Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
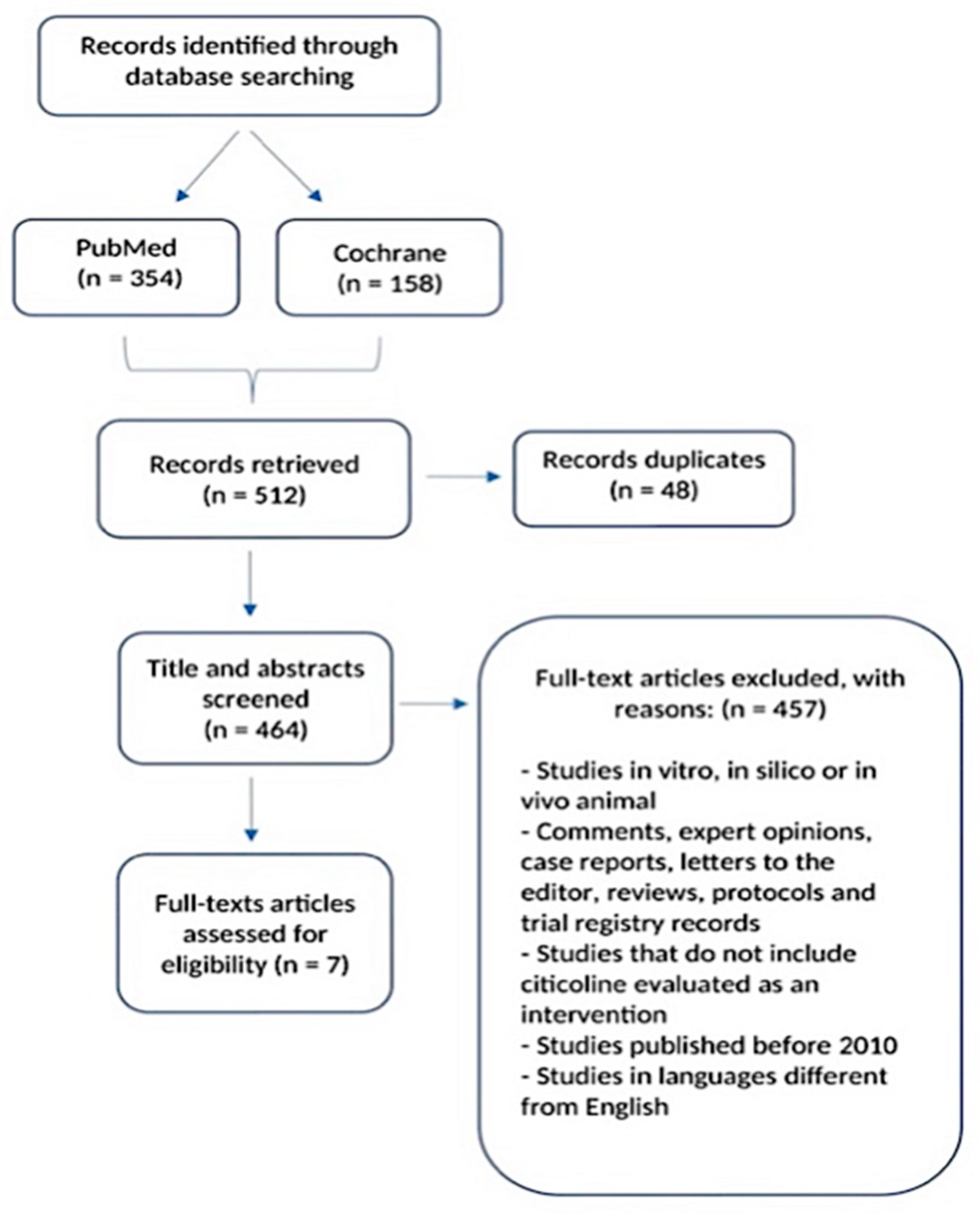
2.1. Search Strategy and Selection Process
2.2. Inclusion Criteria
2.3. Data Collection and Analysis
| Authors, Year | Study Design | Participants and Mean Age | Target Disease | Outcome Measures | Results |
|---|---|---|---|---|---|
| Castagna et al., 2016 [44] | Case–control study | 174 patients (mean age: 81.3 ± 4.5 years) divided into two groups. CASES (rivastigmine + citicoline 1000 mg/day orally): 92 patients (62 affected with AD, 30 with MD; 29% men). CONTROLS (rivastigmine): 82 patients (53 affected with AD, 29 with MD, 28% men). | Alzheimer’s disease and mixed dementia | Primary outcomes: effects of combined administration versus rivastigmine given alone on cognitive functions. Cognitive functions were assessed by MMSEc score which had been administered at baseline, 3 and 9 months. Secondary outcomes: possible side effects of combination therapy versus rivastigmine alone. | MMSEc in AD patients (cases): T0 15.68, DS 3.03, T1 16.79, DS 2.84, T2 16.93, DS 3. MMSEc in AD patients (controls): T0 15.32, DS 3.55, T1 14.81, DS 3.58, T2 13.97, DS 3.56. MMSEc in MD patients (cases): T0 16.04, DS 3.13, T1 16.41, DS 3.26, T2 16.62, DS 3.55. MMSEc in MD patients (controls): T0 14.79, DS 2.75, T1 14.33, DS 2.96, T2 13.2, DS 2.62. |
| Castagna et al., 2021 [45] | Multicentric, case–control study | 170 patients (mean age 76.8 ± 4.93 years, 34.11% men) divided into two groups. CASES (citicoline 1000 mg/day orally + memantine + AChEI): 81 patients (47.65%). CONTROLS (memantine + AChEI): 89 patients (52.35%). | Alzheimer’s disease | Primary outcomes: to assess whether a combined treatment of citicoline, memantine, and AChEI slows cognitive impairment. Cognitive functions were assessed by MMSE score which had been administered at baseline, 6 and 12 months. Secondary outcomes: to assess (a) safety and adverse drug reactions; (b) the possible interactions of citicoline, memantine, and AChEIs with other drugs. | MMSE score in the treated group had a statistically significant increasing trend between T0 and T2: 14.88 (DS 2.95) at T0, 14.95 (DS 2.63) at T1, 15.09 (DS 3) at T2. MMSE score in the control group showed a statistically significant decrease trend: 14.37 (DS 2.63) at T0, 14.19 (DS 2.81) at T1, 14.03 (DS 2.92) at T2. |
