Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Dietary Decanoic Acid
2.3. Coronary Artery Disease
2.4. Potential Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
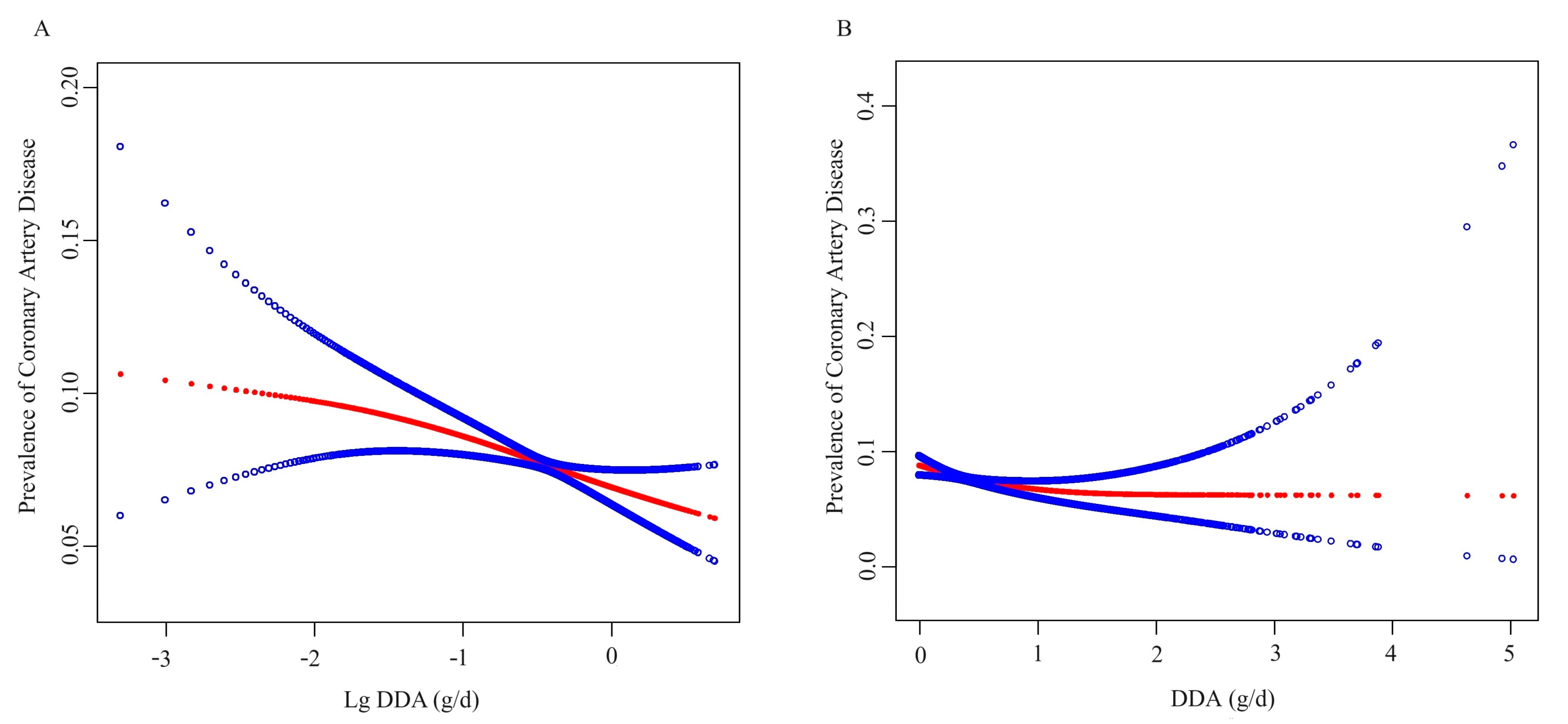
3.2. Association between DDA and CAD Prevalence
3.3. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013, a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Chowdhury, M.; Haque, M.A.; Farhana, Z.; Anik, A.M.; Chowdhury, A.H.; Haque, S.M.; Marjana, L.L.; Bristi, P.D.; Al, M.B.; Uddin, M.J.; et al. Prevalence of cardiovascular disease among Bangladeshi adult population: A systematic review and meta-analysis of the studies. Vasc. Health Risk Manag. 2018, 14, 165–181. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155,722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [PubMed]
- Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; Packard, C.J.; et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [PubMed]
- Wu, L.; Shi, Y.; Kong, C.; Zhang, J.; Chen, S. Dietary Inflammatory Index and Its Association with the Prevalence of Coronary Heart Disease among 45,306 US Adults. Nutrients 2022, 14, 4553. [Google Scholar]
- Zhang, X.Y.; Shu, L.; Si, C.J.; Yu, X.L.; Liao, D.; Gao, W.; Zhang, L.; Zheng, P.F. Dietary Patterns, Alcohol Consumption and Risk of Coronary Heart Disease in Adults: A Meta-Analysis. Nutrients 2015, 7, 6582–6605. [Google Scholar] [CrossRef]
- Martin, K.; Jackson, C.F.; Levy, R.G.; Cooper, P.N. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst. Rev. 2016, 2, CD1903. [Google Scholar]
- Mohammadifard, N.; Haghighatdoost, F.; Rahimlou, M.; Rodrigues, A.; Gaskarei, M.K.; Okhovat, P.; de Oliveira, C.; Silveira, E.A.; Sarrafzadegan, N. The Effect of Ketogenic Diet on Shared Risk Factors of Cardiovascular Disease and Cancer. Nutrients 2022, 14, 3499. [Google Scholar]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.; Walker, M.C.; Williams, R. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar]
- Courchesne-Loyer, A.; Croteau, E.; Castellano, C.A.; St-Pierre, V.; Hennebelle, M.; Cunnane, S.C. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study. J. Cereb. Blood Flow. Metab. 2017, 37, 2485–2493. [Google Scholar] [CrossRef]
- Scheck, A.C.; Abdelwahab, M.G.; Fenton, K.E.; Stafford, P. The ketogenic diet for the treatment of glioma: Insights from genetic profiling. Epilepsy Res. 2012, 100, 327–337. [Google Scholar] [PubMed]
- Likhodii, S.S.; Musa, K.; Mendonca, A.; Dell, C.; Burnham, W.M.; Cunnane, S.C. Dietary fat, ketosis, and seizure resistance in rats on the ketogenic diet. Epilepsia 2000, 41, 1400–1410. [Google Scholar] [PubMed]
- Thavendiranathan, P.; Mendonca, A.; Dell, C.; Likhodii, S.S.; Musa, K.; Iracleous, C.; Cunnane, S.C.; Burnham, W.M. The MCT ketogenic diet: Effects on animal seizure models. Exp. Neurol. 2000, 161, 696–703. [Google Scholar]
