Adipocyte Metabolism and Health after the Menopause: The Role of Exercise
Abstract
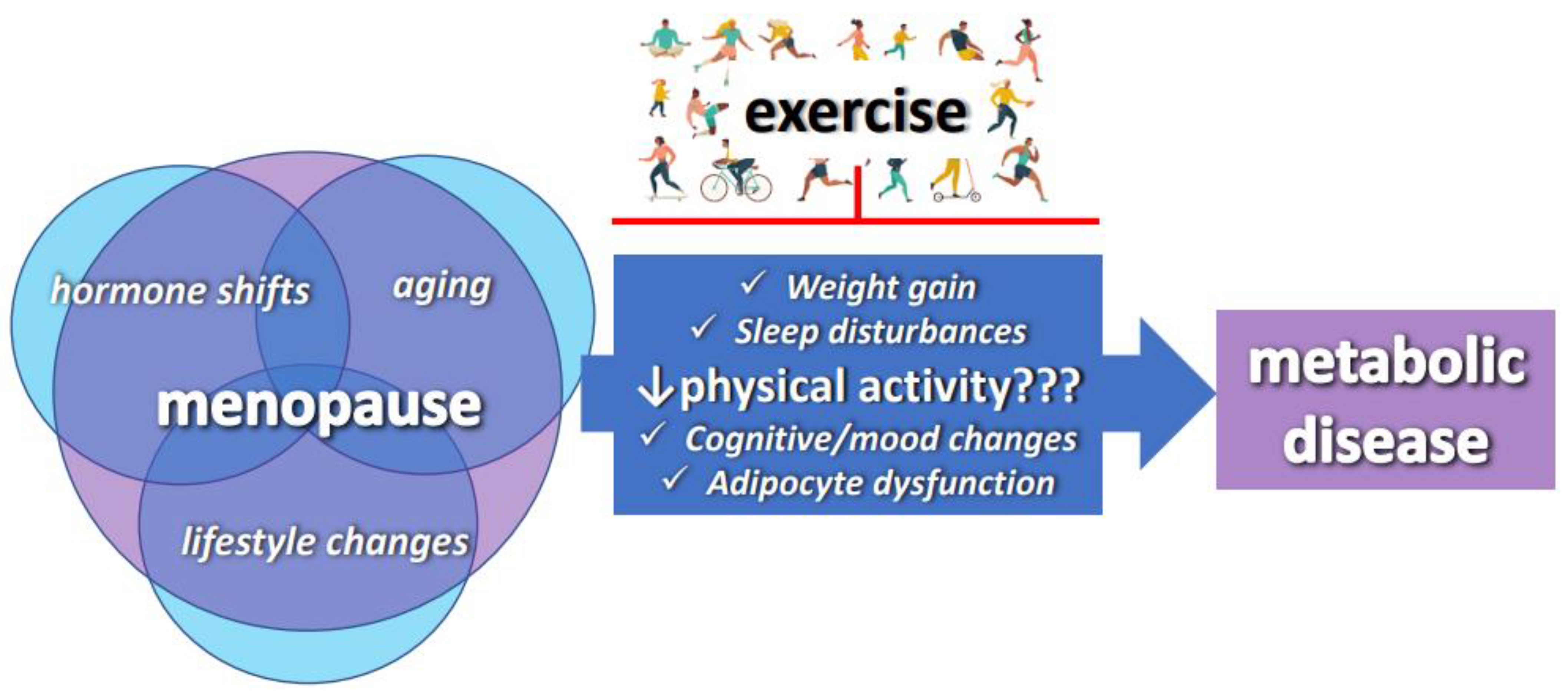
1. Introduction
2. The Menopause Increases the Risk of Metabolic Disorders
2.1. The Menopause Increases Obesity Susceptibility
2.2. The Menopause Causes Dysfunctional Adipose Tissue
2.3. Role of Adipose Tissue Estrogen Receptors in the Link between the Menopause and Metabolic Risk
3. Exercise Improves Metabolic Health Following the Menopause
3.1. Effects of the Menopause on Responsiveness to Exercise and the Role of ERs
3.2. Exercise-Modality-Specific Effects
3.2.1. Strength Training
3.2.2. High-Intensity Interval Training
3.2.3. Alternative Forms of Exercise
4. Nutritional Approaches to Improve Metabolic Health Following the Menopause
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, A.; Tworowska, U.; Demissie, M. Menopausal obesity—Myth or fact? Climacteric 2001, 4, 273–283. [Google Scholar] [PubMed]
- Abildgaard, J.; Pedersen, A.T.; Green, C.J.; Harder-Lauridsen, N.M.; Solomon, T.P.; Thomsen, C.; Juul, A.; Pedersen, M.; Pedersen, J.T.; Mortensen, O.H.; et al. Menopause is associated with decreased whole body fat oxidation during exercise. Am. J. Physiol.-Endocrinol. Metab. 2013, 304, E1227–E1236. [Google Scholar] [CrossRef]
- Abdulnour, J.; Doucet, É.; Brochu, M.; Lavoie, J.M.; Strychar, I.; Rabasa-Lhoret, R.; Prud’homme, D. The effect of the menopausal transition on body composition and cardiometabolic risk factors: A Montreal-Ottawa New Emerging Team group study. Menopause 2012, 19, 760–767. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.; Shi, Z.; Hu, X.; Wu, M.; Guo, Z.; Hussain, A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism 2009, 58, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Ozbey, N.; Sencer, E.; Molvalilar, S.; Orhan, Y. Body fat distribution and cardiovascular disease risk factors in pre- and postmenopausal obese women with similar BMI. Endocr. J. 2002, 49, 503–509. [Google Scholar] [CrossRef]
- Karvonen-Gutierrez, C.; Kim, C. Association of mid-life changes in body size, body composition and obesity status with the menopausal transition healthcare. Healthcare 2016, 4, 42. [Google Scholar] [CrossRef]
- Razmjou, S.; Abdulnour, J.; Bastard, J.; Soraya, F.; Éric, D.; Martin, B.; Jean-Marc, L.; Rémi, R.-L.; Denis, P. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year followup: A MONET study. Menopause 2018, 25, 89–97. [Google Scholar] [CrossRef]
- Elavsky, S. Physical activity, menopause, and quality of life: The role of affect and self-worth across time. Menopause 2009, 16, 265–271. [Google Scholar] [CrossRef]
- Park, Y.M.; Rector, R.S.; Thyfault, J.P.; Zidon, T.M.; Padilla, J.; Welly, R.J.; Meers, G.M.; Morris, M.E.; Britton, S.L.; Koch, L.G.; et al. Effects of ovariectomy and intrinsic aerobic capacity on tissue-specific insulin sensitivity. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E190–E199. [Google Scholar] [CrossRef] [PubMed]
- Sternfeld, B.; Dugan, S. Physical activity and health during the menopausal transition. Obs. Gynecol. Clin. N. Am. 2011, 38, 537–566. [Google Scholar] [CrossRef]
- Kopiczko, A. Bone mineral density in old age: The influence of age at menarche, menopause status and habitual past and present physical activity. Arch. Med. Sci. 2020, 16, 657–665. [Google Scholar] [CrossRef]
- Park, Y.; Kanaley, J.; Zidon, T.; Welly, R.; Scroggins, R.; Britton, S.; Koch, L.; Thyfault, J.; Booth, F.; Padilla, J.; et al. Ovariectomized High Fit Rats Are Protected against Diet-Induced Insulin Resistance. Med. Sci. Sport Exerc. 2016, 48, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Zidon, T.M.; Park, Y.M.; Welly, R.J.; Woodford, M.L.; Scroggins, R.J.; Britton, S.L.; Koch, L.G.; Booth, F.W.; Padilla, J.; Kanaley, J.A.; et al. Voluntary wheel running improves adipose tissue immunometabolism in ovariectomized low-fit rats. Adipocyte 2018, 7, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, O.; Hassager, C.; Christiansen, C. Age- and menopause-associated variations in body composition and fat distribution in healthy women as measured by dual-energy X-ray absorptiometry. Metabolism 1995, 44, 369–373. [Google Scholar] [CrossRef]
- Allal-Elasmi, M.; Taieb, S.H.; Hsairi, M.; Zayani, Y.; Omar, S.; Sanhaji, H.; Jemaa, R.; Feki, M.; Elati, J.; Mebazaa, A.; et al. The metabolic syndrome: Prevalence, main characteristics and association with socio-economic status in adults living in Great Tunis. Diabetes Metab. 2010, 36, 204–208. [Google Scholar] [CrossRef]
- Abildgaard, J.; Tingstedt, J.; Zhao, Y.; Hartling, H.; Pedersen, A.; Lindegaard, B.; Nielsen, S. Increased systemic inflammation and altered distribution of T-cell subsets in postmenopausal women. PLoS ONE 2020, 15, e0234174. [Google Scholar] [CrossRef]