| Alvarez-Sabin et al., 2016 [46] | Open label, randomized, parallel study | 163 patients (83 women, 50.9%), mean age 67.5 ± 10.7 years, divided into two groups. CASES (citicoline 1 g/day orally): 86 patients (52.8%) CONTROLS: 77 patients (47.2%). | Post-stroke (first ischemic stroke) | Primary outcomes: cognitive status and quality of life. | Citicoline group showed a significant improvement in cognitive status during follow up (GCI 43.6% at 1 month, 32.5% at 6 months, 29% at 1 year, 27.9% at 2 years). The untreated group did not show significant changes (42.3% at 1 month, 41% at 6 months, 38% at 1 year, 39% at 2 years). |
| Cotroneo et al., 2013 [47] | Open label, multicentric study | 349 patients were divided into two groups. CASES (Citicoline 500 mg bid orally): 265 patients (122 men and 143 women), mean age 79.9 ± 7.8 years. CONTROLS (no treatment): 84 patients (36 men and 48 women), mean age 78.9 ± 7.01. | Mild vascular cognitive impairment | Primary outcome: the effect of citicoline on cognitive functions. Cognitive functions were assessed by MMSE score which had been administered at baseline, 3 and 9 months. | The MMSE score in the treated group remained essentially unchanged over time (22.4, DS 4 at T0; 22.7, DS 4 at T1; 22.9, DS 4 at T2). The control group showed a decline in MMSE score over the 9 months (21.5, DS 6.9 at T0; 20.4, DS 6.6 at T1 and 19.6, DS 6.3 at T2). |
| Gareri et al., 2016 [48] | Multicentric, case–control study | 448 patients (39.03% men, 60.97% women), mean age 80.03 ± 6.77 years, divided into two groups. CASES (AChEI—donepezil or rivastigmine or galantamine + citicoline 1000 mg/day orally): 251 patients. CONTROLS (AChEI): 197 patents. | Alzheimer’s disease | Primary outcomes: effects of combined administration versus AChEIs given alone on cognitive functions. Cognitive functions were assessed by MMSE score; it was administered at baseline (T0), after 3 (T1), and 9 months (T2). Secondary outcomes: possible side effects or adverse events of combination therapy versus AChEIs alone. | MMSE in the treated group: 16.88 (DS 3.38) at T0, 17.62 (DS 3.64) at T1, 17.89 (DS 3.54) at T2. MMSE in the control group: 16.41 (DS 2.97) at T0, 15.99 (3.16) at T1, 15.41 (DS 3.16) at T2. They compared MMSE scores in the treatment group in order to assess the possible differences among the three different AChEIs. MMSE score in donepezil group (144 patients): 17.15 (DS 3.83) at T0, 18.15 (DS 4.21) at T1, 18.49 (3.98) at T2. MMSE score in rivastigmine group (105 patients): 16.54 (DS 2.64) at T0, 16.89 (DS 2.53) at T1, 17.07 (DS 2.66) at T2. |
