Can Bioactive Food Substances Contribute to Cystic Fibrosis-Related Cardiovascular Disease Prevention?
Abstract
1. Introduction
2. Cardiovascular Disease in Patients with Cystic Fibrosis
2.1. Cardiomyopathy
2.2. Right and Left Ventricular Dysfunction
2.3. Coronary Artery Disease
2.4. Endothelial Dysfunction
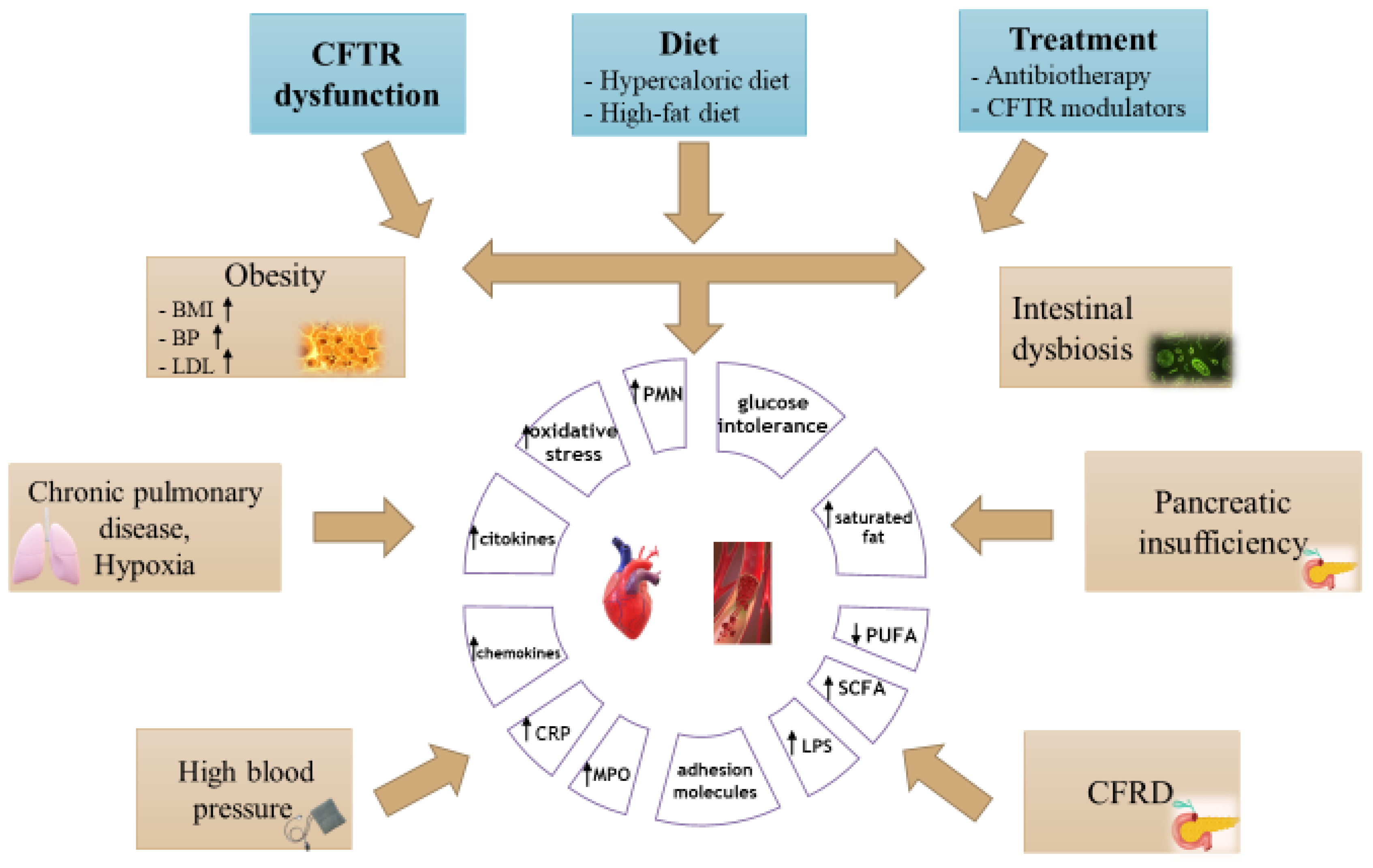
3. Cardiovascular Risk Factors in Cystic Fibrosis
4. Inflammation and Atherosclerotic Process in Cystic Fibrosis
5. Obesity Risk in Patients with CF
6. Novel CFTR Modulator Therapies
7. Impact of Altered Gut Microbiota in Cystic Fibrosis
8. Potential Role of Nutrition in Prevention of Cardiovascular Damage in Cystic Fibrosis
8.1. Phytotherapy in Cystic Fibrosis
8.2. Quercetin
8.3. Curcumin
8.4. Resveratrol
8.5. Allicin
8.6. Green Tea
8.7. Red Yeast Rice
8.8. Cocoa
8.9. Polyunsaturated Fatty Acids
8.10. Vitamins
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunders, T.; Burgner, D.; Ranganathan, S. Identifying and preventing cardiovascular disease in patients with cystic fibrosis. Nat. Cardiovasc. Res. 2022, 1, 187–188. [Google Scholar] [CrossRef]
- Stephenson, A.L.; AMannik, L.; Walsh, S.; Brotherwood, M.; Robert, R.; Darling, P.B.; Nisenbaum, R.; Moerman, J.; Stanojevic, S. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: A population-based cohort study. Am. J. Clin. Nutr. 2013, 97, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, A.; Aliberti, S.; Contarini, M.; Savi, D.; Sotgiu, G.; Majo, F.; Saderi, L.; Lucidi, V.; Amati, F.; Pappalettera, M.; et al. Overweight and obesity in adults with cystic fibrosis: An Italian multicenter cohort study. J. Cyst. Fibros. 2022, 21, 111–114. [Google Scholar] [CrossRef] [PubMed]
- McBennett, K.A.; Davis, P.B.; Konstan, M.W. Increasing life expectancy in cystic fibrosis: Advances and challenges. Pediatr. Pulmonol. 2022, 57 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef]
- Georgiopoulou, V.V.; Denker, A.; Bishop, K.L.; Brown, J.M.; Hirsh, B.; Wolfenden, L.; Sperling, L. Metabolic abnormalities in adults with cystic fibrosis: Metabolic abnormalities in CF. Respirology 2020, 15, 823–829. [Google Scholar] [CrossRef]
- Available online: https://www.cff.org/sites/default/files/2021-11/Patient-Registry-Annual-Data-Report.pdf (accessed on 25 November 2022).