- Després, J.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Freeman, E.; Sammel, M.; Lin, H.; Gracia, C. Obesity and reproductive hormone levels in the transition to menopause. Menopause 2010, 17, 718–726. [Google Scholar] [CrossRef]
- Harzallah, F.; Alberti, H.; Ben Khalifa, F. The metabolic syndrome in an Arab population: A first look at the new International Diabetes Federation criteria. Diabet Med. 2006, 23, 441–444. [Google Scholar] [CrossRef]
- Jaber, L.A.; Brown, M.B.; Hammad, A.; Zhu, Q.; Herman, W.H. The prevalence of the metabolic syndrome among Arab Americans. Diabetes Care 2004, 27, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Meléndez, G.; Gazzinelli, A.; Côrrea-Oliveira, R.; Pimenta, A.M.; Kac, G. Prevalence of metabolic syndrome in a rural area of Brazil. Sao Paulo Med. J. 2007, 125, 155–162. [Google Scholar] [CrossRef]
- D’Eon, T.; Souza, S.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.A.; Al Nuaimi, A.S.; Latif Zainel, A.J.A.; A/Qotba, H.A. Prevalence of metabolic syndrome in primary health settings in Qatar: A cross sectional study. BMC Public Health 2020, 20, 611. [Google Scholar] [CrossRef]
- Mikkola, T.; Gissler, M.; Merikukka, M.; Tuomikoski, P.; Ylikorkala, O. Sex differences in age-related cardiovascular mortality. PLoS ONE 2013, 8, e63347. [Google Scholar] [CrossRef]
- McAloon, C.J.; Boylan, L.M.; Hamborg, T.; Stallard, N.; Osman, F.; Lim, P.B.; Hayat, S.A. The changing face of cardiovascular disease 2000–2012, An analysis of the world health organisation global health estimates data. Int. J. Cardiol. 2016, 224, 256–264. [Google Scholar] [CrossRef]
- Stefanska, A.; Bergmann, K.; Sypniewska, G. Metabolic Syndrome and Menopause: Pathophysiology, Clinical and Diagnostic Significance, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kapoor, E.; Collazo-Clavell, M.L.; Faubion, S.S. Weight gain in women at midlife: A concise review of the pathophysiology and strategies for management. Mayo Clin. Proc. 2017, 92, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Guzman, G. Estrogen deficiency and the origin of obesity during menopause. BioMed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Ogden, C.; Carroll, M.; Curtin, L.; McDowell, M.; Tabak, C.; Flegal, K. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol. Cell. Endocrinol. 2015, 418, 55–65. [Google Scholar] [CrossRef]
- Monteleone, P.; Mascagni, G.; Giannini, A.; Genazzani, A.R.; Simoncini, T. Symptoms of menopause—Global prevalence, physiology and implications. Nat. Rev. Endocrinol. 2018, 14, 199–215. [Google Scholar] [CrossRef]
- Wilson, P.; D’Agostino, R.; Sullivan, L.; Parise, H.; Kannel, W. Overweight and obesity as determinants of cardiovascular risk: The Framingham experience. Arch. Intern Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Mita, V.; Griffin, C.; McKernan, K.; Eter, L.; Abrishami, S.; Singer, K. Female adipose tissue has improved adaptability and metabolic health compared to males in aged obesity. Aging 2020, 12, 1725–1746. [Google Scholar] [CrossRef]
- Brettle, H.; Tran, V.; Drummond, G.R.; Franks, A.E.; Petrovski, S.; Vinh, A.; Jelinic, M. Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front. Immunol. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Clegg, D. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J. Steroid. Biochem. Mol. Biol. 2010, 122, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Eckel, L. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol. Behav. 2011, 104, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Musatov, S.; Chen, W.; Pfaff, D.; Mobbs, C.; Yang, X.; Clegg, D.; Kaplitt, M.; Ogawa, S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Clookey, S.; Welly, R.; Zidon, T.; Gastecki, M.; Woodford, M.; Grunewald, Z.; Winn, N.; Eaton, D.; Karasseva, N.; Sacks, H.; et al. Increased Susceptibility to OVX-Associated Metabolic Dysfunction in UCP1 Null Mice. J. Endocrinol. 2018, 239, 107–120. [Google Scholar] [CrossRef]
- Romero-Picó, A.; Novelle, M.G.; Al-Massadi, O.; Beiroa, D.; Tojo, M.; Heras, V.; Ruiz-Pino, F.; Senra, A.; López, M.; Blouet, C.; et al. Kappa-Opioid Receptor Blockade Ameliorates Obesity Caused by Estrogen Withdrawal via Promotion of Energy Expenditure through mTOR Pathway. Int. J. Mol. Sci. 2022, 23, 3118. [Google Scholar] [CrossRef]
- Gupte, A.; Pownall, H.; Hamilton, D. Estrogen: An emerging regulator of insulin action and mitochondrial function. J. Diabetes Res. 2015, 2015, 916585. [Google Scholar] [CrossRef]
- Guthrie, J.; Dennerstein, L.; Dudley, E. Weight gain and the menopause: A 5-year prospective study. Climacteric 1999, 2, 205–211. [Google Scholar] [CrossRef]