| Castagna et al., 2021 [49] | Multicentric, case–control study | 104 patients (76.04 ± 4.97 years; males 27.88%) divided into two groups. CASES (citicoline 1000 mg/day orally + memantine + rivastigmine): 41 patients. CONTROLS (memantine + rivastigmine): 63 patients. | Alzheimer’s disease | Primary outcome: to assess whether or not triple therapy (citicoline, memantine and rivastigmine) slow cognitive impairment progression. Cognitive functions were assessed by MMSE score; it was administered at baseline (T0), 6 months (T1) and 12 months (T2). Secondary outcomes: safety and possible side effects. | MMSE case group: 13.63, DS 2.46 at T0; 14.17, DS 2.24 at T1; 14.32, DS 2.53 at T2. MMSE control group: 14.25, DS 2.66 at T0; 14.24, DS 2.88 at T1; 14.00, DS 2.97 at T2. |
| Li et al., 2016 [50] | Randomized study | 81 patients divided into two groups (46 males). CASES (citicoline sodium capsules orally 200 mg three times a day): 41 patients. CONTROLS (basic medications such L-dopa or pramipexole with the matching placebo): 40 patients. | Mild cognitive impairment in Parkinson’s disease | Primary outcome: the effect of citicoline adjuvant therapy on mild cognitive impairment in Parkinson’s disease using MoCA and SCOPA-COG evaluations. MoCA and SCOPA-COG evaluations were performed at baseline, 12 and 18 months. | MoCA scale scores (baseline): cases = 24.03, DS 3.22-controls = 23.89, DS 2.27. MoCA scale scores (after 12 months): cases = 23.65, DS 2.55-controls = 22.53, DS 4.14. MoCA scale scores (after 18 months): cases = 23.12, DS 2.8-controls = 21.49, DS 3.99. SCOPA-COG scale scores (baseline): cases = 23.79, DS 2.82-controls = 23.43, DS 2.19. SCOPA-COG scale scores (after 12 months): cases = 21.55, DS 3.05-controls = 20.73, DS 4.14. SCOPA-COG scale scores (after 18 months): cases = 21.09, DS 2.78-controls = 19.25, DS 3.68. |
| Random Sequence | Allocation Concealment | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Competing Risk | Overall Risk of Bias | |
|---|---|---|---|---|---|---|---|---|
| Alvarez-Sabin et al., 2016 [46] |  |  |  |  |  |  |  |  |
| Castagna et al., 2016 [44] |  |  |  |  |  |  |  |  |
| Castagna et al., 2021 [45] |  |  |  |  |  |  |  |  |
