Five Days of Tart Cherry Supplementation Improves Exercise Performance in Normobaric Hypoxia
Abstract
1. Introduction
2. Materials and Methods
2.1. The Sample Size and Participants
2.2. Experimental Procedures
2.3. Supplementation Protocol
2.4. Measurements
2.4.1. Cardiorespiratory Variables
2.4.2. Tissue Oxygenation Profiles
2.4.3. Urine Sample and Analysis
2.5. Data Analysis
2.6. Statistical Analyses
3. Results
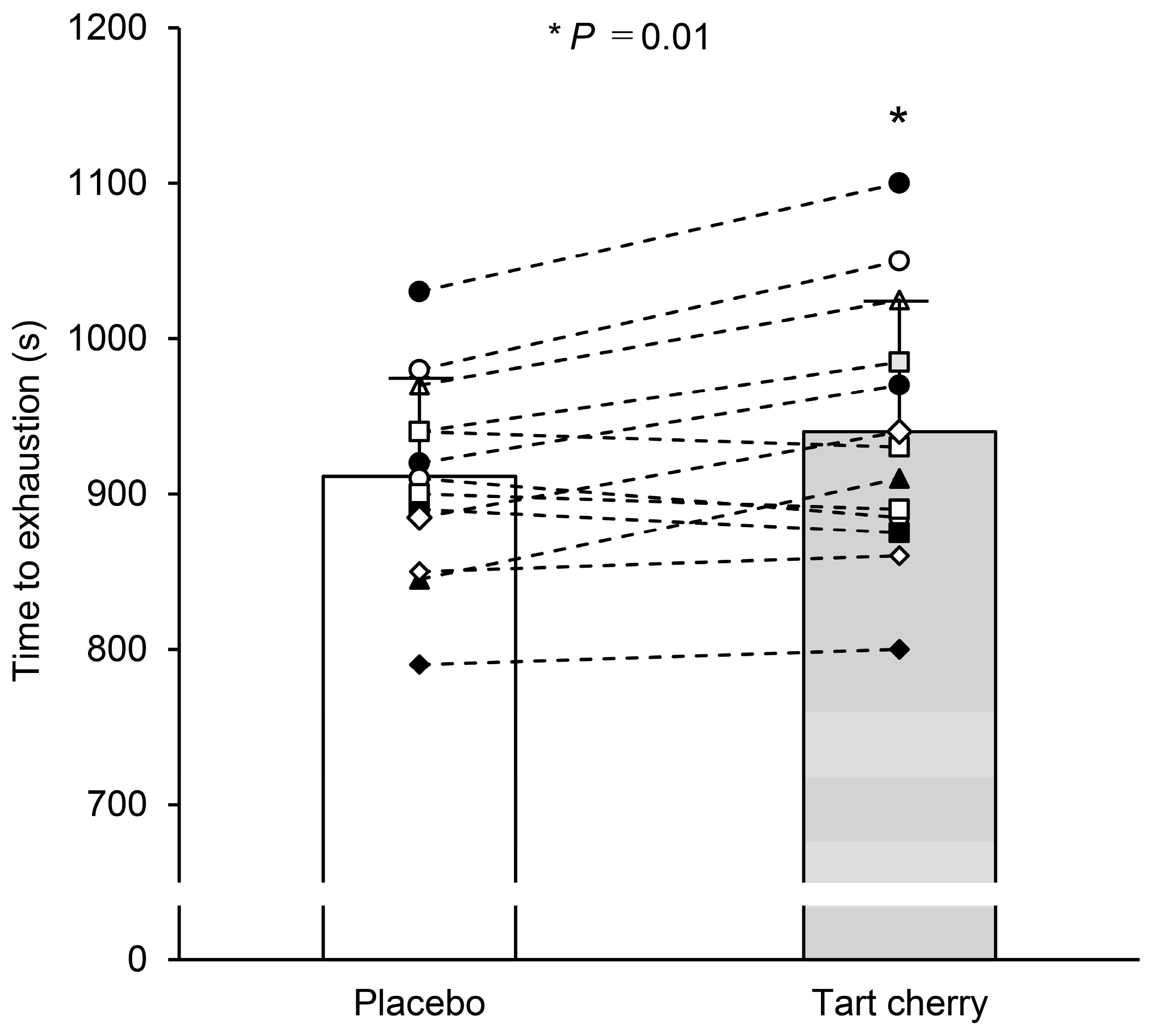
3.1. Exercise Performance in Normobaric Hypoxia
3.2. Cardiorespiratory Responses at Rest and During Exercise
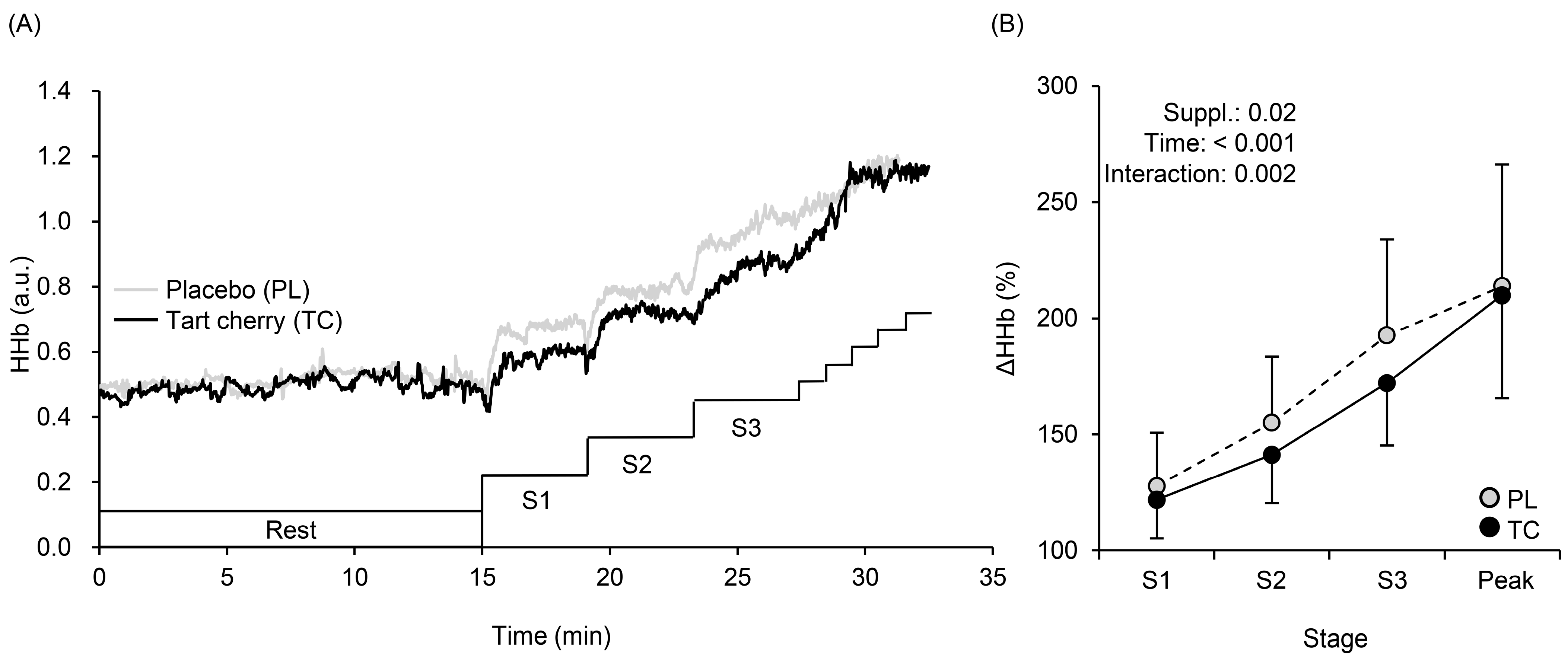
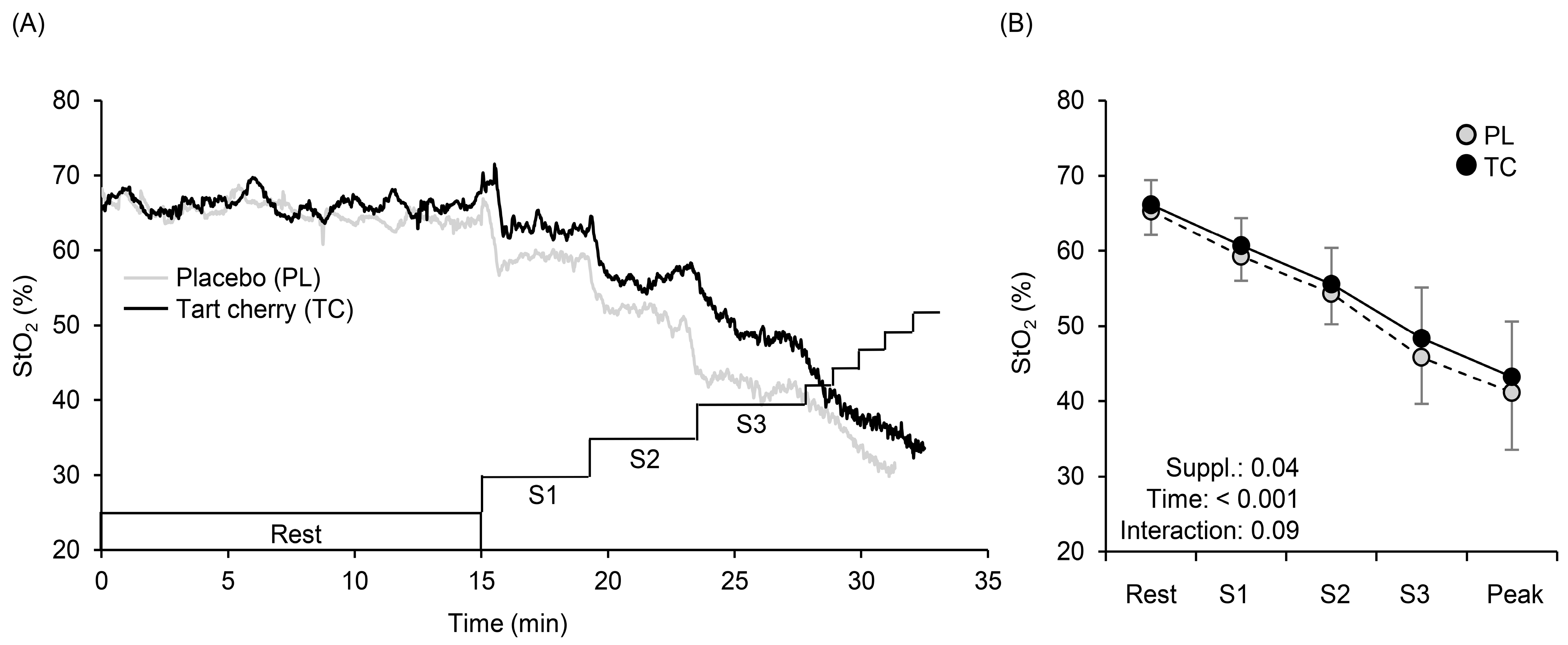
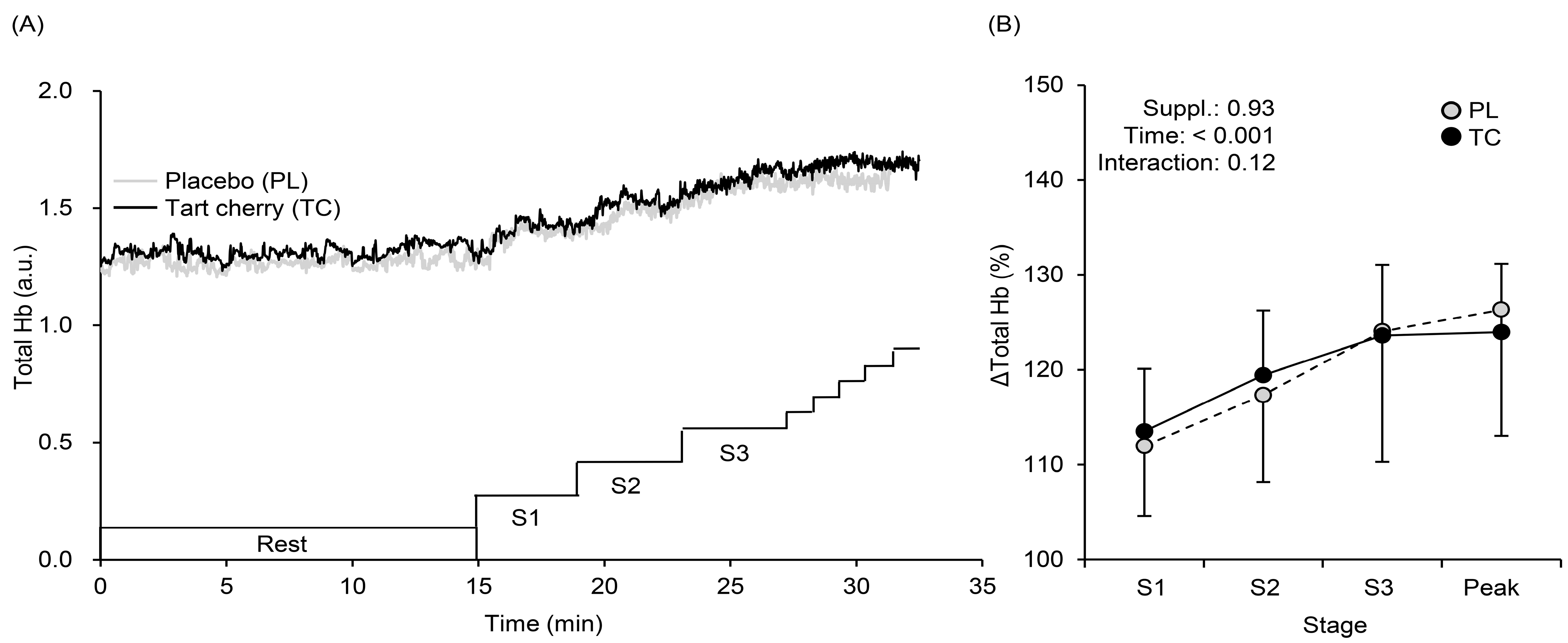
3.3. Muscle Oxygenation Profiles during Exercise
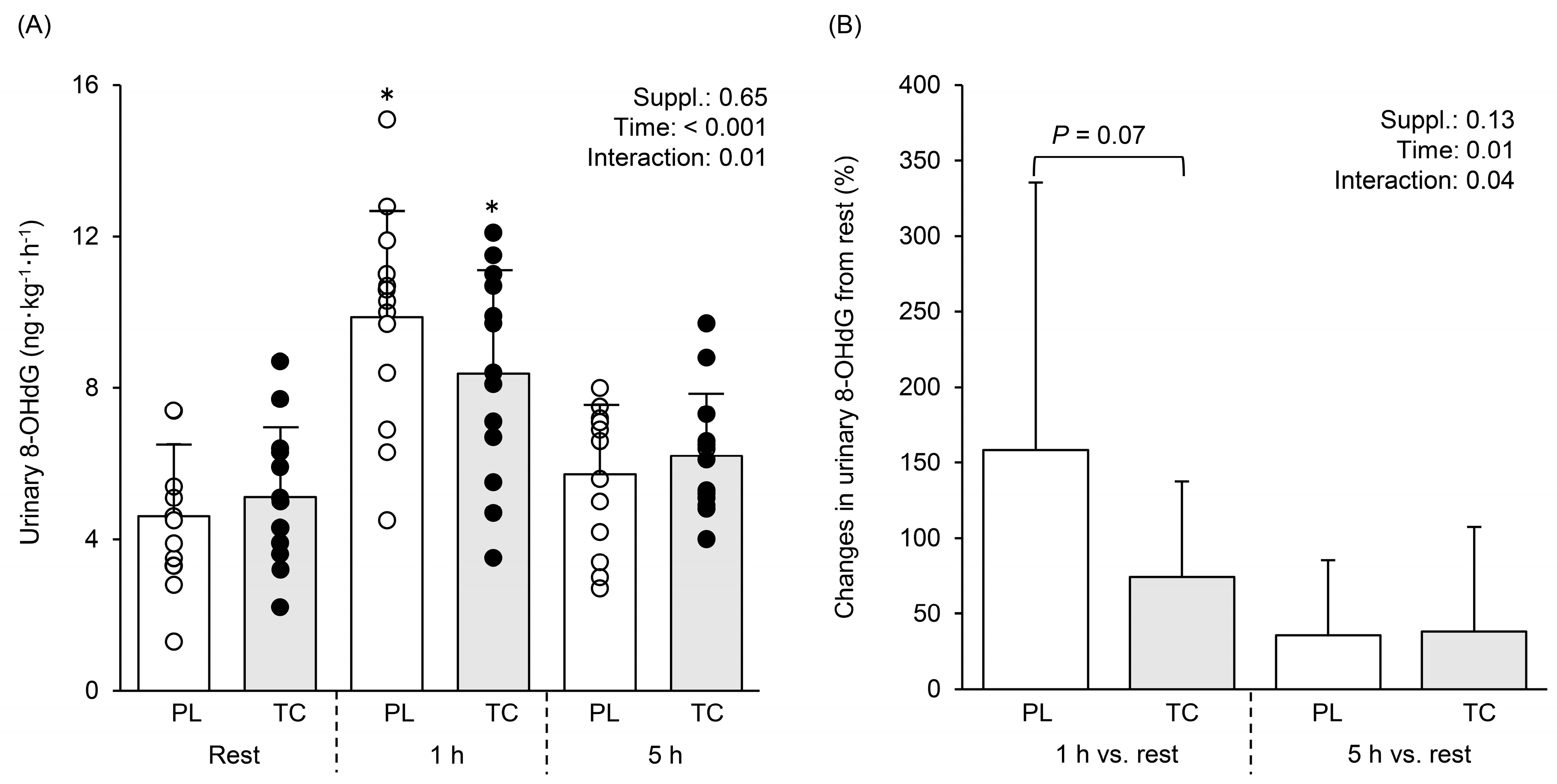
3.4. Oxidative Stress Marker in Urine
4. Discussion
4.1. Exercise Performance in Normobaric Hypoxia
4.2. Systemic Arterial and Local Muscle O2 Saturation
4.3. Effects of Tart Cherry Supplementation on Oxidative Stress
4.4. Methodological Considerations and Potential Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, P.T.; Barton, M.J.; Bowtell, J.L. Montmorency cherry supplementation improves 15-km cycling time-trial performance. Eur. J. Appl. Physiol. 2019, 119, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus cerasus L.) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Gao, R.; Chilibeck, P.D. Effect of Tart Cherry Concentrate on Endurance Exercise Performance: A Meta-analysis. J. Am. Coll. Nutr. 2020, 39, 657–664. [Google Scholar] [CrossRef]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sport. Nutr. 2016, 13, 22. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand J. Med. Sci. Sport. 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Davis, G.R.; Bellar, D. Montmorency cherry supplement does not affect aerobic exercise performance in healthy men. Int. J. Vitam. Nutr. Res. 2020, 90, 403–410. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- Ortega, D.R.; López, A.M.; Amaya, H.M.; de la Rosa, F.J.B. Tart cherry and pomegranate supplementations enhance recovery from exercise-induced muscle damage: A systematic review. Biol. Sport. 2021, 38, 97–111. [Google Scholar] [CrossRef]
- Chao, W.H.; Askew, E.W.; Roberts, D.E.; Wood, S.M.; Perkins, J.B. Oxidative stress in humans during work at moderate altitude. J. Nutr. 1999, 129, 2009–2012. [Google Scholar] [CrossRef]
- Lundby, C.; Pilegaard, H.; van Hall, G.; Sander, M.; Calbet, J.; Loft, S.; Moller, P. Oxidative DNA damage and repair in skeletal muscle of humans exposed to high-altitude hypoxia. Toxicology 2003, 192, 229–236. [Google Scholar] [CrossRef]
- Moller, P.; Loft, S.; Lundby, C.; Olsen, N.V. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001, 15, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, J.M.; Askew, E.W.; Roberts, D.E.; Wood, S.M.; Benson, J.E.; Johnson, S.C.; Freedman, M.S. Effect of antioxidant supplementation on urine and blood markers of oxidative stress during extended moderate-altitude training. Wilderness Environ. Med. 1999, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.C.; Askew, E.W.; Roberts, D.E.; Prior, R.L.; Ensign, W.Y., Jr.; Hesslink, R.E., Jr. Oxidative stress in humans training in a cold, moderate altitude environment and their response to a phytochemical antioxidant supplement. Wilderness Environ. Med. 2002, 13, 94–105. [Google Scholar] [CrossRef]
- Bailey, D.M.; Davies, B.; Young, I.S. Intermittent hypoxic training: Implications for lipid peroxidation induced by acute normoxic exercise in active men. Clin. Sci. 2001, 101, 465–475. [Google Scholar] [CrossRef]
- Magalhaes, J.; Ascensao, A.; Viscor, G.; Soares, J.; Oliveira, J.; Marques, F.; Duarte, J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat. Space Environ. Med. 2004, 75, 16–22. [Google Scholar] [PubMed]