- Hao, L.; Wang, Y.; Duan, Y.; Bu, S. Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. Eur. J. Appl. Physiol. 2010, 109, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Heine, P.; Taylor, J.; Iwamoto, G.; Lubahn, D.; Cooke, P. Increased adipose tissue in male and female estrogen receptor alpha knockout mice. Proc. Natl. Acad. Sci. USA 2000, 97, 12729–12734. [Google Scholar] [CrossRef]
- Jones, M.; Thorburn, A.; Britt, K.; Hewitt, K.; Wreford, N.; Proietto, J.; Oz, O.; Leury, B.; Robertson, K.; Yao, S.; et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc. Natl. Acad. Sci. USA 2000, 97, 12735–12740. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Nair, K. Sarcopenia of aging and its metabolic impact. Curr. Top. Dev. Biol. 2005, 68, 123–148. [Google Scholar]
- Kuk, J.; Saunders, T.; Davidson, L.; Ross, R. Age-related changes in total and regional fat distribution. Ageing Res. Rev. 2009, 8, 339–348. [Google Scholar] [CrossRef]
- Sternfeld, B.; Bhat, A.; Wang, H.; Sharp, T.; Quesenberry, C., Jr. Menopause, physical activity, and body composition/fat distribution in midlife women. Med. Sci. Sport Exerc. 2005, 37, 1195–1202. [Google Scholar] [CrossRef]
- Lafrenière, J.; Prud’homme, D.; Brochu, M.; Rabasa-Lhoret, R.; Lavoie, J.; Doucet, É. Energy Density is Not a Consistent Correlate of Adiposity in Women During the Menopausal Transition. Can. J. Diet. Pract. Res. 2017, 78, 20–25. [Google Scholar] [CrossRef]
- Razmjou, S.; Bastard, J.; Doucet, E.; Rabasa-Lhoret, R.; Fellahi, S.; Lavoie, J.; Prud’homme, D. Effect of the menopausal transition and physical activity energy expenditure on inflammatory markers: A MONET group study. Menopause 2016, 23, 1330–1338. [Google Scholar] [CrossRef]
- Clookey, S.L.; Welly, R.J.; Shay, D.; Woodford, M.L.; Fritsche, K.L.; Rector, R.S.; Padilla, J.; Lubahn, D.B.; Vieira-Potter, V.J. Beta 3 adrenergic receptor activation rescues metabolic dysfunction in female estrogen receptor alpha-null mice. Front. Physiol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Shay, D.; Welly, R.; Givan, S.; Bivens, N.; Kanaley, J.; Marshall, B.; Lubahn, D.; Rosenfeld, C.; Vieira-Potter, V. Changes in nucleus accumbens gene expression accompany sex-specific suppression of spontaneous physical activity in aromatase knockout mice. Horm. Behav. 2020, 121, 104719. [Google Scholar] [CrossRef] [PubMed]
- Vague, J.; Vague, J. La Differenciation Sexuelle Facteur Determinant Des Formes De Lobesite. Press Med. 1947, 55, 339–340. [Google Scholar]
- Pisoni, S.; Marrachelli, V.G.; Morales, J.M.; Maestrini, S.; Di Blasio, A.M.; Monle, D. Sex Dimorphism in the Metabolome of Metabolic Syndrome in Morbidly Obese Individuals. Metabolites 2022, 12, 419. [Google Scholar] [CrossRef]
- Shuster, A.; Patlas, M.; Pinthus, J.; Mourtzakis, M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012, 85, 1–10. [Google Scholar] [CrossRef]
- Kotani, K.; Tokunaga, K.; Fujioka, S.; Kobatake, T.; Keno, Y.; Yoshida, S.; Shimomura, I.; Tarui, S.; Matsuzawa, Y. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 202–207. [Google Scholar]
- Belfki, H.; Ben Ali, S.; Aounallah-Skhiri, H.; Traissac, P.; Bougatef, S.; Maire, B.; Delpeuch, F.; Achour, N.; Ben Romdhane, H. Prevalence and determinants of the metabolic syndrome among Tunisian adults: Results of the Transition and Health Impact in North Africa (TAHINA) project. Public Health Nutr. 2013, 16, 582–590. [Google Scholar] [CrossRef]
- Perry, A.; Wang, X.; Goldberg, R.; Ross, R.; Jackson, L. Androgenic sex steroids contribute to metabolic risk beyond intra-abdominal fat in overweight/obese black and white women. Obesity 2013, 21, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Galmés-Pascual, B.M.; Martínez-Cignoni, M.R.; Morán-Costoya, A.; Bauza-Thorbrügge, M.; Sbert-Roig, M.; Valle, A.; Proenza, A.M.; Lladó, I.; Gianotti, M. 17Β-Estradiol Ameliorates Lipotoxicity-Induced Hepatic Mitochondrial Oxidative Stress and Insulin Resistance. Free Radic. Biol. Med. 2020, 150, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Misso, M.L.; Jang, C.; Adams, J.; Tran, J.; Murata, Y.; Bell, R.; Boon, W.C.; Simpson, E.R.; Davis, S.R. Differential expression of factors involved in fat metabolism with age and the menopause transition. Maturitas 2005, 51, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen signaling in metabolic inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E. Sources of estrogen and their importance. J. Steroid. Biochem. Mol. Biol. 2003, 86, 225–230. [Google Scholar] [CrossRef]