| Gareri et al., 2016 [48] |  |  |  |  |  |  |  |  |
| Cotroneo et al., 2013 [47] |  |  |  |  |  |  |  |  |
| Li et al., 2016 [50] |  |  |  |  |  |  |  |  |
| Castagna et al., 2021 [49] |  |  |  |  |  |  |  |  |
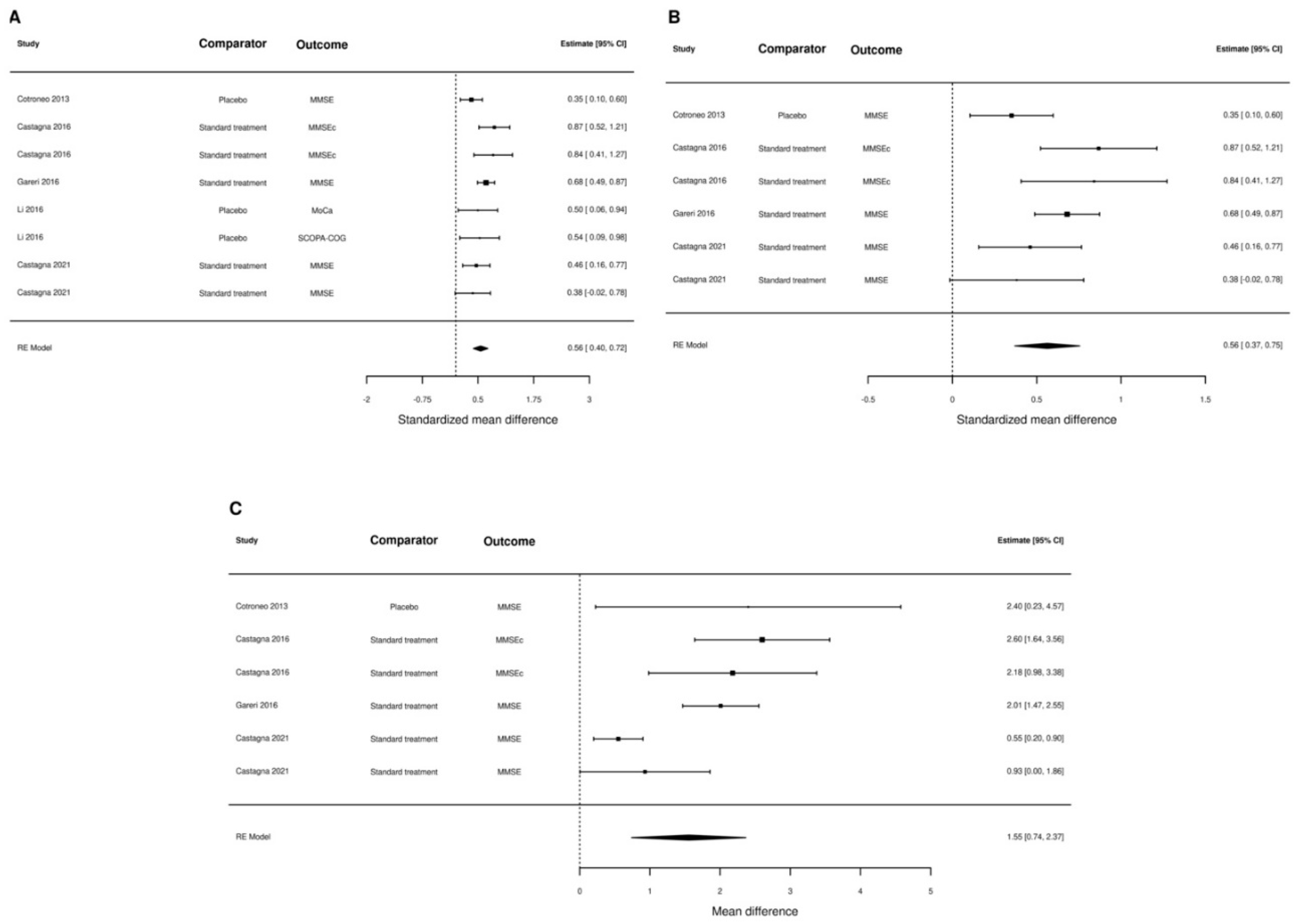
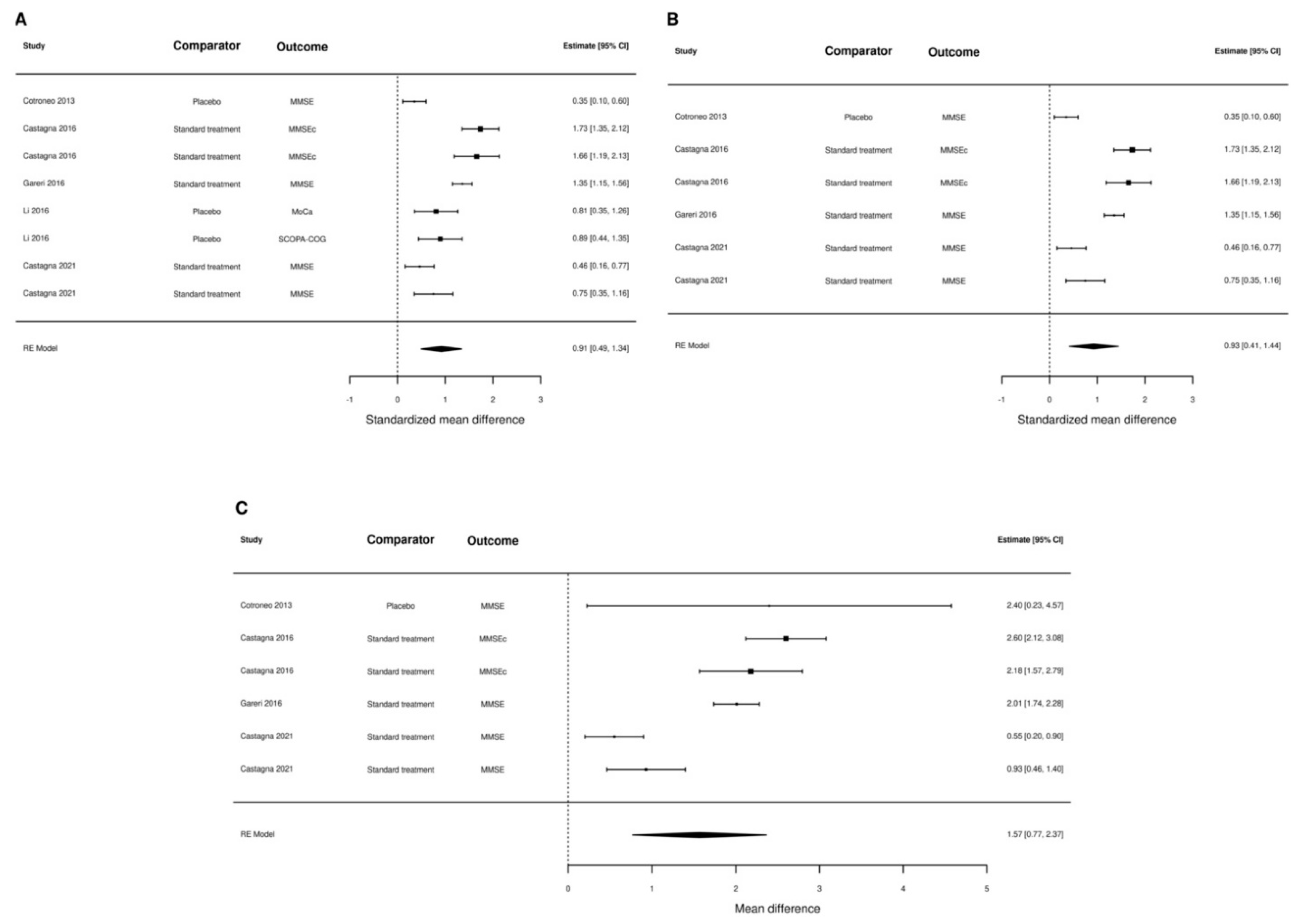
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice Guideline Update Summary: Mild Cognitive Impairment. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué-Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the Early Detection of Dementia in People with Mild Cognitive Impairment (MCI). Cochrane Database Syst. Rev. 2021, 2021, CD010783. [Google Scholar] [CrossRef]
- Lee, J. Effects of Aerobic and Resistance Exercise Interventions on Cognitive and Physiologic Adaptations for Older Adults with Mild Cognitive Impairment: A Systematic Review and Meta-Analysis of Randomized Control Trials. Int. J. Environ. Res. Public Health 2020, 17, 9216. [Google Scholar] [CrossRef] [PubMed]
- Overton, M.; Pihlsgård, M.; Elmståhl, S. Prevalence and Incidence of Mild Cognitive Impairment across Subtypes, Age, and Sex. Dement. Geriatr. Cogn. Disord. 2019, 47, 219–232. [Google Scholar] [CrossRef]
- Etgen, T.; Sander, D.; Bickel, H.; Förstl, H. Mild Cognitive Impairment and Dementia: The Importance of Modifiable Risk Factors. Dtsch. Arztebl. Int. 2011, 108, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Cheng, S.J.; Lin, H.C.; Lee, C.Y.; Chou, C.H. Risk Factors for the Progression of Mild Cognitive Impairment in Different Types of Neurodegenerative Disorders. Behav. Neurol. 2018, 2018, 6929732. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimer’s Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Tan, C.C.; Xu, W.; Hu, H.; Cao, X.P.; Dong, Q.; Tan, L.; Yu, J.T. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef]
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging Concepts in Vascular Dementia: A Review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.W.; Crawford, J.D.; Desmond, D.W.; Bae, H.J.; Lim, J.S.; Godefroy, O.; Roussel, M.; Kang, Y.; Jahng, S.; Köhler, S.; et al. Long-Term Cognitive Decline After Stroke: An Individual Participant Data Meta-Analysis. Stroke 2022, 53, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Sabín, J.; Román, G.C. Citicoline in Vascular Cognitive Impairment and Vascular Dementia after Stroke. Stroke 2011, 42, S40–S43. [Google Scholar] [CrossRef]