- Pialoux, V.; Mounier, R.; Ponsot, E.; Rock, E.; Mazur, A.; Dufour, S.; Richard, R.; Richalet, J.P.; Coudert, J.; Fellmann, N. Effects of exercise and training in hypoxia on antioxidant/pro-oxidant balance. Eur. J. Clin. Nutr. 2006, 60, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Antioxidants and oxidative stress in exercise. Proc. Soc. Exp. Biol. Med. 1999, 222, 283–292. [Google Scholar] [CrossRef]
- Taddei, S.; Virdis, A.; Ghiadoni, L.; Salvetti, G.; Bernini, G.; Magagna, A.; Salvetti, A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 2001, 38, 274–279. [Google Scholar] [CrossRef]
- Thomas, G.D.; Zhang, W.; Victor, R.G. Impaired modulation of sympathetic vasoconstriction in contracting skeletal muscle of rats with chronic myocardial infarctions: Role of oxidative stress. Circ. Res. 2001, 88, 816–823. [Google Scholar] [CrossRef]
- de Groot, A.A.; van Zwieten, P.A.; Peters, S.L. Involvement of reactive oxygen species in angiotensin II-induced vasoconstriction. J. Cardiovasc. Pharmacol. 2004, 43, 154–159. [Google Scholar] [CrossRef]
- Van Guilder, G.P.; Westby, C.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Endothelin-1 vasoconstrictor tone increases with age in healthy men but can be reduced by regular aerobic exercise. Hypertension 2007, 50, 403–409. [Google Scholar] [CrossRef]
- Joyner, M.J.; Casey, D.P. Muscle blood flow, hypoxia, and hypoperfusion. J. Appl. Physiol. 2014, 116, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, Y.; Poole, D.C.; Barstow, T.J.; Kondo, N.; Nishiwaki, M.; Okushima, D.; Koga, S. Reduction of VO2 slow component by priming exercise: Novel mechanistic insights from time-resolved near-infrared spectroscopy. Physiol. Rep. 2015, 3, e12432. [Google Scholar] [CrossRef] [PubMed]
