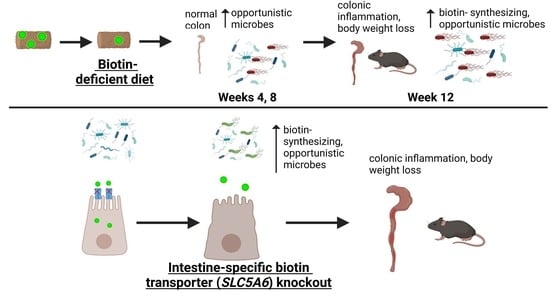
Biotin Deficiency Induces Intestinal Dysbiosis Associated with an Inflammatory Bowel Disease-like Phenotype
Abstract
1. Introduction
2. Materials and Methods
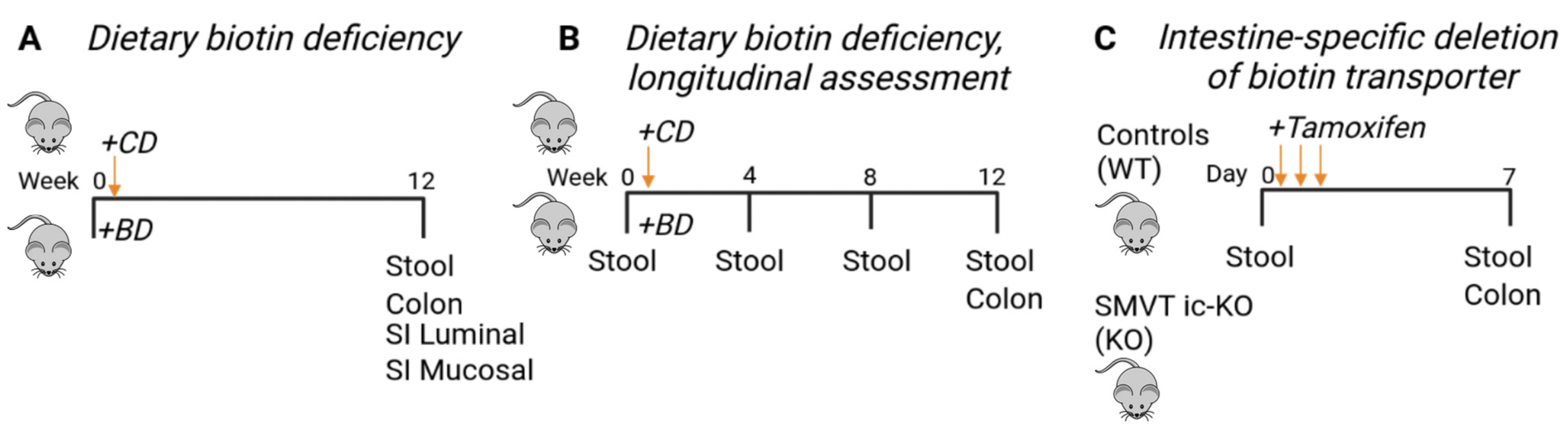
2.1. Dietary Biotin Deficiency Mouse Model
2.1.1. Inducible Biotin Deficiency Mouse Model