- Leeners, B.; Geary, N.; Tobler, P.N.; Asarian, L. Ovarian hormones and obesity. Hum. Reprod. Update 2017, 23, 300–321. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Park, H.; Baek, S.; Lim, J. The association between trunk body composition and spinal bone mineral density in Korean males versus females: A Farmers’ Cohort for Agricultural Work-Related Musculoskeletal Disorders (FARM) study. J. Korean Med. Sci. 2016, 31, 1595–1603. [Google Scholar] [CrossRef]
- Janssen, I.; Powell, L.; Jasielec, M.; Kazlauskaite, R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity 2015, 23, 488–494. [Google Scholar] [CrossRef]
- Ambikairajah, A.; Walsh, E.; Tabatabaei-Jafari, H.; Cherbuin, N. Fat mass changes during menopause: A metaanalysis. Am. J. Obstet. Gynecol. 2019, 221, 393–409.e50. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Lim, Y.-H.; Kim, K.-S.; Gil Kim, S.; Kim, J.H.; Gil Lim, H.; Shin, J. Body fat distribution after menopause and cardiovascular disease risk factors: Korean National Health and Nutrition Examination Survey 2010. J. Women’s Health 2013, 22, 587–594. [Google Scholar] [CrossRef]
- Sowers, M.F.; Zheng, H.; Tomey, K.; Karvonen-Gutierrez, C.; Jannausch, M.; Li, X.; Yosef, M.; Symons, J. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef]
- Tremollieres, F.; Pouilles, J.; Ribot, C. Relative influence of age and menopause on total and regional body composition changes in postmenopausal women. Am. J. Obs. Gynecol. 1996, 175, 1594–1600. [Google Scholar] [CrossRef]
- Torrens, J.; Sutton-Tyrrell, K.; Zhao, X.; Matthews, K.; Brockwell, S.; Sowers, M.; Santoro, N. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: Study of Women’s Health across the Nation. Menopause 2009, 16, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Hermano, E.; Goldberg, R.; Rubinstein, A.M.; Sonnenblick, A.; Maly, B.; Nahmias, D.; Li, J.P.; Bakker, M.A.H.; Van Der Vlag, J.; Vlodavsky, I.; et al. Heparanase accelerates obesity-associated breast cancer progression. Cancer Res. 2019, 79, 5342–5354. [Google Scholar] [CrossRef] [PubMed]
- Goossens, G. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol. Behav. 2008, 94, 206–218. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 DALYs and HALE Collaborators; Murray, C.J.L.; Barber, R.M.; Foreman, K.J.; Ozgoren, A.A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013, Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef]
- Suganami, T.; Tanaka, M.; Ogawa, Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr. J. 2012, 59, 849–857. [Google Scholar] [CrossRef]
- Krotkiewski, M.; Bjorntorp, P.; Sjostrom, L.; Smith, U. Impact of obesity on metabolism in men and women. Importance of regional adipose tissue distribution. J. Clin. Investig. 1983, 72, 1150–1162. [Google Scholar] [CrossRef]
- Weyer, C.; Foley, J.; Bogardus, C.; Tataranni, P.; Pratley, R. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000, 43, 1498–1506. [Google Scholar] [CrossRef]
- Vieira Potter, V.J.; Strissel, K.J.; Xie, C.; Chang, E.; Bennett, G.; Defuria, J.; Obin, M.S.; Greenberg, A.S. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology 2012, 153, 4266–4277. [Google Scholar] [CrossRef]
- Pasarica, M.; Sereda, O.; Redman, L.; Albarado, D.; Hymel, D.; Roan, L.; Rood, J.; Burk, D.H.; Smith, S.S. Reduced adipose tissue oxygenation in human obesity: Evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 2009, 58, 718–725. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clément, K.; Scherer, P. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Fatima, L.A.; Campello, R.S.; Santos, R.D.S.; Freitas, H.S.; Frank, A.P.; Machado, U.F.; Clegg, D.J. Estrogen receptor 1 (ESR1) regulates VEGFA in adipose tissue. Sci. Rep. 2017, 7, 16716. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Moore, T.; Drew, B.; Ribas, V.; Wanagat, J.; Civelek, M.; Segawa, M.; Wolf, D.; Norheim, F.; Seldin, M.; et al. Estrogen receptor α controls metabolism in white and brown adipocytes by regulating Polg1 and mitochondrial remodeling. Sci. Transl. Med. 2020, 12, eaax8096. [Google Scholar] [CrossRef]
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 69. [Google Scholar] [CrossRef]
- Davis, K.; Neinast, M.D.; Sun, K.; Skiles, W.M.; Bills, J.D.; Zehr, J.A.; Zeve, D.; Hahner, L.; Cox, D.W.; Gent, L.M.; et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013, 2, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Rogers, N.; Perfield, J.; Strissel, K.; Obin, M.; Greenberg, A. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009, 150, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Liu, J.; Geletka, L.; Delaney, C.; Delproposto, J.; Desai, A.; Oatmen, K.; Martinez-Santibanez, G.; Julius, A.; Garg, S.; et al. Aging Is Associated with an Increase in T Cells and Inflammatory Macrophages in Visceral Adipose Tissue. J. Immunol. 2011, 187, 6208–6216. [Google Scholar] [CrossRef]