- Labombarda, F.; Saloux, E.; Brouard, J.; Bergot, E.; Milliez, P. Heart involvement in cystic fibrosis: A specific cystic fibrosis-related myocardial changes? Respir. Med. 2016, 118, 31–38. [Google Scholar] [CrossRef]
- Shah, P.H.; Lee, J.H.; Salvi, D.J.; Rabbani, R.; Gavini, D.R.; Hamid, P. Cardiovascular System Involvement in Cystic Fibrosis. Cureus 2021, 13, e16723. [Google Scholar] [CrossRef]
- Poore, T.S.; Taylor-Cousar, J.L.; Zemanick, E.T. Cardiovascular complications in cystic fibrosis: A review of the literature. J. Cyst. Fibros. 2021, 21, 18–25. [Google Scholar] [CrossRef]
- Lagan, J.; Naish, J.H.; Bradley, J.; Fortune, C.; Palmer, C.; Clark, D.; Schelbert, E.B.; Schmitt, M.; Bright-Thomas, R.; Miller, C.A. Cardiac involvement in cystic fibrosis evaluated using cardiopulmonary magnetic resonance. Int. J. Cardiovasc. Imaging 2022, 38, 1121–1131. [Google Scholar] [CrossRef]
- Trandafir, L.M.; Dodi, G.; Frasinariu, O.; Luca, A.C.; Butnariu, L.I.; Tarca, E.; Moisa, S.M. Tackling Dyslipidemia in Obesity from a Nanotechnology Perspective. Nutrients 2022, 14, 3774. [Google Scholar] [CrossRef]
- Lai, S.; Mazzaferro, S.; Mitterhofer, A.P.; Bonci, E.; Marotta, P.G.; Pelligra, F.; Murciano, M.; Celani, C.; Troiani, P.; Cimino, G.; et al. Renal involvement and metabolic alterations in adults patients affected by cystic fibrosis. J. Transl. Med. 2019, 17, 388. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Winn, A.; Seddon, P.; Ranganathan, S. A fat lot of good: Balance and trends in fat intake in children with cystic fibrosis. J. Cyst. Fibros. 2012, 11, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Hayden, H.S.; Eng, A.; Pope, C.E.; Brittnacher, M.J.; Vo, A.T.; Weiss, E.J.; Hager, K.R.; Martin, B.D.; Leung, D.H.; Heltshe, S.L.; et al. Fecal dysbiosis in infants with cystic fibrosis is associated with early linear growth failure. Nat. Med. 2020, 26, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Bass, R.; Brownell, J.N.; Stallings, V.A. The Impact of Highly Effective CFTR Modulators on Growth and Nutrition Status. Nutrients 2021, 13, 2907. [Google Scholar] [CrossRef] [PubMed]
- Pallin, M.; Keating, D.; Kaye, D.M.; Kotsimbos, T.; Wilson, J.W. Subclinical Left Ventricular Dysfunction is Influenced by Genotype Severity in Patients with Cystic Fibrosis. Clin. Med. Insights: Circ. Respir. Pulm. Med. 2018, 12, 1179548418794154. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Skolnik, K.; Levy, R.D.; Wilcox, P.G.; Quon, B.S. Coronary artery disease in cystic fibrosis: An emerging concern? J. Cyst. Fibros. 2016, 15, e70–e71. [Google Scholar] [CrossRef]
- Reverri, E.J.; Morrissey, B.M.; Cross, C.E.; Steinberg, F.M. Inflammation, oxidative stress, and cardiovascular disease risk factors in adults with cystic fibrosis. Free. Radic. Biol. Med. 2014, 76, 261–277. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006, 83, 456S–460S. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Sellers, Z.M.; McGlocklin, L.; Brasch, A. Strain rate echocardiography uncovers subclinical left ventricular dysfunction in cystic fibrosis. J. Cyst. Fibros. 2015, 14, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Kanga, J.F.; Moffett, C.B.; Noonan, J.A. Changes in Left Ventricular Diastolic Filling Patterns by Doppler Echocardiography in Cystic Fibrosis*. Chest 1991, 99, 646–650. [Google Scholar] [CrossRef] [PubMed]
- González, M.P.; Suárez, L.; Camarero, C.; Escobar, H. Fibrosis miocardia en dos niños con fibrosis quística [Myocardial fibrosis in 2 children with cystic fibrosis]. Esp. Pediatr. 1987, 27, 382. (In Spanish) [Google Scholar]
- Sellers, Z.M.; De Arcangelis, V.; Xiang, Y.; Best, P.M. Cardiomyocytes with disrupted CFTR function require CaMKII and Ca2+-activated Cl− channel activity to maintain contraction rate. J. Physiol. 2010, 588, 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.G.; Florea, N.D.; Sharma, R.; Coats, A.J.; Gibson, D.G.; Hodson, M.E.; Henein, M.Y. Right Ventricular Dysfunction in Adult Severe Cystic Fibrosis. Chest 2000, 118, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Garza, E.H.; Stetson, S.J.; Cubillos-Garzon, A.; Vooletich, M.T.; Farmer, J.A.; Torre-Amione, G. Tumor Necrosis Factor-α: A mediator of disease progression in the failing human heart. Chest 1999, 115, 1170–1174. [Google Scholar] [CrossRef]
- Koelling, T.M.; Dec, G.W.; Ginns, L.C.; Semigran, M.J. Left Ventricular Diastolic Function in Patients With Advanced Cystic Fibrosis. Chest 2003, 123, 1488–1494. [Google Scholar] [CrossRef]
- Vizza, C.D.; Lynch, J.P.; Ochoa, L.L.; Richardson, G.; Trulock, E.P. Right and Left Ventricular Dysfunction in Patients With Severe Pulmonary Disease. Chest 1998, 113, 576–583. [Google Scholar] [CrossRef]
- Baño-Rodrigo, A.; Salcedo-Posadas, A.; Villa-Asensi, J.R.; Tamariz-Martel, A.; Lopez-Neyra, A.; Blanco-Iglesias, E. Right ventricular dysfunction in adolescents with mild cystic fibrosis. J. Cyst. Fibros. 2012, 11, 274–280. [Google Scholar] [CrossRef]
- Chipps, B.E.; Alderson, P.O.; Roland, J.-M.A.; Yang, S.; van Aswegen, A.; Martinez, C.R.; Rosenstein, B.J. Noninvasive evaluation of ventricular function in cystic fibrosis. J. Pediatr. 1979, 95, 379–384. [Google Scholar] [CrossRef]
- Labombarda, F.; Pellissier, A.; Ellafi, M.; Creveuil, C.; Ribault, V.; Laurans, M.; Guillot, M.; Bergot, E.; Grollier, G.; Milliez, P.; et al. Myocardial Strain Assessment in Cystic Fibrosis. J. Am. Soc. Echocardiogr. 2011, 24, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, P.; Chazalettes, J.P.; Viard, L.; Raynaud, M.; Faugere, G.; Noirclerc, M.; Bernard, P.J. Left ventricular involvement in mucoviscidosis after 2 years of age. Arch. Fr. De Pediatr. 1993, 50, 653–656. (In French) [Google Scholar]