- McDonough, P.; Behnke, B.J.; Padilla, D.J.; Musch, T.I.; Poole, D.C. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J. Physiol. 2005, 563, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M.; Howatson, G. Effects of montmorency tart cherry (L. Prunus Cerasus) consumption on nitric oxide biomarkers and exercise performance. Scand J. Med. Sci. Sport. 2018, 28, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Horiuchi, M.; Stoner, L. Macrovascular and microvascular responses to prolonged sitting with and without bodyweight exercise interruptions: A randomized cross-over trial. Vasc. Med. 2022, 27, 127–135. [Google Scholar] [CrossRef]
- Dobashi, S.; Horiuchi, M.; Endo, J.; Kiuchi, M.; Koyama, K. Cognitive Function and Cerebral Oxygenation During Prolonged Exercise Under Hypoxia in Healthy Young Males. High Alt. Med. Biol. 2016, 17, 214–221. [Google Scholar] [CrossRef]
- Horiuchi, M.; Endo, J.; Dobashi, S.; Handa, Y.; Kiuchi, M.; Koyama, K. Muscle oxygenation profiles between active and inactive muscles with nitrate supplementation under hypoxic exercise. Physiol. Rep. 2017, 5, e13475. [Google Scholar] [CrossRef]
- Koga, S.; Poole, D.C.; Ferreira, L.F.; Whipp, B.J.; Kondo, N.; Saitoh, T.; Ohmae, E.; Barstow, T.J. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J. Appl. Physiol. 2007, 103, 2049–2056. [Google Scholar] [CrossRef]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time resolved reflectance and transmittance for the non-invasive measurement of tissue optical properties. Appl. Opt. 1989, 28, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Schalcher, T.R.; Borges, R.S.; Coleman, M.D.; Batista Junior, J.; Salgado, C.G.; Vieira, J.L.; Romao, P.R.; Oliveira, F.R.; Monteiro, M.C. Clinical oxidative stress during leprosy multidrug therapy: Impact of dapsone oxidation. PLoS ONE 2014, 9, e85712. [Google Scholar] [CrossRef] [PubMed]