- Chang, P.; Augustin, K.; Boddum, K.; Williams, S.; Sun, M.; Terschak, J.A.; Hardege, J.D.; Chen, P.E.; Walker, M.C.; Williams, R.S. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain 2016, 139, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.D.; Kanabus, M.; Anderson, G.; Hargreaves, I.P.; Rutherford, T.; O’Donnell, M.; Cross, J.H.; Rahman, S.; Eaton, S.; Heales, S.J. The ketogenic diet component decanoic acid increases mitochondrial citrate synthase and complex I activity in neuronal cells. J. Neurochem. 2014, 129, 426–433. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S. Triglycerides of medium-chain fatty acids: A concise review. J. Food Sci. Technol. 2023, 60, 2143–2152. [Google Scholar]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef]
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar]
- Skipina, T.M.; Patel, N.; Upadhya, B.; Soliman, E.Z. Cannabis use is associated with prevalent coronary artery disease. Am. J. Med. Sci. 2022, 364, 304–308. [Google Scholar]
- Hui, G.; Koch, B.; Calara, F.; Wong, N.D. Angina in Coronary Artery Disease Patients with and without Diabetes: US National Health and Nutrition Examination Survey 2001–2010. Clin. Cardiol. 2016, 39, 30–36. [Google Scholar]
- Greenland, S. Modeling and variable selection in epidemiologic analysis. Am. J. Public Health 1989, 79, 340–349. [Google Scholar] [PubMed]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet. in Renal Disease Study Group. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Petiot, E. Thresholds for Hypertension Definition, Treatment Initiation, and Treatment Targets: Recent Guidelines at a Glance. Circulation 2022, 146, 805–807. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar]
- Dyńka, D.; Kowalcze, K.; Charuta, A.; Paziewska, A. The Ketogenic Diet and Cardiovascular Diseases. Nutrients 2023, 15, 3368. [Google Scholar]
- Luong, T.V.; Abild, C.B.; Bangshaab, M.; Gormsen, L.C.; Søndergaard, E. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients 2022, 14, 1322. [Google Scholar] [CrossRef]
- Rusek, M.; Pluta, R.; Ułamek-Kozioł, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar]
- Dallérac, G.; Moulard, J.; Benoist, J.F.; Rouach, S.; Auvin, S.; Guilbot, A.; Lenoir, L.; Rouach, N. Non-ketogenic combination of nutritional strategies provides robust protection against seizures. Sci. Rep. 2017, 7, 5496. [Google Scholar]
- Chang, P.; Terbach, N.; Plant, N.; Chen, P.E.; Walker, M.C.; Williams, R.S. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology 2013, 69, 105–114. [Google Scholar]
- Kanabus, M.; Fassone, E.; Hughes, S.D.; Bilooei, S.F.; Rutherford, T.; Donnell, M.O.; Heales, S.; Rahman, S. The pleiotropic effects of decanoic acid treatment on mitochondrial function in fibroblasts from patients with complex I deficient Leigh syndrome. J. Inherit. Metab. Dis. 2016, 39, 415–426. [Google Scholar] [PubMed]