- Benson, L.N.; Newth, C.J.; DeSouza, M.; Lobraico, R.; Kartodihardjo, W.; Corkey, C.; Gilday, D.; Olley, P.M. Radionuclide assessment of right and left ventricular function during bicycle exercise in young patients with cystic fibrosis. Am. Rev. Respir. Dis. 1984, 130, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Bers, D. Borlaug. Mechanisms of Cardiac Contractions and Relaxation. In Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Elsevier: Amsterdam, The Netherlands, 2014; Volume 22, pp. 418–441. [Google Scholar]
- Cross, C.E.; Reverri, E.J.; Morrissey, B.M. Joining the Crowd: Cystic fibrosis and cardiovascular disease risk factors. Chest 2013, 143, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Poore, S.; Berry, B.; Eidson, D.; McKie, K.T.; Harris, R.A. Evidence of Vascular Endothelial Dysfunction in Young Patients With Cystic Fibrosis. Chest 2013, 143, 939–945. [Google Scholar] [CrossRef]
- Zouk, A.N.; Gulati, S.; Xing, D.; Wille, K.M.; Rowe, S.M.; Wells, J.M. Pulmonary artery enlargement is associated with pulmonary hypertension and decreased survival in severe cystic fibrosis: A cohort study. PLoS ONE 2020, 15, e0229173. [Google Scholar] [CrossRef]
- Jiang, K.; Jiao, S.; Vitko, M.; Darrah, R.; Flask, C.A.; Hodges, C.A.; Yu, X. The impact of Cystic Fibrosis Transmembrane Regulator Disruption on cardiac function and stress response. J. Cyst. Fibros. 2016, 15, 34–42. [Google Scholar] [CrossRef]
- Eising, J.B.; van der Ent, C.K.; Evelein, A.M.V.; Uiterwaal, C.S.P.M. The association between lung function and arterial stiffness in young childhood. Eur. Respir. J. 2014, 44, 530–532. [Google Scholar] [CrossRef]
- Noe, J.; Petrusca, D.; Rush, N.; Deng, P.; VanDemark, M.; Berdyshev, E.; Gu, Y.; Smith, P.; Schweitzer, K.; Pilewsky, J.; et al. CFTR Regulation of Intracellular pH and Ceramides Is Required for Lung Endothelial Cell Apoptosis. Am. J. Respir. Cell Mol. Biol. 2009, 41, 314–323. [Google Scholar] [CrossRef]
- Wells, J.M.; Farris, R.F.; A Gosdin, T.; Dransfield, M.T.; E Wood, M.; Bell, S.C.; Rowe, S.M. Pulmonary artery enlargement and cystic fibrosis pulmonary exacerbations: A cohort study. Lancet Respir. Med. 2016, 4, 636–645. [Google Scholar] [CrossRef]
- Monroe, E.J.; Pierce, D.B.; Ingraham, C.R.; Johnson, G.E.; Shivaram, G.M.; Valji, K. An Interventionalist’s Guide to Hemoptysis in Cystic Fibrosis. Radiographics 2018, 38, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Hull, J.H.; Garrod, R.; Ho, T.B.; Knight, R.K.; Cockcroft, J.R.; Shale, D.J.; Bolton, C.E. Increased augmentation index in patients with cystic fibrosis. Eur. Respir. J. 2009, 34, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Buehler, T.; Steinmann, M.; Singer, F.; Regamey, N.; Casaulta, C.; Schoeni, M.H.; Simonetti, G.D. Increased arterial stiffness in children with cystic fibrosis. Eur. Respir. J. 2016, 39, 1536–1537. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-Y.; Mok, Y.; Kim, G.W.; Baek, S.-J.; Yun, Y.D.; Jee, S.H.; Kim, D.S. Association between idiopathic pulmonary fibrosis and coronary artery disease: A case-control study and cohort analysis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2015, 31, 289–296. [Google Scholar]
- Karpouzas, G.A.; Malpeso, J.; Choi, T.-Y.; Li, D.; Munoz, S.; Budoff, M.J. Prevalence, extent and composition of coronary plaque in patients with rheumatoid arthritis without symptoms or prior diagnosis of coronary artery disease. Ann. Rheum. Dis. 2014, 73, 1797–1804. [Google Scholar] [CrossRef]
- Perrin, F.M.; Serino, W. Ischaemic heart disease–a new issue in cystic fibrosis? J. R. Soc. Med. 2010, 103 (Suppl. S1), 44–48. [Google Scholar] [CrossRef]
- Thambuluru, S.R.; Kyazimzade, S.; Despotes, K.A.; Kirk, D.; Goralski, J.L. Acute ST-elevation myocardial infarction in two young women with cystic fibrosis and cystic fibrosis-related diabetes. J. Cyst. Fibros. 2022, 21, e44–e47. [Google Scholar] [CrossRef]
- Sandouk, Z.; Nachawi, N.; Simon, R.; Wyckoff, J.; Putman, M.S.; Kiel, S.; Soltman, S.; Moran, A.; Moheet, A. Coronary artery disease in patients with cystic fibrosis–A case series and review of the literature. J. Clin. Transl. Endocrinol. 2022, 30, 100308. [Google Scholar] [CrossRef]
- Uhl, F.E.; Vanherle, L.; Meissner, A. Cystic fibrosis transmembrane regulator correction attenuates heart failure-induced lung inflammation. Front. Immunol. 2022, 13, 928300. [Google Scholar] [CrossRef]
- Moss, T.J.; Austin, G.E.; Moss, A.J. Preatherosclerotic aortic lesions in cystic fibrosis. J. Pediatr. 1979, 94, 32–37. [Google Scholar] [CrossRef]
- Bright-Thomas, R.J.; Webb, A.K. The heart in cystic fibrosis. J. R. Soc. Med. 2002, 95 (Suppl. S41), 2–10. [Google Scholar] [PubMed]
- Onady, G.M.; Farinet, C.L. An adult cystic fibrosis patient presenting with persistent dyspnea: Case report. BMC Pulm. Med. 2006, 6, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, B.A.; Goldsweig, B.K.; Sidhaye, A.; Blackman, S.M.; Schindler, T.; Moran, A. Cystic fibrosis related diabetes: Nutrition and growth considerations. J. Cyst. Fibros. 2019, 18, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Strandvik, B. Nutrition in Cystic Fibrosis—Some Notes on the Fat Recommendations. Nutrients 2022, 14, 853. [Google Scholar] [CrossRef]
- Goetz, D.M.; Savant, A.P. Review of CFTR modulators. Pediatr. Pulmonol. 2021, 56, 3595–3606. [Google Scholar] [CrossRef] [PubMed]