- Ohlsson, C.; Hammarstedt, A.; Vandenput, L.; Saarinen, N.; Ryberg, H.; Windahl, S.H.; Farman, H.H.; Jansson, J.O.; Movérare-Skrtic, S.; Smith, U.; et al. Increased adipose tissue aromatase activity improves insulin sensitivity and reduces adipose tissue inflammation in male mice. Am. J. Physiol.-Endocrinol. Metab. 2017, 313, E450–E462. [Google Scholar] [CrossRef]
- Muller, M.; Grobbee, D.; den Tonkelaar, I.; Lamberts, S.; van der Schouw, Y. Endogenous sex hormones and metabolic syndrome in aging men. J. Clin. Endocrinol. Metab. 2005, 90, 2618–2623. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.; Kiens, B. Gender differences in skeletal muscle substrate metabolism—Molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef]
- Nuutila, P.; Knuuti, M.; Maki, M.; Laine, H.; Ruotsalainen, U.; Teräs, M.; Haaparanta, M.; Solin, O.; Yki-Järvinen, H. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes 1995, 44, 31–36. [Google Scholar] [CrossRef]
- Ferrara, C.; Goldberg, A.; Nicklas, B.; Sorkin, J.; Ryan, A. Sex differences in insulin action and body fat distribution in overweight and obese middle-aged and older men and women. Appl. Physiol. Nutr. Metab. 2008, 33, 784–790. [Google Scholar] [CrossRef]
- Garaulet, M.; Perex-Llamas, F.; Fuente, T.; Zamora, S.; Tebar, F.J. Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. Eur. J. Endocrinol. 2000, 143, 657–666. [Google Scholar] [CrossRef]
- Girousse, A.; Tavernier, G.; Valle, C.; Moro, C.; Mejhert, N.; Dinel, A.; Houssier, M.; Roussel, B.; Besse-Patin, A.; Combes, M.; et al. Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol. 2013, 11, e1001485. [Google Scholar] [CrossRef]
- Park, Y.M.; Pereira, R.I.; Erickson, C.B.; Swibas, T.A.; Cox-York, K.A.; Van Pelt, R.E. Estradiol-mediated improvements in adipose tissue insulin sensitivity are related to the balance of adipose tissue estrogen receptor α and β in postmenopausal women. PLoS ONE 2017, 12, e0176446. [Google Scholar] [CrossRef]
- Piche, M.; Weisnagel, S.; Corneau, L.; Nadeau, A.; Bergeron, J.; Lemieux, S. Contribution of abdominal visceral obesity and insulin resistance to the cardiovascular risk profile of postmenopausal women. Diabetes 2005, 54, 770–777. [Google Scholar] [CrossRef]
- Macotela, Y.; Boucher, J.; Tran, T.; Kahn, C. Sex and Depot Differences in Adipocyte Insulin Sensitivity and Glucose Metabolism. Diabetes 2009, 58, 803–812. [Google Scholar] [CrossRef]
- Herrmann, B.L.; Saller, B.; Janssen, O.E.; Gocke, P.; Bockisch, A.; Sperling, H.; Mann, K.; Broecker, M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J. Clin. Endocrinol. Metab. 2002, 87, 5476–5484. [Google Scholar] [CrossRef]
- Smith, E.P.; Boyd, J.; Frank, G.; Frank, G.R.; Takahashi, H.; Cohen, R.M.; Specker, B.; Williams, T.C.; Lubahn, D.B.; Korach, K.S. Estrogen resistance caused by a mutation in the estrogen receptor gene in a man. N. Engl. J. Med. 1994, 331, 1056–1061. [Google Scholar] [CrossRef]
- Bracht, J.; Vieira-Potter, V.; De Souza Santos, R.; Öz, O.; Palmer, B.; Clegg, D. The role of estrogens in the adipose tissue milieu. Ann. N. Y. Acad. Sci. 2020, 1461, 127–143. [Google Scholar] [CrossRef]
- Clegg, D.; Hevener, A.L.; Moreau, K.L.; Morselli, E.; Criollo, A.; Van Pelt, R.E.; Vieira-Potter, V.J. Sex hormones and cardiometabolic health: Role of estrogen and estrogen receptors. Endocrinology 2017, 158, 1095–1105. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 2012, 14, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Handgraaf, S.; Riant, E.; Fabre, A.; Waget, A.; Burcelin, R.; Lière, P.; Krust, A.; Chambon, P.; Arnal, J.-F. Pierre Gourdy Prevention of obesity and insulin resistance by estrogens requires ERα activation function-2 (ERαAF-2), whereas ERαAF-1 is dispensable. Diabetes 2013, 62, 4098–4108. [Google Scholar] [CrossRef]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and Inflammation: New Insights into Breast Cancer Development and Progression. Am. Soc. Clin. Oncol. Educ. B 2013, 33, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.D.; Perez, E.; Yang, S.; Liu, R.; Covey, D.F.; Simpkins, J.W. The assessment of non-feminizing estrogens for use in neuroprotection. Brain Res. 2011, 1379, 61–70. [Google Scholar] [CrossRef] [PubMed]
- González-Granillo, M.; Savva, C.; Li, X.; Ghosh Laskar, M.; Angelin, B.; Gustafsson, J.Å.; Korach-André, M. Selective estrogen receptor (ER)β activation provokes a redistribution of fat mass and modifies hepatic triglyceride composition in obese male mice. Mol. Cell. Endocrinol. 2020, 502, 110672. [Google Scholar] [CrossRef]