- Morillas-Ruiz, J.; Zafrilla, P.; Almar, M.; Cuevas, M.J.; Lopez, F.J.; Abellan, P.; Villegas, J.A.; Gonzalez-Gallego, J. The effects of an antioxidant-supplemented beverage on exercise-induced oxidative stress: Results from a placebo-controlled double-blind study in cyclists. Eur. J. Appl. Physiol. 2005, 95, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, A.A.D.; Ebine, N.; Nakae, S.; Hojo, T.; Fukuoka, Y. Application of Molecular Hydrogen as an Antioxidant in Responses to Ventilatory and Ergogenic Adjustments during Incremental Exercise in Humans. Nutrients 2021, 13, 459. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.; Koga, S.; Barstow, T.J. Dynamics of noninvasively estimated microvascular O2 extraction during ramp exercise. J. Appl. Physiol. 2007, 103, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Burnley, M.; Doust, J.H.; Ball, D.; Jones, A.M. Effects of prior heavy exercise on VO(2) kinetics during heavy exercise are related to changes in muscle activity. J. Appl. Physiol. 2002, 93, 167–174. [Google Scholar] [CrossRef]
- DeLorey, D.S.; Kowalchuk, J.M.; Heenan, A.P.; Dumanoir, G.R.; Paterson, D.H. Prior exercise speeds pulmonary O2 uptake kinetics by increases in both local muscle O2 availability and O2 utilization. J. Appl. Physiol. 2007, 103, 771–778. [Google Scholar] [CrossRef]
- Jones, A.M.; Fulford, J.; Wilkerson, D.P. Influence of prior exercise on muscle [phosphorylcreatine] and deoxygenation kinetics during high-intensity exercise in men. Exp. Physiol. 2008, 93, 468–478. [Google Scholar] [CrossRef]
- Koga, S.; Poole, D.C.; Kondo, N.; Oue, A.; Ohmae, E.; Barstow, T.J. Effects of increased skin blood flow on muscle oxygenation/deoxygenation: Comparison of time-resolved and continuous-wave near-infrared spectroscopy signals. Eur. J. Appl. Physiol. 2015, 115, 335–343. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Fulford, J.; Bailey, S.J.; Blackwell, J.R.; Winyard, P.G.; Jones, A.M. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. J. Physiol. 2011, 589, 5517–5528. [Google Scholar] [CrossRef]
