Effects of Vitamin D Deficiency on Sepsis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Study Population, and Data Collection
2.2. Definition
2.3. Statistical Analysis
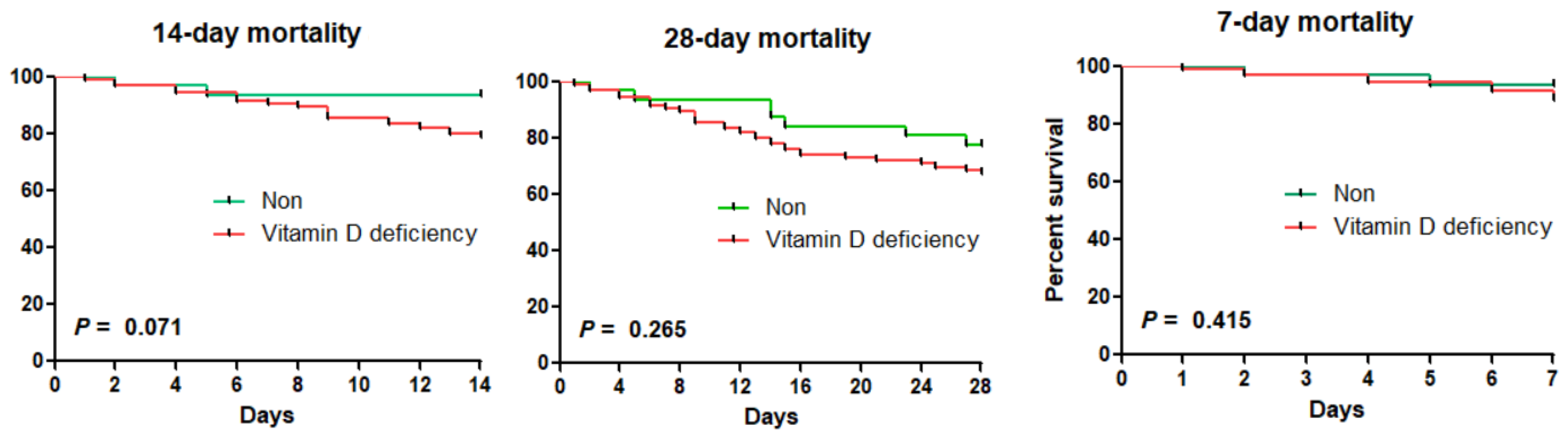
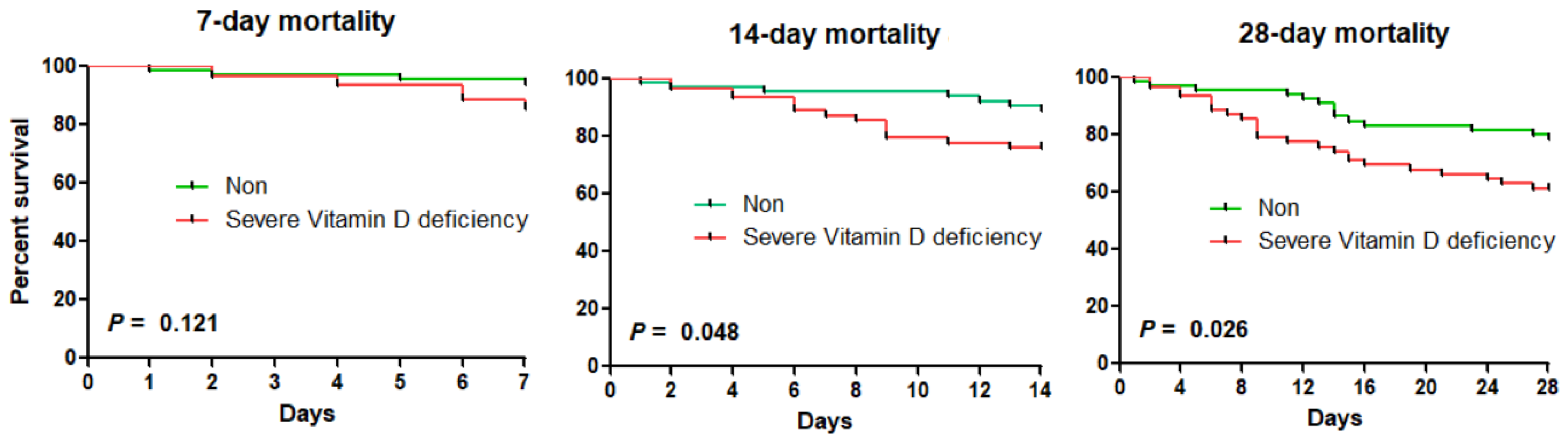
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Ward, P.A. The inflammatory response in sepsis. Trends Immunol. 2013, 34, 129–136. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the global burden of disease study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Im, Y.; Kang, D.; Ko, R.E.; Lee, Y.J.; Lim, S.Y.; Park, S.; Na, S.J.; Chung, C.R.; Park, M.H.; Oh, D.K.; et al. Time-to-antibiotics and clinical outcomes in patients with sepsis and septic shock: A prospective nationwide multicenter cohort study. Crit. Care 2022, 26, 19. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Song, J.; Jeon, J.H.; Choi, H.K.; Choi, W.S.; Moon, S.; Park, D.W. Timing of antibiotics in septic patients: A prospective cohort study. Clin. Microbiol. Infect. 2020, 26, 1495–1500. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Mankowski, R.T.; Anton, S.D.; Ghita, G.L.; Brumback, B.; Cox, M.C.; Mohr, A.M.; Leeuwenburgh, C.; Moldawer, L.L.; Efron, P.A.; Brakenridge, S.C.; et al. Older sepsis survivors suffer persistent disability burden and poor long-term survival. J. Am. Geriatr. Soc. 2020, 68, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Liu, M.C.; Camargo, C.A., Jr. Demographic differences and trends of vitamin d insufficiency in the US population, 1988–2004. Arch. Intern. Med. 2009, 169, 626–632. [Google Scholar] [CrossRef]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin d deficiency in us adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Park, H.Y.; Lim, Y.H.; Park, J.B.; Rhie, J.; Lee, S.J. Environmental and occupation factors associated with vitamin d deficiency in korean adults: The korea national health and nutrition examination survey (knhanes) 2010–2014. Int. J. Environ. Res. Public Health 2020, 17, 9166. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin d deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, M.Y.; Kim, J.M.; Kim, D.W.; Kim, C.B. Vitamin d deficiency in nursing home elderly in korea. J. Korean Geriatr. Soc. 2016, 20, 102–107. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin d and marine omega 3 fatty acid supplementation and incident autoimmune disease: Vital randomized controlled trial. BMJ (Clin. Res. Ed.) 2022, 376, e066452. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The effect of vitamin d supplementation on glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Nutrients 2018, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory actions of vitamin d in various immune-related disorders: A comprehensive review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin d: Nutrient, hormone, and immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef]
- Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin d and infectious diseases: Simple bystander or contributing factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef]
- Yang, C.; Lu, Y.; Wan, M.; Xu, D.; Yang, X.; Yang, L.; Wang, S.; Sun, G. Efficacy of high-dose vitamin d supplementation as an adjuvant treatment on pneumonia: Systematic review and a meta-analysis of randomized controlled studies. Nutr. Clin. Pract. 2021, 36, 368–384. [Google Scholar] [CrossRef]
- Urashima, M.; Segawa, T.; Okazaki, M.; Kurihara, M.; Wada, Y.; Ida, H. Randomized trial of vitamin d supplementation to prevent seasonal influenza a in schoolchildren. Am. J. Clin. Nutr. 2010, 91, 1255–1260. [Google Scholar] [CrossRef]
- Braun, A.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Liu, Y.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin d levels and mortality in the critically ill. Crit. Care Med. 2011, 39, 671–677. [Google Scholar] [CrossRef]
- Braun, A.B.; Gibbons, F.K.; Litonjua, A.A.; Giovannucci, E.; Christopher, K.B. Low serum 25-hydroxyvitamin d at critical care initiation is associated with increased mortality. Crit. Care Med. 2012, 40, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, X.; Ying, J.; Zhou, Y.; Li, X.; Mu, D.; Qu, Y. Association between vitamin d status and sepsis in children: A meta-analysis of observational studies. Clin. Nutr. 2020, 39, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Eisman, J.A.; Center, J.R. Vitamin d deficiency in critically ill patients. N. Engl. J. Med. 2009, 360, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Münch, A.; Warnkross, H.; et al. Effect of high-dose vitamin d3 on hospital length of stay in critically ill patients with vitamin d deficiency: The vitdal-icu randomized clinical trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef]
- Erdoğan, M.; Fındıklı, H.A. Novel biomarker for predicting sepsis mortality: Vitamin d receptor. J. Int. Med. Res. 2021, 49, 3000605211034733. [Google Scholar] [CrossRef]
- Han, J.; Zhang, X.; Saraf, S.L.; Gowhari, M.; Molokie, R.E.; Hassan, J.; Jain, S.; Shah, B.N.; Abbasi, T.; Machado, R.F.; et al. Risk factors for vitamin d deficiency in sickle cell disease. Br. J. Haematol. 2018, 181, 828–835. [Google Scholar] [CrossRef]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin d promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J. Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef]