- Ponnusamy, S.; Tran, Q.; Harvey, I.; Smallwood, H.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.; Dalton, J.; Sullivan, R.; Miller, D.; et al. Pharmacologic activation of estrogen receptor β increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB 2017, 31, 266–281. [Google Scholar] [CrossRef]
- Sims, S.T.; Kubo, J.; Desai, M.; Bea, J.; Beasley, J.M.; Manson, J.E.; Allison, M.; Seguin, R.A.; Chen, Z.; Michael, Y.L.; et al. Changes in physical activity and body composition in postmenopausal women over time. Med. Sci. Sports Exerc. 2013, 45, 1486–1492. [Google Scholar] [CrossRef]
- Lakka, T.; Laaksonen, D. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef]
- Maréchal, R.; Ghachem, A.; Prud’homme, D.; Rabasa-Lhoret, R.; Dionne, I.; Brochu, M. Physical activity energy expenditure and fat-free mass: Relationship with metabolic syndrome in overweight or obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2021, 46, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Engin, B.; Willis, S.; Malaikah, S.; Sargeant, J.; Yates, T.; Gray, L.; Aithal, G.; Stensel, D.; King, J. The effect of exercise training on adipose tissue insulin sensitivity: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13445. [Google Scholar] [CrossRef]
- Serra, M.C.; Blumenthal, J.B.; Addison, O.R.; Miller, A.J.; Goldberg, A.P.; Ryan, A.S. Effects of Weight Loss with and without Exercise on Regional Body Fat Distribution in Postmenopausal Women. Ann. Nutr. Metab. 2017, 70, 312–320. [Google Scholar] [CrossRef]
- Gonzalo-Encabo, P.; McNeil, J.; Pérez-López, A.; Valadés, D.; Courneya, K.S.; Friedenreich, C.M. Dose-response effects of aerobic exercise on adiposity markers in postmenopausal women: Pooled analyses from two randomized controlled trials. Int. J. Obes. 2021, 45, 1298–1309. [Google Scholar] [CrossRef]
- De Magalhães, A.C.L.; Carvalho, V.F.; da Cruz, S.P.; Ramalho, A. Dose–Response Relationship of Resistance Training on Metabolic Phenotypes, Body Composition and Lipid Profile in Menopausal Women. Int. J. Environ. Res. Public Health 2022, 19, 10369. [Google Scholar] [CrossRef] [PubMed]
- Earnest, C.P.; Johannsen, N.M.; Swift, D.L.; Lavie, C.J.; Blair, S.N.; Church, T.S. Dose effect of cardiorespiratory exercise on metabolic syndrome in postmenopausal women. Am. J. Cardiol. 2013, 111, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.; Jungbluth Rodriguez, K.; Sabag, A.; Mavros, Y.; Parker, H.M.; Keating, S.E.; Johnson, N.A. Effect of aerobic exercise on waist circumference in adults with overweight or obesity: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13446. [Google Scholar] [CrossRef]
- Park, Y.M.; Kanaley, J.A.; Padilla, J.; Zidon, T.; Welly, R.J.; Will, M.J.; Britton, S.L.; Koch, L.G.; Ruegsegger, G.N.; Booth, F.W.; et al. Effects of intrinsic aerobic capacity and ovariectomy on voluntary wheel running and nucleus accumbens dopamine receptor gene expression. Physiol. Behav. 2016, 1, 383–389. [Google Scholar] [CrossRef]
- Sato, Y.; Nagasaki, M.; Nakai, N.; Fushimi, T. Physical exercise improves glucose metabolism in lifestyle-related diseases. Exp. Biol. Med. 2003, 228, 1208–1212. [Google Scholar] [CrossRef]
- Dehghan, A.; Vasan, S.K.; Fielding, B.A.; Karpe, F. A prospective study of the relationships between change in body composition and cardiovascular risk factors across the menopause. Menopause 2021, 28, 400–406. [Google Scholar] [CrossRef]
- Hyvärinen, M.; Juppi, H.K.; Taskinen, S.; Karppinen, J.E.; Karvinen, S.; Tammelin, T.H.; Kovanen, V.; Aukee, P.; Kujala, U.M.; Rantalainen, T.; et al. Metabolic health, menopause, and physical activity—A 4-year follow-up study. Int. J. Obes. 2022, 46, 544–554. [Google Scholar] [CrossRef]
- Kravitz, H.; Kazlauskaite, R.; Joffe, H. Sleep, Health, and Metabolism in Midlife Women and Menopause: Food for Thought. Obs. Gynecol. Clin. N. Am. 2018, 45, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M.; El Refaye, G.E.; Rabiee, A.; Abouzeid, S.; Elsisi, H.F. Aerobic exercise with diet induces hormonal, metabolic, and psychological changes in postmenopausal obese women. Heliyon 2022, 8, e09165. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Silva, I.; Dorneles, G.; Lira, F.; Romão, P.; Peres, A. Reduced Fat Oxidation During Exercise in Post-Menopausal Overweight-Obese Women with Higher Lipid Accumulation Product Index. Exp. Clin. Endocrinol. Diabetes 2020, 128, 556–562. [Google Scholar] [CrossRef]
- Porter, J.W.; Barnas, J.L.; Welly, R.; Spencer, N.; Pitt, J.; Vieira-Potter, V.J.; Kanaley, J.A. Estrogen Receptors in Individuals with Obesity. Obesity 2020, 28, 1698–1707. [Google Scholar] [CrossRef]