- Donato, A.J.; Uberoi, A.; Bailey, D.M.; Wray, D.W.; Richardson, R.S. Exercise-induced brachial artery vasodilation: Effects of antioxidants and exercise training in elderly men. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H671–H678. [Google Scholar] [CrossRef]
- Trinity, J.D.; Wray, D.W.; Witman, M.A.; Layec, G.; Barrett-O’Keefe, Z.; Ives, S.J.; Conklin, J.D.; Reese, V.; Zhao, J.; Richardson, R.S. Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H765–H774. [Google Scholar] [CrossRef] [PubMed]
- Wray, D.W.; Nishiyama, S.K.; Harris, R.A.; Zhao, J.; McDaniel, J.; Fjeldstad, A.S.; Witman, M.A.; Ives, S.J.; Barrett-O’Keefe, Z.; Richardson, R.S. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 2012, 59, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Crecelius, A.R.; Kirby, B.S.; Voyles, W.F.; Dinenno, F.A. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J. Physiol. 2011, 589, 3671–3683. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.P.; Gonzalez-Alonso, J.; Damsgaard, R.; Saltin, B.; Hellsten, Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J. Physiol. 2007, 581, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R.; Langberg, H.; Gemmer, C.; Olesen, J.; Crameri, R.; Scheede, C.; Sander, M.; Kjaer, M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J. Physiol. 2002, 543, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E. NO-synthase independent NO generation in mammals. Biochem. Biophys. Res. Commun. 2010, 396, 39–45. [Google Scholar] [CrossRef]
- Masschelein, E.; Thienen, R.V.; Wang, X.; Schepdael, A.V.; Thomis, M.; Hespel, P. Dietary nitrate improves muscle but not cerebral oxygenation status during exercise in hypoxia. J. Appl. Physiol. 2012, 113, 736–745. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black soybean improves the vascular function through an increase in nitric oxide and a decrease in oxidative stress in healthy women. Arch. Biochem. Biophys. 