- Bock, M.A.; Tanner, C.M. The Epidemiology of Cognitive Function in Parkinson’s Disease. Prog. Brain Res. 2022, 269, 3–37. [Google Scholar] [CrossRef]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson Disease-Associated Cognitive Impairment. Nat. Rev. Dis. Prim. 2021, 7, 47. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Anderson, N.D. State of the Science on Mild Cognitive Impairment (MCI). CNS Spectr. 2019, 24, 78–87. [Google Scholar] [CrossRef]
- Honarvar, B.; Khaksar, E.; Jafari, F.; Zahedroozegar, M.H.; Amiri, S. Quality of Life in Elders with Suspected Alzheimer Disease: An Urban Health Centers-Based Study from Iran. Dement. Geriatr. Cogn. Dis. Extra 2020, 10, 143–153. [Google Scholar] [CrossRef]
- Kasper, S.; Bancher, C.; Eckert, A.; Förstl, H.; Frölich, L.; Hort, J.; Korczyn, A.D.; Kressig, R.W.; Levin, O.; Palomo, M.S.M. Management of Mild Cognitive Impairment (MCI): The Need for National and International Guidelines. World J. Biol. Psychiatry 2020, 21, 579–594. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M. Nutritional Prevention of Cognitive Decline and Dementia. Acta Biomed. 2018, 89, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Martínez, V.G.; Salas, A.A.; Ballestín, S.S. Vitamin Supplementation and Dementia: A Systematic Review. Nutrients 2022, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of Folic Acid and Vitamin B12 Supplementation on Cognitive Impairment and Inflammation in Patients with Alzheimer’s Disease: A Randomized, Single-Blinded, Placebo-Controlled Trial. J. Prev. Alzheimer’s Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of Vitamin D Supplementation on Cognitive Function and Blood Aβ-Related Biomarkers in Older Adults with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled Trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Schneider, L.S.; Sano, M.; Diaz-Arrastia, R.; Van Dyck, C.H.; Weiner, M.F.; Bottiglieri, T.; Jin, S.; Stokes, K.T.; Thomas, R.G.; et al. High-Dose B Vitamin Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Controlled Trial. JAMA 2008, 300, 1774–1783. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive Response to Fish Oil, Blueberry, and Combined Supplementation in Older Adults with Subjective Cognitive Impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef]