- Ahmed, F.; Hetty, S.; Vranic, M.; Fanni, G.; Kullberg, J.; Pereira, M.J.; Eriksson, J.W. ESR2 expression in subcutaneous adipose tissue is related to body fat distribution in women, and knockdown impairs preadipocyte differentiation. Adipocyte 2022, 11, 434–447. [Google Scholar] [CrossRef]
- Dirkes, R.K.; Winn, N.C.; Jurrissen, T.J.; Lubahn, D.B.; Vieira-Potter, V.J.; Padilla, J.; Hinton, P.S. Voluntary wheel running partially compensates for the effects of global estrogen receptor-α knockout on cortical bone in young male mice. Int. J. Mol. Sci. 2021, 22, 1734. [Google Scholar] [CrossRef] [PubMed]
- Queathem, E.D.; Fitzgerald, M.; Welly, R.; Rowles, C.C.; Schaller, K.; Bukhary, S.; Baines, C.P.; Rector, R.S.; Padilla, J.; Manrique-Acevedo, C.; et al. Suppression of estrogen receptor beta classical genomic activity enhances systemic and adipose-specific response to chronic beta-3 adrenergic receptor (β3AR) stimulation. Front. Physiol. 2022, 13, 1–20. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen Receptor β Controls Muscle Growth and Regeneration in Young Female Mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef]
- Zidon, T.; Padilla, J.; Fritsche, K.; Welly, R.; McCabe, L.; Stricklin, O.; Frank, A.; Park, Y.; Clegg, D.; Lubahn, D.; et al. Effects of ERβ and ERα on OVX-induced changes in adiposity and insulin resistance. J. Endocrinol. 2020, 245, 165–178. [Google Scholar] [CrossRef]
- Clart, L.M.; Welly, R.J.; Queathem, E.D.; Rector, R.S.; Padilla, J.; Baines, C.P.; Kanaley, J.A.; Lubahn, D.B.; Vieira-Potter, V.J. Role of ERβ in adipocyte metabolic response to wheel running following ovariectomy. J. Endocrinol. 2021, 249, 233–237. [Google Scholar] [CrossRef]
- Winn, N.C.; Jurrissen, T.J.; Grunewald, Z.I.; Cunningham, R.P.; Woodford, M.L.; Kanaley, J.A.; Lubahn, D.B.; Manrique-Acevedo, C.; Rector, R.S.; Vieira-Potter, V.J.; et al. Estrogen receptor-α signaling maintains immunometabolic function in males and is obligatory for exercise-induced amelioration of nonalcoholic fatty liver. Am. J. Physiol. Metab. 2019, 316, E156–E167. [Google Scholar] [CrossRef]
- Queathem, E.D.; Welly, R.J.; Clart, L.M.; Rowles, C.C.; Timmons, H.; Fitzgerald, M.; Eichen, P.A.; Lubahn, D.B.; Vieira-Potter, V.J. White adipose tissue depots respond to chronic beta-3 adrenergic receptor activation in a sexually dimorphic and depot divergent manner. Cells 2021, 10, 3453. [Google Scholar] [CrossRef]
- Bea, J.W.; Cussler, E.C.; Going, S.B.; Blew, R.M.; Metcalfe, L.L.; Lohman, T.G. Resistance training predicts 6-yr body composition change in postmenopausal women. Med. Sci. Sports Exerc. 2010, 42, 1286–1295. [Google Scholar] [CrossRef]
- Dam, T.V.; Dalgaard, L.B.; Thomsen, C.B.; Hjortebjerg, R.; Ringgaard, S.; Johansen, F.T.; Bengtsen, M.B.; Mose, M.; Lauritsen, K.M.; Søndergaard, E.; et al. Estrogen modulates metabolic risk profile after resistance training in early postmenopausal women: A randomized controlled trial. Menopause 2021, 28, 1214–1224. [Google Scholar] [CrossRef]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sport Med. 2018, 48, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Dupuit, M.; Maillard, F.; Pereira, B.; Marquezi, M.L.; Lancha, A.H.; Boisseau, N. Effect of high intensity interval training on body composition in women before and after menopause: A meta-analysis. Exp. Physiol. 2020, 105, 1470–1490. [Google Scholar] [CrossRef] [PubMed]
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Pereira, B.; Bonnet, A.; Maillard, F.; Duclos, M.; Boisseau, N. Moderate-Intensity Continuous Training or High-Intensity Interval Training with or without Resistance Training for Altering Body Composition in Postmenopausal Women. Med. Sci. Sport. Exerc. 2020, 52, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.; Arigo, D. Meaningful weight loss in obese postmenopausal women: A pilot study of high-intensity interval training and wearable technology. Menopause 2018, 25, 465–470. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, C.; Chen, X.; Sun, X.; Wang, Y.; Yang, J.; Wang, F. The effects of HIIT compared to MICT on endothelial function and hemodynamics in postmenopausal females. J. Sci. Med. Sport 2022, 25, 364–371. [Google Scholar] [CrossRef]