2020, 688, 108408. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakamura, A.; Nanba, F.; Saito, S.; Toda, T.; Nakagawa, J.; Ashida, H. Black Soybean Improves Vascular Function and Blood Pressure: A Randomized, Placebo Controlled, Crossover Trial in Humans. Nutrients 2020, 12, 2755. [Google Scholar] [CrossRef] [PubMed]
- Owuor, E.D.; Kong, A.N.T. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002, 64, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef] [PubMed]
- Rossman, M.J.; Trinity, J.D.; Garten, R.S.; Ives, S.J.; Conklin, J.D.; Barrett-O’Keefe, Z.; Witman, M.A.; Bledsoe, A.D.; Morgan, D.E.; Runnels, S.; et al. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H977–H985. [Google Scholar] [CrossRef] [PubMed]
- Loft, S.; Fischer-Nielsen, A.; Jeding, I.B.; Vistisen, K.; Poulsen, H.E. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J. Toxicol. Environ. Health 1993, 40, 391–404. [Google Scholar] [CrossRef]

| Placebo | Tart Cherry | Two-Way Repeated Measures ANOVA Results | ||||
|---|---|---|---|---|---|---|
| Suppl. | Time | Interaction | ||||
| O2 (mL·min−1) | ||||||
| Rest | 271 ± 29 | 269 ± 32 | F | 1.27 | 327.17 | 1.95 |
| Stage 1 | 785 ± 110 | 752 ± 104 | p | 0.28 | <0.001 | 0.12 |
| Stage 2 | 1175 ± 161 | 1153 ± 127 | η2 | 0.000 | 0.918 | 0.000 |
| Stage 3 | 1577 ± 151 | 1558 ± 167 | ||||
| Peak | 2058 ± 340 | 2080 ± 376 | ||||
| CO2 (mL·min−1) | ||||||
| Rest | 261 ± 33 | 261 ± 34 | F | 0.432 | 204.22 | 1.516 |
| Stage 1 | 801 ± 126 | 757 ± 119 | p | 0.52 | <0.001 | 0.21 |
| Stage 2 | 1291 ± 194 | 1256 ± 161 | η2 | 0.000 | 0.891 | 0.002 |
| Stage 3 | 1813 ± 216 | 1691 ± 494 | ||||
| Peak | 2547 ± 426 | 2651 ± 525 | ||||
| E (L·min−1) | ||||||
| Rest | 11.0 ± 1.8 | 11.3 ± 1.3 | F | 0.471 | 474.06 | 1.907 |
| Stage 1 | 28.2 ± 3.5 | 27.2 ± 3.4 | p | 0.51 | <0.001 | 0.13 |
| Stage 2 | 44.6 ± 5.1 | 43.9 ± 4.6 | η2 | 0.000 | 0.958 | 0.000 |