- Litvin, M.; Yoon, J.C. Nutritional excess in cystic fibrosis: The skinny on obesity. J. Cyst. Fibros. 2020, 19, 3–5. [Google Scholar] [CrossRef]
- April-Sanders, A.K.; Rodriguez, C.J. Metabolically Healthy Obesity Redefined. JAMA Netw. Open 2021, 4, e218860. [Google Scholar] [CrossRef]
- Harindhanavudhi, T.; Wang, Q.; Dunitz, J.; Moran, A.; Moheet, A. Prevalence and factors associated with overweight and obesity in adults with cystic fibrosis: A single-center analysis. J. Cyst. Fibros. 2020, 19, 139–145. [Google Scholar] [CrossRef]
- Bonhoure, A.; Boudreau, V.; Litvin, M.; Colomba, J.; Bergeron, C.; Mailhot, M.; Tremblay, F.; Lavoie, A.; Rabasa-Lhoret, R. Overweight, obesity and significant weight gain in adult patients with cystic fibrosis association with lung function and cardiometabolic risk factors. Clin. Nutr. 2020, 39, 2910–2916. [Google Scholar] [CrossRef]
- Kutney, K.A.; Sandouk, Z.; Desimone, M.; Moheet, A. Obesity in cystic fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100276. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.; Com, G.; Deutz, N. Protein is an important but undervalued macronutrient in the nutritional care of patients with cystic fibrosis. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, V.; Chou, F.Y.; Lam, G.Y.; Hamilton, C.M.; Wilcox, P.G.; Quon, B.S. The Extrapulmonary Effects of Cystic Fibrosis Transmembrane Conductance Regulator Modulators in Cystic Fibrosis. Ann. Am. Thorac. Soc. 2020, 17, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Van Dorst, J.M.; Tam, R.Y.; Ooi, C.Y. What Do We Know about the Microbiome in Cystic Fibrosis? Is There a Role for Probiotics and Prebiotics? Nutrients 2022, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, L.M.; Anton-Paduraru, D.T.; Miron, I.; Indrei, L.L. Psychosocial Implications of Childhood Obesity. Rev. De Cercet. Si Interv. Soc. 2015, 49, 205–215. [Google Scholar]
- Bilan, N.; Marefat, E.; Nikniaz, L.; Farhangi, M.A.; Nikniaz, Z. Does synbiotic supplementation affect the quality of life in children with cystic fibrosis? A pilot randomized controlled clinical trial. BMC Nutr. 2020, 6, 44. [Google Scholar] [CrossRef]
- Butnariu, L.I.; Țarcă, E.; Cojocaru, E.; Rusu, C.; Moisă, M.; Constantin, M.-M.L.; Gorduza, E.V.; Trandafir, L.M. Genetic Modifying Factors of Cystic Fibrosis Phenotype: A Challenge for Modern Medicine. J. Clin. Med. 2021, 10, 5821. [Google Scholar] [CrossRef]
- Trandafir, L.M.; Leon, M.M.; Frasinariu, O.; Baciu, G.; Dodi, G.; Cojocaru, E. Current Practices and Potential Nanotechnology Perspectives for Pain Related to Cystic Fibrosis. J. Clin. Med. 2019, 8, 1023. [Google Scholar] [CrossRef]
- Tam, R.Y.; van Dorst, J.M.; McKay, I.; Coffey, M.; Ooi, C.Y. Intestinal Inflammation and Alterations in the Gut Microbiota in Cystic Fibrosis: A Review of the Current Evidence, Pathophysiology and Future Directions. J. Clin. Med. 2022, 11, 649. [Google Scholar] [CrossRef]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Matamouros, S.; Hayden, H.S.; Hager, K.R.; Brittnacher, M.J.; Lachance, K.; Weiss, E.J.; Pope, C.E.; Imhaus, A.-F.; McNally, C.P.; Borenstein, E.; et al. Adaptation of commensal proliferating Escherichia coli to the intestinal tract of young children with cystic fibrosis. Proc. Natl. Acad. Sci. USA 2018, 115, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.; Hayden, H.S.; Pope, C.E.; Brittnacher, M.J.; Vo, A.T.; Weiss, E.J.; Hager, K.R.; Leung, D.H.; Heltshe, S.L.; Raftery, D.; et al. Infants with cystic fibrosis have altered fecal functional capacities with potential clinical and metabolic consequences. BMC Microbiol. 2021, 21, 247. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Needham, B.; Leach, S.T.; Day, A.S.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Disrupted progression of the intestinal microbiota with age in children with cystic fibrosis. Sci. Rep. 2016, 6, 24857. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Syed, S.A.; Rossi, L.; Garg, M.; Needham, B.; Avolio, J.; Young, K.; Surette, M.G.; Gonska, T. Impact of CFTR modulation with Ivacaftor on Gut Microbiota and Intestinal Inflammation. Sci. Rep. 2018, 8, 1783. [Google Scholar] [CrossRef]
- Schippa, S.; Iebba, V.; Santangelo, F.; Gagliardi, A.; De Biase, R.V.; Stamato, A.; Bertasi, S.; Lucarelli, M.; Conte, M.P.; Quattrucci, S. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Allelic Variants Relate to Shifts in Faecal Microbiota of Cystic Fibrosis Patients. PLoS ONE 2013, 8, e61176. [Google Scholar] [CrossRef]
- Thavamani, A.; Salem, I.; Sferra, T.; Sankararaman, S. Impact of Altered Gut Microbiota and Its Metabolites in Cystic Fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef]
- Coffey, M.J.; Nielsen, S.; Wemheuer, B.; Kaakoush, N.O.; Garg, M.; Needham, B.; Pickford, R.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Gut Microbiota in Children With Cystic Fibrosis: A Taxonomic and Functional Dysbiosis. Sci. Rep. 2019, 9, 18593. [Google Scholar] [CrossRef]
- Albenberg, L.; Kelsen, J. Advances in Gut Microbiome Research and Relevance to Pediatric Diseases. J. Pediatr. 2016, 178, 16–23. [Google Scholar] [CrossRef]
- Kristensen, M.; Prevaes, S.M.; Kalkman, G.; Tramper-Stranders, G.A.; Hasrat, R.; de Winter-de Groot, K.M.; Janssens, H.M.; Tiddens, H.A.; van Westreenen, M.; Sanders, E.A.; et al. Development of the gut microbiota in early life: The impact of cystic fibrosis and antibiotic treatment. J. Cyst. Fibros. 2020, 19, 553–561. [Google Scholar] [CrossRef]