- Araya-Quintanilla, F.; Gutiérrez-Espinoza, H.; Sánchez-Montoya, U.; Muñoz-Yañez, M.J.; Baeza-Vergara, A.; Petersen-Yanjarí, M.; Fernández-Lecaros, L. Effectiveness of Omega-3 Fatty Acid Supplementation in Patients with Alzheimer Disease: A Systematic Review and Meta-Analysis. Neurologia 2020, 35, 105–114. [Google Scholar] [CrossRef]
- Mccleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.S.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and Mineral Supplementation for Preventing Dementia or Delaying Cognitive Decline in People with Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Swaminathan, A.; Jicha, G.A. Nutrition and Prevention of Alzheimer’s Dementia. Front. Aging Neurosci. 2014, 6, 282. [Google Scholar] [CrossRef]
- Savica, R.; Petersen, R.C. Prevention of Dementia. Psychiatr. Clin. North Am. 2011, 34, 127–145. [Google Scholar] [CrossRef]
- Aisen, P.S.; Jimenez-Maggiora, G.A.; Rafii, M.S.; Walter, S.; Raman, R. Early-Stage Alzheimer Disease: Getting Trial-Ready. Nat. Rev. Neurol. 2022, 18, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, M.; Yanagi, M. Cytidinediphosphocholine (CDP Choline) for Cognitive and Behavioural Disturbances Associated with Chronic Cerebral Disorders in the Elderly. Cochrane Database Syst. Rev. 2004, 2, CD000269. [Google Scholar] [CrossRef]
- Gareri, P.; Castagna, A.; Cotroneo, A.M.; Putignano, S.; De Sarro, G.; Bruni, A.C. The Role of Citicoline in Cognitive Impairment: Pharmacological Characteristics, Possible Advantages, and Doubts for an Old Drug with New Perspectives. Clin. Interv. Aging 2015, 10, 1421–1429. [Google Scholar] [CrossRef]
- Grieb, P. Neuroprotective Properties of Citicoline: Facts, Doubts and Unresolved Issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Research Paper: Citicoline Improves Human Vigilance and Visual Working Memory: The Role of Neuronal Activation and Oxidative Stress. Basic Clin. Neurosci. 2020, 11, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Piamonte, B.L.C.; Espiritu, A.I.; Anlacan, V.M.M. Effects of Citicoline as an Adjunct Treatment for Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2020, 76, 725–732. [Google Scholar] [CrossRef]
- Que, D.L.S.; Jamora, R.D.G. Citicoline as Adjuvant Therapy in Parkinson’s Disease: A Systematic Review. Clin. Ther. 2021, 43, e19–e31. [Google Scholar] [CrossRef]