| Stage 3 | 65.8 ± 7.2 | 67.0 ± 6.8 | ||||
| Peak | 107.9 ± 14.2 | 111.5 ± 13.2 | ||||
| RER | ||||||
| Rest | 0.96 ± 0.05 | 0.97 ± 0.05 | F | 0.693 | 121.83 | 1.662 |
| Stage 1 | 1.02 ± 0.06 | 1.01 ± 0.08 | p | 0.42 | <0.001 | 0.17 |
| Stage 2 | 1.10 ± 0.07 | 1.09 ± 0.06 | η2 | 0.001 | 0.699 | 0.005 |
| Stage 3 | 1.15 ± 0.11 | 1.17 ± 0.07 | ||||
| Peak | 1.24 ± 0.06 | 1.27 ± 0.07 | ||||
| HR (bpm) | ||||||
| Rest | 80 ± 8 | 79 ± 8 | F | 0.426 | 608.78 | 0.686 |
| Stage 1 | 114 ± 9 | 112 ± 9 | p | 0.53 | <0.001 | 0.61 |
| Stage 2 | 135 ± 10 | 134 ± 11 | η2 | 0.000 | 0.926 | 0.000 |
| Stage 3 | 156 ± 11 | 155 ± 9 | ||||
| Peak | 175 ± 11 | 176 ± 11 | ||||
| SpO2 (%) | ||||||
| Rest | 86.6 ± 1.8 | 87.1 ± 1.9 | F | 18.53 | 40.10 | 1.75 |
| Stage 1 | 82.5 ± 3.1 | 83.3 ± 3.3 | p | 0.001 | <0.001 | 0.15 |
| Stage 2 | 80.8 ± 3.0 | 81.6 ± 3.9 | η2 | 0.009 | 0.482 | 0.002 |
| Stage 3 | 78.2 ± 3.8 | 79.9 ± 4.6 | ||||
| Peak | 75.8 ± 5.0 | 76.8 ± 5.7 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horiuchi, M.; Fukuoka, Y.; Koyama, K.; Oliver, S.J. Five Days of Tart Cherry Supplementation Improves Exercise Performance in Normobaric Hypoxia. Nutrients 2023, 15, 388. https://doi.org/10.3390/nu15020388
Horiuchi M, Fukuoka Y, Koyama K, Oliver SJ. Five Days of Tart Cherry Supplementation Improves Exercise Performance in Normobaric Hypoxia. Nutrients. 2023; 15(2):388. https://doi.org/10.3390/nu15020388
Chicago/Turabian StyleHoriuchi, Masahiro, Yoshiyuki Fukuoka, Katsuhiro Koyama, and Samuel J. Oliver. 2023. "Five Days of Tart Cherry Supplementation Improves Exercise Performance in Normobaric Hypoxia" Nutrients 15, no. 2: 388. https://doi.org/10.3390/nu15020388
APA StyleHoriuchi, M., Fukuoka, Y., Koyama, K., & Oliver, S. J. (2023). Five Days of Tart Cherry Supplementation Improves Exercise Performance in Normobaric Hypoxia. Nutrients, 15(2), 388. https://doi.org/10.3390/nu15020388