- Wang, Z.; Joshi, A.; Leopold, K.; Jackson, S.; Christensen, S.; Nayfeh, T.; Mohammed, K.; Creo, A.; Tebben, P.; Kumar, S. Association of vitamin d deficiency with covid-19 infection severity: Systematic review and meta-analysis. Clin. Endocrinol. 2022, 96, 281–287. [Google Scholar] [CrossRef]
- Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; Hyzy, R.C.; Khan, A.; et al. Early high-dose vitamin d(3) for critically ill, vitamin d-deficient patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [CrossRef]
- Riché, F.; Gayat, E.; Barthélémy, R.; Le Dorze, M.; Matéo, J.; Payen, D. Reversal of neutrophil-to-lymphocyte count ratio in early versus late death from septic shock. Crit. Care 2015, 19, 439. [Google Scholar] [CrossRef]
- Gaieski, D.F.; Edwards, J.M.; Kallan, M.J.; Carr, B.G. Benchmarking the incidence and mortality of severe sepsis in the united states. Crit. Care Med. 2013, 41, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Horie, S.; Laffey, J.G. Role of the adaptive immune response in sepsis. Intensive Care Med. Exp. 2020, 8, 20. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 129) | Vitamin D Insufficiency (n = 119) | Vitamin D Deficiency (n = 96) | Severe Vitamin D Deficiency (n = 62) | |
|---|---|---|---|---|
| Age, years | 74 ± 13 (66–83) | 74 ± 13 (66–83) | 75 ± 13 (68–83) | 74 ± 15 (66–83) |
| Male | 60 (46.5) | 54 (45.4) | 42 (43.8) | 27 (4.35) |
| Vitamin D level (ng/mL) | 14.5 (6.2–20.8) | 11.1 (6.0–17.8) | 8.5 (5.0–14.1) | 6.1 (4.6–8.4) |
| Charlson comorbidity index | 5 (3–6) | 5 (3–6) | 5 (3–6) | 5 (3–6) |
| Grade of infection | ||||
| High-grade | 83 (64.3) | 76 (63.9) | 60 (62.5) | 40 (64.5) |
| Low-grade | 46 (35.7) | 43 (36.1) | 36 (37.5) | 22 (36.1) |
| SOFA score | 7 (5–10) | 7 (5–10) | 7 (5–10) | 7 (5–9) |
| Septic shock | 60 (46.5) | 57 (47.9) | 6 (47.9) | 29 (46.8) |
| Bacteremia | 45 (34.9) | 41 (34.5) | 36 (37.5) | 21 (33.9) |
| ICU admission | 78 (60.5) | 75 (63.0) | 63 (65.6) | 39 (62.9) |
| CRP (mg/dL) * | 10.5 (4.2–21.5) | 11.2 (4.4–22.2) | 10.2 (4.4–22.1) | 11.2 (4.5–20.4) |
| Procalcitonin (ng/mL) * | 1.0 (0.3–7.1) | 1.15 (0.28–10.51) | 1.08 (0.27–7.02) | 0.90 (0.27–5.39) |
| Initial lactate (mmol/L) * | 2.4 (1.7–5.9) | 2.5 (1.7–6.0) | 2.7 (1.8–6.1) | 2.3 (1.7–5.8) |
| Time to antibiotics administration, minutes | 119 (72–240) | 119 (72–230) | 115 (72–213) | 130 (72–220) |