- Sengupta, A.; Ghosh, M. Comparison of native and capric acid-enriched mustard oil effects on oxidative stress and antioxidant protection in rats. Br. J. Nutr. 2012, 107, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Lowery, R. The Ketogenic Bibleed; Victory Belt Publishing Inc.: Las Vegas, NV, USA, 2017. [Google Scholar]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar]
- Poff, A.; Kesl, S.; Koutnik, A.; Ward, N.; Ari, C.; Deblasi, J.; D’Agostino, D. Characterizing the metabolic effects of exogenous ketone supplementation an alternative or adjuvant to the ketogenic diet. FASEB J. 2017, 311, 970.7. [Google Scholar]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [PubMed]
- Kelly, E.; Sharma, D.; Wilkinson, C.J.; Williams, R. Diacylglycerol kinase (DGKA) regulates the effect of the epilepsy and bipolar disorder treatment valproic acid in Dictyostelium discoideum. Dis. Model. Mech. 2018, 11, dmm035600. [Google Scholar]
- Yen, C.L.; Stone, S.J.; Koliwad, S.; Harris, C.; Farese, R.J. Thematic review series: Glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 2008, 49, 2283–2301. [Google Scholar]
- Marx, N.; Duez, H.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors and atherogenesis: Regulators of gene expression in vascular cells. Circ. Res. 2004, 94, 1168–1178. [Google Scholar]
- Chang, P.; Orabi, B.; Deranieh, R.M.; Dham, M.; Hoeller, O.; Shimshoni, J.A.; Yagen, B.; Bialer, M.; Greenberg, M.L.; Walker, M.C.; et al. The antiepileptic drug valproic acid and other medium-chain fatty acids acutely reduce phosphoinositide levels independently of inositol in Dictyostelium. Dis. Model. Mech. 2012, 5, 115–124. [Google Scholar]
- Malkin, C.J.; Pugh, P.J.; West, J.N.; van Beek, E.J.; Jones, T.H.; Channer, K.S. Testosterone therapy in men with moderate severity heart failure: A double-blind randomized placebo controlled trial. Eur. Heart J. 2006, 27, 57–64. [Google Scholar]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef] [PubMed]


| Variables a,* | Non-CAD (N = 31,501) | CAD (N = 2685) | p-Value |
|---|---|---|---|
| Age, years | 46.85 ± 16.43 | 65.61 ± 12.49 | <0.0001 |
| Sex, % | <0.0001 | ||
| Male | 47.15 | 59.91 | |
| Female | 52.85 | 40.09 | |
| Race, % | <0.0001 | ||
| Mexican American | 8.05 | 3.70 | |
| Other Hispanic | 5.17 | 3.15 | |
| Non-Hispanic white | 69.05 | 78.90 | |
| Non-Hispanic black | 11.07 | 8.57 | |
| Other race | 6.67 | 5.69 | |
| Education level, % | <0.0001 | ||
| <9th grade | 4.80 | 8.66 | |
| 9–11th grade | 9.87 | 14.24 | |
| High school | 23.42 | 28.02 | |
| College | 31.66 | 29.56 | |
| Graduate or above | 30.26 | 19.53 | |
| PIR | 3.07 ± 1.63 | 2.70 ± 1.56 | <0.0001 |
| BMI, kg/m2 | 28.93 ± 6.81 | 30.37 ± 6.81 | <0.0001 |
| Hypertension, % | <0.0001 | ||
| No | 64.51 | 25.07 | |
| Yes | 35.43 | 74.93 | |
| Diabetes, % | <0.0001 | ||
| No | 88.02 | 62.77 | |
| Yes | 11.98 | 37.23 | |
| Smoking status, % | <0.0001 | ||
| Never smoking | 55.86 | 35.65 | |
| Quit smoking | 22.16 | 41.94 | |
| Current smoking | 21.98 | 22.41 | |
| Drinking status, % | <0.0001 | ||
| Has never drunk | 19.65 | 23.61 | |