- Burke, D.; Fouhy, F.; Harrison, M.J.; Rea, M.C.; Cotter, P.D.; O’Sullivan, O.; Stanton, C.; Hill, C.; Shanahan, F.; Plant, B.J.; et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017, 17, 58. [Google Scholar] [CrossRef]
- Ballarini, S.; Rossi, G.; Principi, N.; Esposito, S. Dysbiosis in Pediatrics Is Associated with Respiratory Infections: Is There a Place for Bacterial-Derived Products? Microorganisms 2021, 9, 448. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, E.; Callegari, M.L.; Raia, V.; Viscovo, S.; Scotto, R.; Ferrari, S.; Morelli, L.; Buccigrossi, V.; Vecchio, A.L.; Ruberto, E.; et al. Disrupted Intestinal Microbiota and Intestinal Inflammation in Children with Cystic Fibrosis and Its Restoration with Lactobacillus GG: A Randomised Clinical Trial. PLoS ONE 2014, 9, e87796. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, G.; Huys, G.; Boulanger, L.; De Boeck, K.; Vandamme, P. Amoxicillin–clavulanic acid resistance in fecal Enterobacteriaceae from patients with cystic fibrosis and healthy siblings. J. Cyst. Fibros. 2013, 12, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Levy, R.; Pope, C.E.; Hayden, H.S.; Brittnacher, M.J.; Carr, R.; Radey, M.C.; Hager, K.R.; Heltshe, S.L.; Ramsey, B.W.; et al. Metagenomic evidence for taxonomic dysbiosis and functional imbalance in the gastrointestinal tracts of children with cystic fibrosis. Sci. Rep. 2016, 6, 22493. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.; Zaccari, P.; Rocco, G.; Scalese, G.; Panetta, C.; Porowska, B.; Pontone, S.; Severi, C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019, 25, 2706–2719. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Hu, L.; Amirault, J.; Khatwa, U.; Ward, D.V.; Onderdonk, A. 16S Community Profiling Identifies Proton Pump Inhibitor Related Differences in Gastric, Lung, and Oropharyngeal Microflora. J. Pediatr. 2015, 166, 917–923. [Google Scholar] [CrossRef]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Crawford, M.; Whisner, C.; Al-Nakkash, L.; Sweazea, K.L. Six-Week High-Fat Diet Alters the Gut Microbiome and Promotes Cecal Inflammation, Endotoxin Production, and Simple Steatosis without Obesity in Male Rats. Lipids 2019, 54, 119–131. [Google Scholar] [CrossRef]
- Anitha, M.; Reichardt, F.; Tabatabavakili, S.; Nezami, B.G.; Chassaing, B.; Mwangi, S.; Vijay-Kumar, M.; Gewirtz, A.; Srinivasan, S. Intestinal Dysbiosis Contributes to the Delayed Gastrointestinal Transit in High-Fat Diet Fed Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 328–339. [Google Scholar] [CrossRef]
- Debyser, G.; Mesuere, B.; Clement, L.; Van de Weygaert, J.; Van Hecke, P.; Duytschaever, G.; Aerts, M.; Dawyndt, P.; De Boeck, K.; Vandamme, P.; et al. Faecal proteomics: A tool to investigate dysbiosis and inflammation in patients with cystic fibrosis. J. Cyst. Fibros. 2015, 15, 242–250. [Google Scholar] [CrossRef]
- Loman, B.R.; Shrestha, C.L.; Thompson, R.; Groner, J.A.; Mejias, A.; Ruoff, K.L.; O’Toole, G.A.; Bailey, M.T.; Kopp, B.T. Age and environmental exposures influence the fecal bacteriome of young children with cystic fibrosis. Pediatr. Pulmonol. 2020, 55, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Nobili, R.M.; Alicandro, G. Challenges with optimizing nutrition in cystic fibrosis. Expert Rev. Respir. Med. 2019, 13, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Katz, T.; Liu, V.; Quintano, J.; Brunner, R.; Tong, C.W.; Collins, C.E.; Ooi, C.Y. Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lerma, J.; Hulst, J.; Boon, M.; Martins, T.; Ruperto, M.; Colombo, C.; Fornés-Ferrer, V.; Woodcock, S.; Claes, I.; Asseiceira, I.; et al. The Relative Contribution of Food Groups to Macronutrient Intake in Children with Cystic Fibrosis: A European Multicenter Assessment. J. Acad. Nutr. Diet. 2019, 119, 1305–1319. [Google Scholar] [CrossRef]
- Bass, R.M.; Tindall, A.; Sheikh, S. Utilization of the Healthy Eating Index in Cystic Fibrosis. Nutrients 2022, 14, 834. [Google Scholar] [CrossRef]
- McDonald, C.M.; Bowser, E.K.; Farnham, K.; Alvarez, J.A.; Padula, L.; Rozga, M. Dietary Macronutrient Distribution and Nutrition Outcomes in Persons with Cystic Fibrosis: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2020, 121, 1574–1590.e3. [Google Scholar] [CrossRef]
- Eilat-Adar, S.; Sinai, T.; Yosefy, C.; Henkin, Y. Nutritional Recommendations for Cardiovascular Disease Prevention. Nutrients 2013, 5, 3646–3683. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- Li, H.-Y.; Gan, R.-Y.; Shang, A.; Mao, Q.-Q.; Sun, Q.-C.; Wu, D.-T.; Geng, F.; He, X.-Q.; Li, H.-B. Plant-Based Foods and Their Bioactive Compounds on Fatty Liver Disease: Effects, Mechanisms, and Clinical Application. Oxidative Med. Cell. Longev. 2021, 2021, 6621644. [Google Scholar] [CrossRef]
- Moroşan, E.; Mihailovici, M.S.; Giuşcă, S.E.; Cojocaru, E.; Avădănei, E.R.; Căruntu, I.D.; Teleman, S. Hepatic steatosis background in chronic hepatitis B and C-significance of similarities and differences. Rom. J. Morphol. Embryol. 2014, 55 (Suppl. S3), 1041–1047. [Google Scholar]
- Ramírez-Moreno, E.; Arias-Rico, J.; Jiménez-Sánchez, R.C.; Estrada-Luna, D.; Jiménez-Osorio, A.S.; Zafra-Rojas, Q.Y.; Ariza-Ortega, J.A.; Flores-Chávez, O.R.; Morales-Castillejos, L.; Sandoval-Gallegos, E.M. Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods 2022, 11, 1232. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; McKenzie, K.; Floyd, A.D.; Kwon, E.; Zeitlin, P.L. Modulation of ΔF508 Cystic Fibrosis Transmembrane Regulator Trafficking and Function with 4-Phenylbutyrate and Flavonoids. Am. J. Respir. Cell Mol. Biol. 2004, 31, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and In-flammatory Biomarkers in Women with Type 2 Diabetes: A double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Chaudhary, N.; Ueno-Shuto, K.; Ono, T.; Ohira, Y.; Watanabe, K.; Nasu, A.; Fujikawa, H.; Nakashima, R.; Takahashi, N.; Suico, M.A.; et al. Curcumin Down-Regulates Toll-Like Receptor-2 Gene Expression and Function in Human Cystic Fibrosis Bronchial Epithelial Cells. Biol. Pharm. Bull. 2019, 42, 489–495. [Google Scholar] [CrossRef]