- Steckling, F.; Farinha, J.; Figueiredo, F.; Santos, D.; Bresciani, G.; Kretzmann, N.; Stefanello, S.; Courtes, A.; Beck, M.; Sangoi Cardoso, M.; et al. High-intensity interval training improves inflammatory and adipokine profiles in postmenopausal women with metabolic syndrome. Arch. Physiol. Biochem. 2019, 125, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Fortaleza, A.; Rossi, F.; Diniz, T.; Codogno, J.; Gobbo, L.; Gobbi, S.; Freitas, I.J. Functional training reduces body fat and improves functional fitness and cholesterol levels in postmenopausal women: A randomized clinical trial. J. Sport Med. Phys. 2017, 57, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Barbat-Artigas, S.; Filion, M.; Dupontgand, S.; Karelis, A.; Aubertin-Leheudre, M. Effects of tai chi training in dynapenic and nondynapenic postmenopausal women. Menopause 2011, 18, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Susanti, H.; Sonko, I.; Chang, P.; Chuang, Y.; Chung, M. Effects of yoga on menopausal symptoms and sleep quality across menopause statuses: A randomized controlled trial. Nurs. Health Sci. 2022, 24, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.; Santaella, D.; Pontes, I.; Shiramizu, V.; Nascimento, E.; Cabral, A.; Lemos, T.; Silva, R.; Ribeiro, A. Hatha Yoga practice decreases menopause symptoms and improves quality of life: A randomized controlled trial. Complement. Ther. Med. 2016, 26, 128–135. [Google Scholar] [CrossRef]
- Duval, K.; Prud’Homme, D.; Rabasa-Lhoret, R.; Strychar, I.; Brochu, M.; Lavoie, J.M.; Doucet, É. Effects of the menopausal transition on energy expenditure: A MONET Group Study. Eur. J. Clin. Nutr. 2013, 67, 407–411. [Google Scholar] [CrossRef]
- Howard, B.; Van Horn, L.; Hsia, J.; Manson, J.; Stefanick, M.; Wassertheil-Smoller, S.; Kuller, L.; LaCroix, A.; Langer, R.; Lasser, N.; et al. Low-fat dietary pattern and risk of cardiovascular disease: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006, 295, 655–666. [Google Scholar] [CrossRef]
- Prentice, R.; Caan, B.; Chlebowski, R.; Patterson, R.; Kuller, L.; Ockene, J.; Margolis, K.; Limacher, M.; Manson, J.; Parker, L.; et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006, 295, 629–642. [Google Scholar] [CrossRef]
- Howard, B.V.; Aragaki, A.K.; Tinker, L.F.; Allison, M.; Hingle, M.D.; Johnson, K.C.; Manson, J.A.E.; Shadyab, A.H.; Shikany, J.M.; Snetselaar, L.G.; et al. A low-fat dietary pattern and diabetes: A secondary analysis from the women’s health initiative dietary modification trial. Diabetes Care 2018, 41, 680–687. [Google Scholar] [CrossRef]
- Prentice, R.L.; Aragaki, A.K.; Howard, B.V.; Chlebowski, R.T.; Thomson, C.A.; Van Horn, L.; Tinker, L.F.; Manson, J.E.; Anderson, G.L.; Kuller, L.E.; et al. Low-fat dietary pattern among postmenopausal women influences long-Term cancer, cardiovascular disease, and diabetes outcomes. J. Nutr. 2019, 149, 1565–1574. [Google Scholar] [CrossRef]
- Prentice, R.L.; Mossavar-Rahmani, Y.; Huang, Y.; Van Horn, L.; Beresford, S.A.A.; Caan, B.; Tinker, L.; Schoeller, D.; Bingham, S.; Eaton, C.B.; et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am. J. Epidemiol. 2011, 174, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Baber, R.J. Treating menopause—MHT and beyond. Nat. Rev. Endocrinol. 2022, 18, 490–502. [Google Scholar] [CrossRef]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef]
- Bermingham, K.M.; Linenberg, I.; Hall, W.L.; Kadé, K.; Franks, P.W.; Davies, R.; Wolf, J.; Hadjigeorgiou, G.; Asnicar, F.; Segata, N.; et al. Menopause is associated with postprandial metabolism, metabolic health and lifestyle: The ZOE PREDICT study. EBioMedicine 2022, 85, 104303. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Lambrinoudaki, I.; Lumsden, M.; Mishra, G.D.; Pal, L.; Rees, M.; Santoro, N.; Simoncini, T. Menopause. Nat. Rev. Dis. Prim. 2015, 1, 15004. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, Y.; Kartsonaki, C. Associations of adiposity and weight change with recurrence and survival in breast cancer patients: A systematic review and meta-analysis. Breast Cancer 2022, 29, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, E.; Furtado, G.E.; Jones, J.G.; Portincasa, P.; Vieira-Pedrosa, A.; Teixeira, A.M.; Barros, M.P.; Bachi, A.L.L.; Sardão, V.A. The advantages of physical exercise as a preventive strategy against NAFLD in postmenopausal women. Eur. J. Clin. Investig. 2022, 52, e13731. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsh, M.L.; Oliveira, M.N.; Vieira-Potter, V.J. Adipocyte Metabolism and Health after the Menopause: The Role of Exercise. Nutrients 2023, 15, 444. https://doi.org/10.3390/nu15020444
Marsh ML, Oliveira MN, Vieira-Potter VJ. Adipocyte Metabolism and Health after the Menopause: The Role of Exercise. Nutrients. 2023; 15(2):444. https://doi.org/10.3390/nu15020444
Chicago/Turabian StyleMarsh, Megan L., Marta Novaes Oliveira, and Victoria J. Vieira-Potter. 2023. "Adipocyte Metabolism and Health after the Menopause: The Role of Exercise" Nutrients 15, no. 2: 444. https://doi.org/10.3390/nu15020444
APA StyleMarsh, M. L., Oliveira, M. N., & Vieira-Potter, V. J. (2023). Adipocyte Metabolism and Health after the Menopause: The Role of Exercise. Nutrients, 15(2), 444. https://doi.org/10.3390/nu15020444