- Jasielski, P.; Piędel, F.; Piwek, M.; Rocka, A.; Petit, V.; Rejdak, K. Application of Citicoline in Neurological Disorders: A Systematic Review. Nutrients 2020, 12, 3113. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions|Cochrane Training. Available online: https://training.cochrane.org/handbook/current (accessed on 27 November 2022).
- Castagna, A.; Cotroneo, A.M.; Ruotolo, G.; Gareri, P. The CITIRIVAD Study: CITIcoline plus RIVAstigmine in Elderly Patients Affected with Dementia Study. Clin. Drug Investig. 2016, 36, 1059–1065. [Google Scholar] [CrossRef]
- Castagna, A.; Fabbo, A.; Manzo, C.; Lacava, R.; Ruberto, C.; Ruotolo, G. A Retrospective Study on the Benefits of Combined Citicoline, Memantine, and Acetylcholinesterase Inhibitor Treatments in Older Patients Affected with Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 79, 1509–1515. [Google Scholar] [CrossRef]
- Alvarez-Sabín, J.; Santamarina, E.; Maisterra, O.; Jacas, C.; Molina, C.; Quintana, M. Long-Term Treatment with Citicoline Prevents Cognitive Decline and Predicts a Better Quality of Life after a First Ischemic Stroke. Int. J. Mol. Sci. 2016, 17, 390. [Google Scholar] [CrossRef]
- Cotroneo, A.M.; Castagna, A.; Putignano, S.; Lacava, R.; Fantò, F.; Monteleone, F.; Rocca, F.; Malara, A.; Gareri, P. Effectiveness and Safety of Citicoline in Mild Vascular Cognitive Impairment: The IDEALE Study. Clin. Interv. Aging 2013, 8, 131–137. [Google Scholar] [CrossRef]
- Gareri, P.; Castagna, A.; Cotroneo, A.M.; Putignano, D.; Conforti, R.; Santamaria, F.; Marino, S.; Putignano, S. The Citicholinage Study: Citicoline Plus Cholinesterase Inhibitors in Aged Patients Affected with Alzheimer’s Disease Study. J. Alzheimer’s Dis. 2017, 56, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Manzo, C.; Fabbo, A.; Lacava, R.; Ruberto, C.; Ruotolo, G. The CITIMERIVA Study: CITIcoline plus MEmantina plus RIVAstigmine in Older Patients Affected with Alzheimer’s Disease. Clin. Drug Investig. 2021, 41, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, P.; Yu, Z.; Sun, H.; Zhang, J.; Zhang, J.; Cong, Y.; Sun, C.; Zhang, Y.; Ju, X. Effect of Citicoline Adjuvant Therapy on Mild Cognitive Impairment in Parkinson’s Disease. Int. J. Clin. Exp. Med. 2016, 9, 4593–4598. [Google Scholar]
- Synoradzki, K.; Grieb, P. Citicoline: A Superior Form of Choline? Nutrients 2019, 11, 1569. [Google Scholar] [CrossRef]