- Abdelhaleem, I.A.; Brakat, A.M.; Adayel, H.M.; Asla, M.M.; Rizk, M.A.; Aboalfetoh, A.Y. The effects of resveratrol on glycemic control and cardiometabolic parameters in patients with T2DM: A systematic review and meta-analysis. Med. Clínica 2022, 158, 576–585. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Randeva, H.S.; Pfeiffer, A.F.H.; Weickert, M.O. Implications of Resveratrol in Obesity and Insulin Resistance: A State-of-the-Art Review. Nutrients 2022, 14, 2870. [Google Scholar] [CrossRef]
- Lu, B.; Corey, D.A.; Kelley, T.J. Resveratrol restores intracellular transport in cystic fibrosis epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L1145–L1157. [Google Scholar] [CrossRef]
- Manali, C.; Srujana, M. Nutraceutical Approach to the Management of Cystic Fibrosis. Curr. Opin. Solid State Mater. Sci. 2022, 18, 814–826. [Google Scholar]
- Shi, X.; Zhou, X.; Chu, X.; Wang, J.; Xie, B.; Ge, J.; Guo, Y.; Li, X.; Yang, G. Allicin Improves Metabolism in High-Fat Diet-Induced Obese Mice by Modulating the Gut Microbiota. Nutrients 2019, 11, 2909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, X.; Sheng, Y.; Xu, J.; Yang, C.; Zheng, S.; Liu, J.; Li, H.; Ge, J.; Yang, M.; et al. Allicin Regulates Energy Homeostasis through Brown Adipose Tissue. iScience 2020, 23, 101113. [Google Scholar] [CrossRef] [PubMed]
- Panyod, S.; Wu, W.-K.; Chen, P.-C.; Chong, K.-V.; Yang, Y.-T.; Chuang, H.-L.; Chen, C.-C.; Chen, R.-A.; Liu, P.-Y.; Chung, C.-H.; et al. Atherosclerosis amelioration by allicin in raw garlic through gut microbiota and trimethylamine-N-oxide modulation. NPJ Biofilms Microbiomes 2022, 8, 4. [Google Scholar] [CrossRef]
- Ried, K. Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review. J. Nutr. 2016, 146, 389S–396S. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Quercegen Pharmaceuticals LLC Obtains FDA GRAS Notified Status for Its QU995™ Quercetin with Purity Greater Than 99.5%. 2011. Available online: https://www.businesswire.com/news/home/20110203006895/en/Quercegen-Pharmaceuticals-LLC-Obtains-FDA-GRAS-Notified-Status-for-Its-QU995%E2%84%A2-Quercetin-with-Purity-Greater-Than-99.5 (accessed on 21 November 2022).
- Peters, W.; Kusche-Vihrog, K.; Oberleithner, H.; Schillers, H. Cystic fibrosis transmembrane conductance regulator is involved in polyphenol-induced swelling of the endothelial glycocalyx. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1521–1530. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, Y.; Jin, L.; Ma, T.; Yang, H. Role of Quercetin in Modulating Chloride Transport in the Intestine. Front. Physiol. 2016, 7, 549. [Google Scholar] [CrossRef]
- Sirinupong, N.; Yang, Z. Bioactive Food Components as Dietary Intervention for Cystic Fibrosis. Curr. Drug Targets 2015, 16, 988–992. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxidative Med. Cell. Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxidative Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [PubMed]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef] [PubMed]
- Giacco, R.; Costabile, G.; Fatati, G.; Frittitta, L.; Maiorino, M.I.; Marelli, G.; Parillo, M.; Pistis, D.; Tubili, C.; Vetrani, C.; et al. Effects of polyphenols on cardio-metabolic risk factors and risk of type 2 diabetes. A joint position statement of the Diabetes and Nutrition Study Group of the Italian Society of Diabetology (SID), the Italian Association of Dietetics and Clinical Nu-trition (ADI) and the Italian Association of Medical Diabetologists (AMD). Nutr. Metab. Cardiovasc. Dis. 2020, 30, 355–367. [Google Scholar] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Sohma, Y.; Yu, Y.C.; Hwang, T.C. Curcumin and Genistein: The Combined Effects on Disease-associated CFTR Mutants and their Clinical Implications. Curr. Pharm. Des. 2013, 19, 3521–3528. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sahebkar, A.; Iranshahi, M.; Amini, M.; Khojasteh, R.; Ghayour-Mobarhan, M.; Ferns, G.A. Effects of Supplementation with Curcuminoids on Dyslipidemia in Obese Patients: A Randomized Crossover Trial. Phytother. Res. 2012, 27, 374–379. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Kumar, G.; Dey, S.K.; Kundu, S. Herbs and their bioactive ingredients in cardio-protection: Underlying molecular mechanisms and evidences from clinical studies. Phytomedicine 2021, 92, 153753. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef]
- Egan, M.E.; Pearson, M.; Weiner, S.A.; Rajendran, V.; Rubin, D.; Glöckner-Pagel, J.; Canny, S.; Du, K.; Lukacs, G.L.; Caplan, M.J. Curcumin, a Major Constituent of Turmeric, Corrects Cystic Fibrosis Defects. Science 2004, 304, 600–602. [Google Scholar] [CrossRef]
- Yu, Y.-C.; Miki, H.; Nakamura, Y.; Hanyuda, A.; Matsuzaki, Y.; Abe, Y.; Yasui, M.; Tanaka, K.; Hwang, T.-C.; Bompadre, S.G.; et al. Curcumin and genistein additively potentiate G551D-CFTR. J. Cyst. Fibros. 2011, 10, 243–252. [Google Scholar] [CrossRef]
- Kubow, S.; Fung, M.; Naqvi, N.; Lands, L.C. Chapter 20-The Emergence of Polyphenols in the Potentiation of Treatment Modality in Cystic Fibrosis. In Diet and Exercise in Cystic Fibrosis; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 159–169. [Google Scholar]