| Appropriateness of empirical antibiotics administration | 99 (76.7) | 92 (77.3) | 72 (75.0) | 50 (80.6) |
| All-cause 7-day mortality | 13 (10.1) | 13 (10.9) | 11 (11.5) | 9 (14.8) |
| All-cause 14-day mortality | 22 (17.1) | 22 (18.5) | 20 (20.8) | 15 (24.6) |
| All-cause 28-day mortality | 38 (29.5) | 36 (30.3) | 31 (32.3) | 24 (38.7) |
| In-hospital mortality | 36 (27.9) | 34 (28.6) | 30 (31.3) | 22 (35.5) |
| 7-Day Mortality | 14-Day Mortality | 28-Day Mortality | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | |
| Vitamin D deficiency | 1.78 (0.39–8.08) | 0.454 | 3.20 (0.74–13.81) | 0.120 | 1.51 (0.66–3.45) | 0.332 | 1.63 (0.66–4.01) | 0.287 |
| Age | 1.02 (0.97–1.07) | 0.487 | 0.98 (0.95–1.02) | 0.361 | 0.99 (0.97–1.02) | 0.517 | 0.98 (0.95–1.01) | 0.182 |
| CCI | 1.10 (0.85–1.42) | 0.479 | 1.30 (1.11–1.53) | 0.002 | 1.22 (1.07–1.39) | 0.003 | 1.31 (1.14–1.50) | <0.001 |
| SOFA | 1.22 (1.02–1.44) | 0.025 | 1.08 (0.96–1.22) | 0.203 | 1.11 (1.01–1.22) | 0.027 | 1.10 (0.99–1.22) | 0.060 |
| Shock | 1.33 (0.66–2.69) | 0.430 | 1.38 (0.85–2.24) | 0.198 | 1.25 (0.83–1.90) | 0.285 | 1.17 (0.75–1.83) | 0.483 |
| 7-Day Mortality | 14-Day Mortality | 28-Day Mortality | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | aHR (95% CI) | p-Value | |
| Severe vitamin D deficiency | 2.80 (0.85–9.23) | 0.090 | 2.57 (1.03–6.43) | 0.043 | 2.28 (1.17–4.45) | 0.016 | 2.11 (1.02–4.36) | 0.044 |
| Age | 1.02 (0.97–1.07) | 0.496 | 0.98 (0.95–1.02) | 0.336 | 0.99 (0.96–1.02) | 0.486 | 0.98 (0.96–1.01) | 0.244 |
| CCI | 1.10 (0.85–1.43) | 0.467 | 1.31 (1.12–1.54) | 0.001 | 1.23 (1.08–1.41) | 0.002 | 1.30 (1.13–1.49) | <0.001 |
| SOFA | 1.24 (1.04–1.48) | 0.016 | 1.12 (0.99–1.27) | 0.077 | 1.13 (1.03–1.24) | 0.010 | 1.12 (1.01–1.24) | 0.034 |
| Shock | 1.31 (0.67–2.56) | 0.437 | 1.33 (0.81–2.20) | 0.267 | 1.21 (0.80–1.82) | 0.365 | 1.10 (0.70–1.74) | 0.676 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, H.; Kim, J.; Choi, W.S.; Park, D.W. Effects of Vitamin D Deficiency on Sepsis. Nutrients 2023, 15, 4309. https://doi.org/10.3390/nu15204309
Seok H, Kim J, Choi WS, Park DW. Effects of Vitamin D Deficiency on Sepsis. Nutrients. 2023; 15(20):4309. https://doi.org/10.3390/nu15204309
Chicago/Turabian StyleSeok, Hyeri, Jooyun Kim, Won Suk Choi, and Dae Won Park. 2023. "Effects of Vitamin D Deficiency on Sepsis" Nutrients 15, no. 20: 4309. https://doi.org/10.3390/nu15204309
APA StyleSeok, H., Kim, J., Choi, W. S., & Park, D. W. (2023). Effects of Vitamin D Deficiency on Sepsis. Nutrients, 15(20), 4309. https://doi.org/10.3390/nu15204309