- Andrews, J.S.; Desai, U.; Kirson, N.Y.; Zichlin, M.L.; Ball, D.E.; Matthews, B.R. Disease Severity and Minimal Clinically Important Differences in Clinical Outcome Assessments for Alzheimer’s Disease Clinical Trials. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 354. [Google Scholar] [CrossRef] [PubMed]
| Patient (P) | Adults with normal cognition, MCI, Alzheimer’s disease or vascular dementia. There were no restrictions on sex, ethnicity or severity of the cognitive impairment at baseline. |
| Intervention (I) | Citicoline as dietary supplements. Co-interventions with citicoline and standard treatment were allowed. |
| Comparison (C) | Standard of care, no intervention or placebo. |
| Outcome (O) |
|
| Type of studies (S) |
|
| Scheme. | A Priori Ranking | Upgrade/Downgrade | Final Grade | Factors Affecting Recommendation | Make Recommendation |
|---|---|---|---|---|---|
| Castagna 2016 [44] | LOW | Downgrade | VERY LOW | Low side effects | Weak for using |
| Castagna 2021 [49] | LOW | Downgrade | VERY LOW | Low side effects | Weak for using |
| Castagna 2021 [45] | LOW | Downgrade | VERY LOW | Low side effects | Weak for using |
| Gareri 2016 [48] | LOW | Downgrade | VERY LOW | Low side effects | Weak for using |
| Alvarez-Sabin 2016 [46] | LOW | Downgrade | VERY LOW | Low side effects | Weak for using |
| Cotroneo 2013 [47] | LOW | Downgrade | VERY LOW | Low side effects | Weak for using |
| Li 2016 [50] | HIGH | Downgrade | MODERATE | Low side effects | Strong for using |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonvicini, M.; Travaglini, S.; Lelli, D.; Antonelli Incalzi, R.; Pedone, C. Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis. Nutrients 2023, 15, 386. https://doi.org/10.3390/nu15020386
Bonvicini M, Travaglini S, Lelli D, Antonelli Incalzi R, Pedone C. Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis. Nutrients. 2023; 15(2):386. https://doi.org/10.3390/nu15020386
Chicago/Turabian StyleBonvicini, Maria, Silvia Travaglini, Diana Lelli, Raffaele Antonelli Incalzi, and Claudio Pedone. 2023. "Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis" Nutrients 15, no. 2: 386. https://doi.org/10.3390/nu15020386
APA StyleBonvicini, M., Travaglini, S., Lelli, D., Antonelli Incalzi, R., & Pedone, C. (2023). Is Citicoline Effective in Preventing and Slowing Down Dementia?—A Systematic Review and a Meta-Analysis. Nutrients, 15(2), 386. https://doi.org/10.3390/nu15020386