- Dey, I.; Shah, K.; ABradbury, N. Natural Compounds as Therapeutic Agents in the Treatment Cystic Fibrosis. J. Genet. Syndr. Gene Ther. 2016, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Moliteo, E.; Sciacca, M.; Palmeri, A.; Papale, M.; Manti, S.; Parisi, G.F.; Leonardi, S. Cystic Fibrosis and Oxidative Stress: The Role of CFTR. Molecules 2022, 27, 5324. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Spencer, E.; Heneghan, C.; Thompson, M. The effect of green tea on blood pressure and lipid profile: A systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 823–836. [Google Scholar] [CrossRef]
- Babu, P.V.; Liu, D. Green tea catechins and cardiovascular health: An update. Curr. Med. Chem. 2008, 15, 1840–1850. [Google Scholar] [CrossRef]
- Banach, M.; Catapano, A.L.; Cicero, A.F.; Escobar, C.; Foger, B.; Katsiki, N.; Latkovskis, G.; Rakowski, M.; Reiner, Z.; Sahebkar, A.; et al. Red yeast rice for dyslipidaemias and cardiovascular risk reduction: A position paper of the International Lipid Expert Panel. Pharmacol. Res. 2022, 183, 106370. [Google Scholar] [CrossRef]
- Cicero, A.F.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 2013, 33, 622–628. [Google Scholar] [CrossRef]
- Erdman, J.W.; Carson, L.; Kwik-Uribe, C.; Evans, E.M.; Allen, R.R. Effects of cocoa flavanols on risk factors for cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 284–287. [Google Scholar]
- Grassi, D.; Ferri, C. Chapter 78-Cocoa, Flavonoids and Cardiovascular Protection. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1009–1023. [Google Scholar]
- Amirpoor, A.; Zavar, R.; Amerizadeh, A.; Asgary, S.; Moradi, S.; Farzaei, M.H.; Masoumi, G.; Sadeghi, M. Effect of Beetroot Consumption on Serum Lipid Profile: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 100887. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L. Dietary n−6 and n−3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Portal, C.; Gouyer, V.; Léonard, R.; Husson, M.-O.; Gottrand, F.; Desseyn, J.-L. Long-term dietary (n-3) polyunsaturated fatty acids show benefits to the lungs of Cftr F508del mice. PLoS ONE 2018, 13, e0197808. [Google Scholar] [CrossRef] [PubMed]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Schwartz, J. Role of polyunsaturated fatty acids in lung disease. Am. J. Clin. Nutr. 2000, 71 (Suppl. S1), 393S–396S. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Shah, A.K.; Dhalla, N.S. Effectiveness of Some Vitamins in the Prevention of Cardiovascular Disease: A Narrative Review. Front. Physiol. 2021, 12, 729255. [Google Scholar] [CrossRef]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free. Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Nemerovski, C.W.; Dorsch, M.P.; Simpson, R.U.; Bone, H.G.; Aaronson, K.D.; EBleske, B. Vitamin D and Cardiovascular Disease. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2009, 29, 691–708. [Google Scholar] [CrossRef]
- Moser, M.A.; Chun, O.K. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int. J. Mol. Sci. 2016, 17, 1328. [Google Scholar] [CrossRef]
- Gale, C.R.; Martyn, C.N.; Winter, P.D.; Cooper, C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 1995, 310, 1563–1566. [Google Scholar] [CrossRef]
- Wilcken, D.E.L.; Wilcken, B. B vitamins and homocysteine in cardiovascular disease and aging. Ann. New York Acad. Sci. 1998, 854, 361–370. [Google Scholar] [CrossRef]
- Zittermann, A.; Koerfer, R. Protective and toxic effects of vitamin D on vascular calcification: Clinical implications. Mol. Asp. Med. 2009, 29, 423–432. [Google Scholar] [CrossRef]
- Gori, T.; Münzel, T. Oxidative stress and endothelial dysfunction: Therapeutic implications. Ann. Med. 2011, 43, 259–272. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, C.; Williams, D. New Insights About Vitamin D and Cardiovascular Disease. Ann. Intern. Med. 2011, 155, 820–826. [Google Scholar] [CrossRef]
- Rafnsson, S.B.; Orrell, M.; D’Orsi, E.; Hogervorst, E.; Steptoe, A. Loneliness, Social Integration, and Incident Dementia Over 6 Years: Prospective Findings From the English Longitudinal Study of Ageing. J. Gerontol. Series. B Psychol. Sci. Soc. Sci. 2017, 75, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Chen, J.; He, J.; Hamm, L.; Batuman, V.; Whelton, P.K. Serum Antioxidant Vitamins and Blood Pressure in the United States Population. Hypertension 2002, 40, 810–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; Zhou, C.; Zhang, Z.; He, P.; Li, Q.; Liu, C.; Qin, X. Inverse association between dietary vitamin A intake and new-onset hypertension. Clin. Nutr. 2021, 40, 2868–2875. [Google Scholar] [CrossRef] [PubMed]
- Ozkanlar, S.; Akcay, F. Antioxidant vitamins in atherosclerosis-animal experiments and clinical studies. Adv. Clin. Exp. Med. 2012, 21, 115–123. [Google Scholar] [PubMed]
- Ahmad, A.; Riaz, S.; Nadeem, M.S.; Mubeen, U.; Maham, K. Role of Carotenoids in Cardiovascular Disease. In Carotenoids-New Perspectives and Application; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093. [Google Scholar] [CrossRef]
- Bahadorpour, S.; Hajhashemy, Z.; Saneei, P. Serum 25-hydroxyvitamin D levels and dyslipidemia: A systematic review and dose-response meta-analysis of epidemiologic studies. Nutr. Rev. 2022, 81, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Skaaby, T.; Thuesen, B.H.; Linneberg, A. Vitamin D, Cardiovascular Disease and Risk Factors. Adv. Exp. Med. Biol. 2017, 996, 221–230. [Google Scholar] [CrossRef]
