126.79 ab |
| Indicators | Acetic Acid | Propionic Acid | Butyric Acid | Isobutyric Acid | Valeric Acid | Isovaleric Acid |
|---|---|---|---|---|---|---|
| Gastrointestinal transit rate | 0.768 * | 0.264 | 0.400 | 0.504 | 0.111 | 0.750 * |
| Stool pellet number | 0.309 | −0.499 | 0.211 | 0.409 | 0.116 | 0.570 * |
| Defecation time | −0.552 * | 0.077 | −0.247 | −0.382 | −0.241 | −0.502 |
| Stool weight | 0.236 | −0.389 | 0.393 | 0.514 * | 0.050 | 0.554 * |
| Stool water content | 0.657 * | 0.054 | 0.289 | 0.439 | 0.007 | 0.543 * |
| GAS | 0.318 | −0.650 * | −0.071 | −0.061 | 0.604 * | 0.268 |
| MTL | 0.814 * | 0.114 | 0.679 * | 0.614 * | −0.061 | 0.704 * |
| SP | 0.324 | −0.147 | 0.574 * | 0.390 | 0.225 | 0.357 |
| VIP | −0.717 * | −0.311 | −0.794 * | −0.519 * | 0.249 | −0.524 * |
| SS | −0.579 * | −0.400 | −0.575 * | −0.221 | 0.311 | −0.271 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, Z.; Liu, X.; Hu, W.; Wu, W. Gastrointestinal Fermentable Polysaccharide Is Beneficial in Alleviating Loperamide-Induced Constipation in Mice. Nutrients 2023, 15, 4364. https://doi.org/10.3390/nu15204364
Liu B, Zhang Z, Liu X, Hu W, Wu W. Gastrointestinal Fermentable Polysaccharide Is Beneficial in Alleviating Loperamide-Induced Constipation in Mice. Nutrients. 2023; 15(20):4364. https://doi.org/10.3390/nu15204364
Chicago/Turabian StyleLiu, Buyu, Zhiguo Zhang, Xingquan Liu, Weiwei Hu, and Weicheng Wu. 2023. "Gastrointestinal Fermentable Polysaccharide Is Beneficial in Alleviating Loperamide-Induced Constipation in Mice" Nutrients 15, no. 20: 4364. https://doi.org/10.3390/nu15204364
APA StyleLiu, B., Zhang, Z., Liu, X., Hu, W., & Wu, W. (2023). Gastrointestinal Fermentable Polysaccharide Is Beneficial in Alleviating Loperamide-Induced Constipation in Mice. Nutrients, 15(20), 4364. https://doi.org/10.3390/nu15204364