| 1–5 drinks/month | 41.06 | 42.33 | |
| 5–10 drinks/month | 7.86 | 3.42 | |
| 10+ drinks/month | 14.13 | 12.90 | |
| Unknown | 17.30 | 17.73 | |
| FBG, mmol/L | 5.44 ± 1.74 | 6.31 ± 2.38 | <0.0001 |
| HbA1c, % | 5.57 ± 0.87 | 6.11 ± 1.14 | <0.0001 |
| TC, mmol/L | 5.10 ± 1.07 | 4.64 ± 1.19 | <0.0001 |
| Triglyceride, mmol/L | 1.70 ± 1.49 | 1.91 ± 1.29 | <0.0001 |
| ALT, U/L | 25.37± 18.54 | 24.78 ± 33.37 | 0.1943 |
| BUN, mg/dL | 4.77 ± 1.83 | 6.13 ± 2.95 | <0.0001 |
| UA, umol/L | 320.66 ± 82.13 | 353.00 ± 91.18 | <0.0001 |
| SCR, umol/L | 78.17 ± 30.13 | 94.16 ± 47.65 | <0.0001 |
| eGFR, mL/min/1.73 m2 | 92.84 ± 23.57 | 76.25 ± 24.51 | <0.0001 |
| Energy, kcal | 2113.30 ± 829.80 | 1904.67 ± 758.04 | <0.0001 |
| Protein, g/d | 82.71 ± 36.51 | 74.36 ± 32.95 | <0.0001 |
| Carbohydrate, g/d | 252.80 ± 111.84 | 228.45 ± 98.73 | <0.0001 |
| Fiber, g/d | 16.80 ± 9.10 | 15.96 ± 8.66 | <0.0001 |
| Total fat, g/d | 82.04 ± 40.15 | 74.22 ± 37.10 | <0.0001 |
| SFA, g/d | 26.68 ± 14.61 | 24.04 ± 12.88 | <0.0001 |
| MUFA, g/d | 29.22 ± 13.87 | 26.63 ± 14.09 | <0.0001 |
| PUFA, g/d | 18.36 ± 9.85 | 16.89 ± 9.96 | <0.0001 |
| Vitamin E, mg/d | 8.37 ± 5.69 | 7.57 ± 4.94 | <0.0001 |
| Vitamin A, μg/d | 647.79 ± 566.36 | 623.16 ± 489.08 | 0.0658 |
| Vitamin B6, mg/d | 2.11 ± 1.41 | 1.90 ± 1.35 | <0.0001 |
| Vitamin C, mg/d | 82.86 ± 79.01 | 80.80 ± 78.80 | 0.2369 |
| Vitamin K, μg/d | 114.01 ± 215.49 | 99.73 ± 107.70 | 0.0029 |
| DDA, g/d | 0.49 ± 0.37 | 0.43 ± 0.35 | <0.0001 |
| DDA, g/d | Coronary Artery Disease OR (95% CI), p-Value | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| DDA | 0.60 (0.49, 0.72) < 0.001 | 0.69 (0.56, 0.84) < 0.001 | 0.79 (0.61, 1.02) 0.078 |
| Lg DDA | 0.66 (0.59, 0.74) < 0.001 | 0.74 (0.64, 0.84) < 0.001 | 0.81 (0.69, 0.96) 0.015 |
| DDA Tertile | |||
| T1 (0.00–0.24) | Reference | Reference | Reference |
| T2 (0.24–0.49) | 0.79 (0.71, 0.88) < 0.001 | 0.82 (0.73, 0.93) 0.002 | 0.85 (0.75, 0.97) 0.014 |
| T3 (0.49–5.03) | 0.66 (0.58, 0.75) < 0.001 | 0.75 (0.65,0.86) < 0.001 | 0.83 (0.69, 1.00) 0.056 |
| P for trend | <0.001 | <0.001 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Yang, W.; Li, M.; Li, F.; Gong, R.; Wu, Y. Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study. Nutrients 2023, 15, 4308. https://doi.org/10.3390/nu15204308
Wu Z, Yang W, Li M, Li F, Gong R, Wu Y. Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study. Nutrients. 2023; 15(20):4308. https://doi.org/10.3390/nu15204308
Chicago/Turabian StyleWu, Zhijian, Weichang Yang, Meng Li, Fengyuan Li, Ren Gong, and Yanqing Wu. 2023. "Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study" Nutrients 15, no. 20: 4308. https://doi.org/10.3390/nu15204308
APA StyleWu, Z., Yang, W., Li, M., Li, F., Gong, R., & Wu, Y. (2023). Relationship between Dietary Decanoic Acid and Coronary Artery Disease: A Population-Based Cross-Sectional Study. Nutrients, 15(20), 4308. https://doi.org/10.3390/nu15204308