- Qi, K.-J.; Zhao, Z.-T.; Zhang, W.; Yang, F. The impacts of vitamin D supplementation in adults with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2022, 13, 1033026. [Google Scholar] [CrossRef]
- Han, L.; Xu, X.-J.; Zhang, J.-S.; Liu, H.-M. Association between Vitamin D Deficiency and Levels of Renin and Angiotensin in Essential Hypertension. Int. J. Clin. Pract. 2022, 2022, 8975396. [Google Scholar] [CrossRef]
- Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants 2022, 11, 1785. [Google Scholar] [CrossRef]
- Bellinge, J.W.; Dalgaard, F.; Murray, K.; Connolly, E.; Blekkenhorst, L.C.; Bondonno, C.P.; Lewis, J.R.; Sim, M.; Croft, K.D.; Gislason, G.; et al. Vitamin K Intake and Atherosclerotic Cardiovascular Disease in the Danish Diet Cancer and Health Study. J. Am. Heart Assoc. 2021, 10, e020551. [Google Scholar] [CrossRef]
- Hariri, E.; Kassis, N.; Iskandar, J.-P.; Schurgers, L.J.; Saad, A.; Abdelfattah, O.; Bansal, A.; Isogai, T.; Harb, S.C.; Kapadia, S. Vitamin K2—A neglected player in cardiovascular health: A narrative review. Open Heart 2021, 8, e001715. [Google Scholar] [CrossRef]
- Jeon, J.; Park, K. Dietary Vitamin B6 Intake Associated with a Decreased Risk of Cardiovascular Disease: A Prospective Cohort Study. Nutrients 2019, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Jamil, R.T.; Attia, F.N. Vitamin C (Ascorbic Acid). [Updated 2022 May 8]; In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Ima-Nirwana, S. Vitamin C: A Review on its Role in the Management of Metabolic Syndrome. Int. J. Med. Sci. 2020, 17, 1625–1638. [Google Scholar] [CrossRef]
- Heller, R.; Münscher-Paulig, F.; Gräbner, R.; Till, U. l-Ascorbic Acid Potentiates Nitric Oxide Synthesis in Endothelial Cells. J. Biol. Chem. 1999, 274, 8254–8260. [Google Scholar] [CrossRef] [PubMed]
- D’Uscio, L.V.; Milstien, S.; Richardson, D.; Smith, L.; Katusic, Z.S. Long-term vitamin C treatment increases vascular tetra-hydrobiopterin levels and nitric oxide synthase activity. Circ. Res. 2003, 92, 88–95. [Google Scholar] [CrossRef] [PubMed]

| Anatomy | Clinical Impact | Management Options |
|---|---|---|
| Heart | RV and LV dysfunction | Exercise Glycemic control for CFRD/IGT In selected cases: anti-fibrotic agents such as spironolactone or angiotensin-converting enzyme inhibitors, positive inotropic agents |
| Coronary Arteries | Atherosclerosis | Exercise Glycemic control for CFRD/IGT Dietary modifications Oxygen iNO Sildenafil Sleep optimization Statins Coronary stenting |
| Pulmonary Vasculature | Pulmonary hypertension Angiogenesis/remodeling Enlarged pulmonary arteries Increased levels of pro-oxidant cytokines | Pulmonary hypertension-specific treatment Sildenafil Fasudil Lung transplantation |
| Peripheral Vasculature | Impaired dilatation Increased stiffness Impaired endothelial function Blood pressure differences ROS production Dysregulate NOS activities | Exercise CFRD treatment/screen Flavonoids Calcium channel blockers PUFA, vitamin E Blood pressure monitoring Salt intake and hydration |
| Non-Modifiable Risk Factors |
|---|
| Chronic inflammation Pro-oxidative states CFTR-related vascular dysfunction Pulmonary hypertension Chronic pulmonary, cardiac, and renal disease Hyperglycemia and CFRD Pro-inflammatory macrophages Increased levels of cellular adhesion molecules CFTR-related plaque instability |
| Modifiable Risk Factors |
| High-calorie, high-fat diet Lung infection Solid-organ transplant Novel CFTR modulator therapies Indwelling vascular devices Pro-thrombotic coagulation |
| Nutraceuticals | Clinical and Biological Effects |
|---|---|
| RESVERATROL |
|
| CURCUMIN |
|
| QUERCETIN |
|
| ALLICIN/AGED GARLIC EXTRACT |
|
| GREEN TEA |
|
| RED YEAST RICE |
|
| BEETROOT JUICE |
|
| COCOA |
|
| Name | Sources | Roles |
|---|---|---|
| Omega-3 LC-PUFA | ||
| Alpha linoleic acid (ALA) |
|
|
| Decohexanoic acid (DHA) |
| The same roles as ALA. |
| Eicosapentanoic acid (EPA) |
| The same roles as ALA. |
| Omega-6 LC-PUFA | ||
| Linoleic acid (LA) |
|
|
| Gamma-linoleic acid (GLA) |
|
|
| Arachidonic acid (AA) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trandafir, L.M.; Frăsinariu, O.E.; Țarcă, E.; Butnariu, L.I.; Leon Constantin, M.M.; Moscalu, M.; Temneanu, O.R.; Melinte Popescu, A.S.; Popescu, M.G.M.; Stârcea, I.M.; et al. Can Bioactive Food Substances Contribute to Cystic Fibrosis-Related Cardiovascular Disease Prevention? Nutrients 2023, 15, 314. https://doi.org/10.3390/nu15020314
Trandafir LM, Frăsinariu OE, Țarcă E, Butnariu LI, Leon Constantin MM, Moscalu M, Temneanu OR, Melinte Popescu AS, Popescu MGM, Stârcea IM, et al. Can Bioactive Food Substances Contribute to Cystic Fibrosis-Related Cardiovascular Disease Prevention? Nutrients. 2023; 15(2):314. https://doi.org/10.3390/nu15020314
Chicago/Turabian StyleTrandafir, Laura Mihaela, Otilia Elena Frăsinariu, Elena Țarcă, Lăcrămioara Ionela Butnariu, Maria Magdalena Leon Constantin, Mihaela Moscalu, Oana Raluca Temneanu, Alina Sinziana Melinte Popescu, Marian George Melinte Popescu, Iuliana Magdalena Stârcea, and et al. 2023. "Can Bioactive Food Substances Contribute to Cystic Fibrosis-Related Cardiovascular Disease Prevention?" Nutrients 15, no. 2: 314. https://doi.org/10.3390/nu15020314
APA StyleTrandafir, L. M., Frăsinariu, O. E., Țarcă, E., Butnariu, L. I., Leon Constantin, M. M., Moscalu, M., Temneanu, O. R., Melinte Popescu, A. S., Popescu, M. G. M., Stârcea, I. M., Cojocaru, E., & Moisa, S. M. (2023). Can Bioactive Food Substances Contribute to Cystic Fibrosis-Related Cardiovascular Disease Prevention? Nutrients, 15(2), 314. https://doi.org/10.3390/nu15020314