2.1.2. Histologic Analysis
2.1.3. Fecal Calprotectin Measurement
2.2. Microbiome Analysis
2.2.1. 16S rRNA Gene Sequencing and Library Preparation
2.2.2. Sequence Data Pre-Processing
2.2.3. Data Analysis, Statistics, and Figure Generation
3. Results
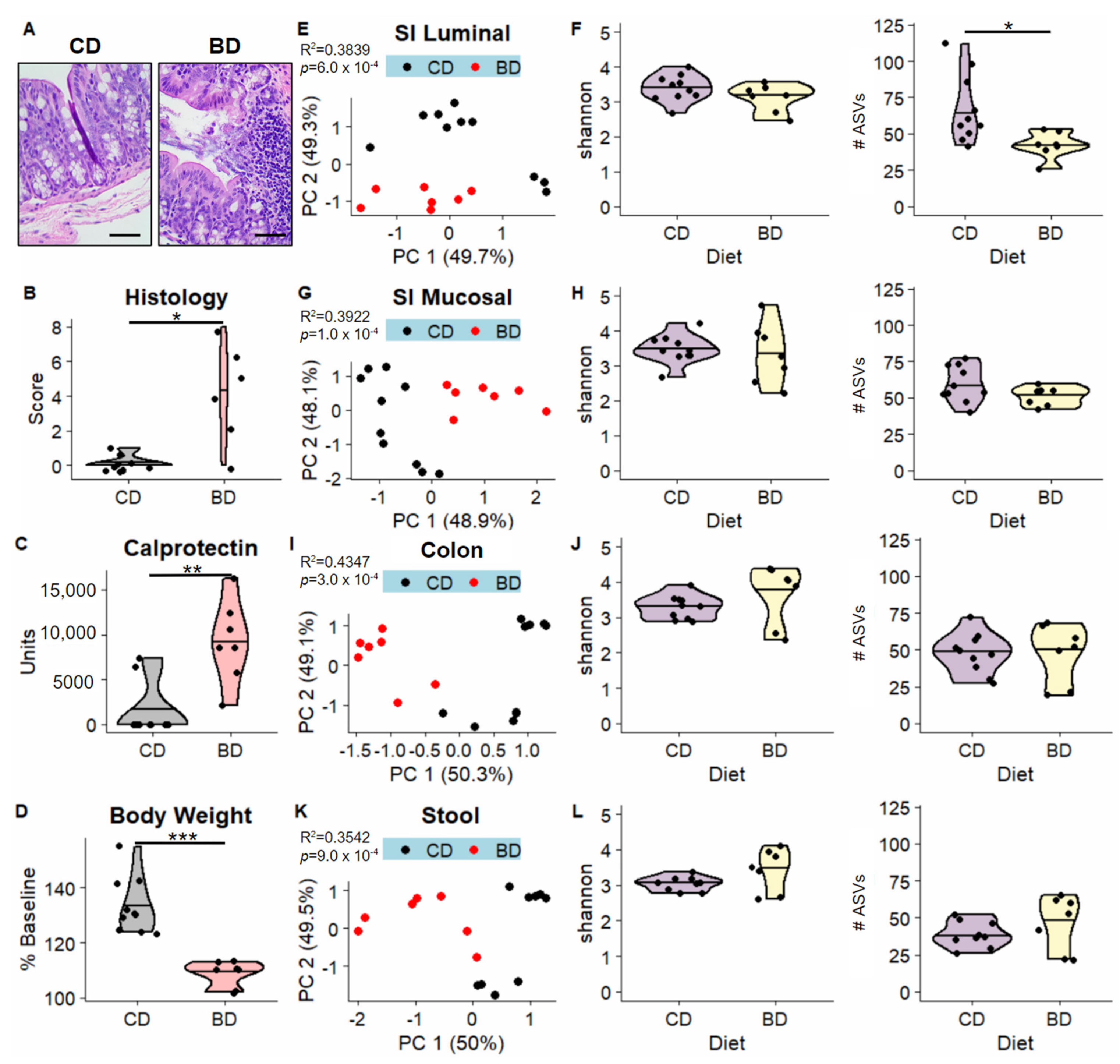
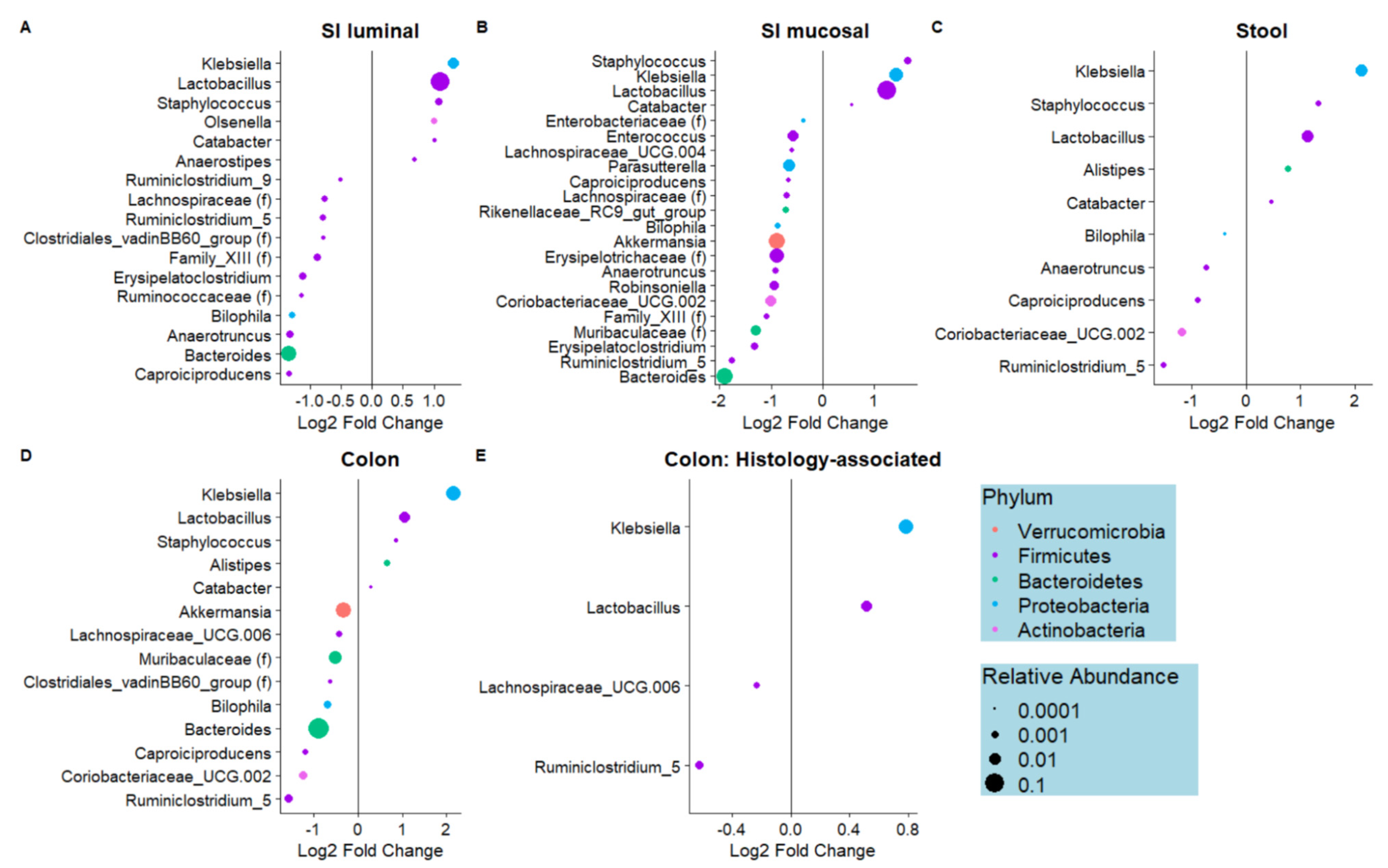
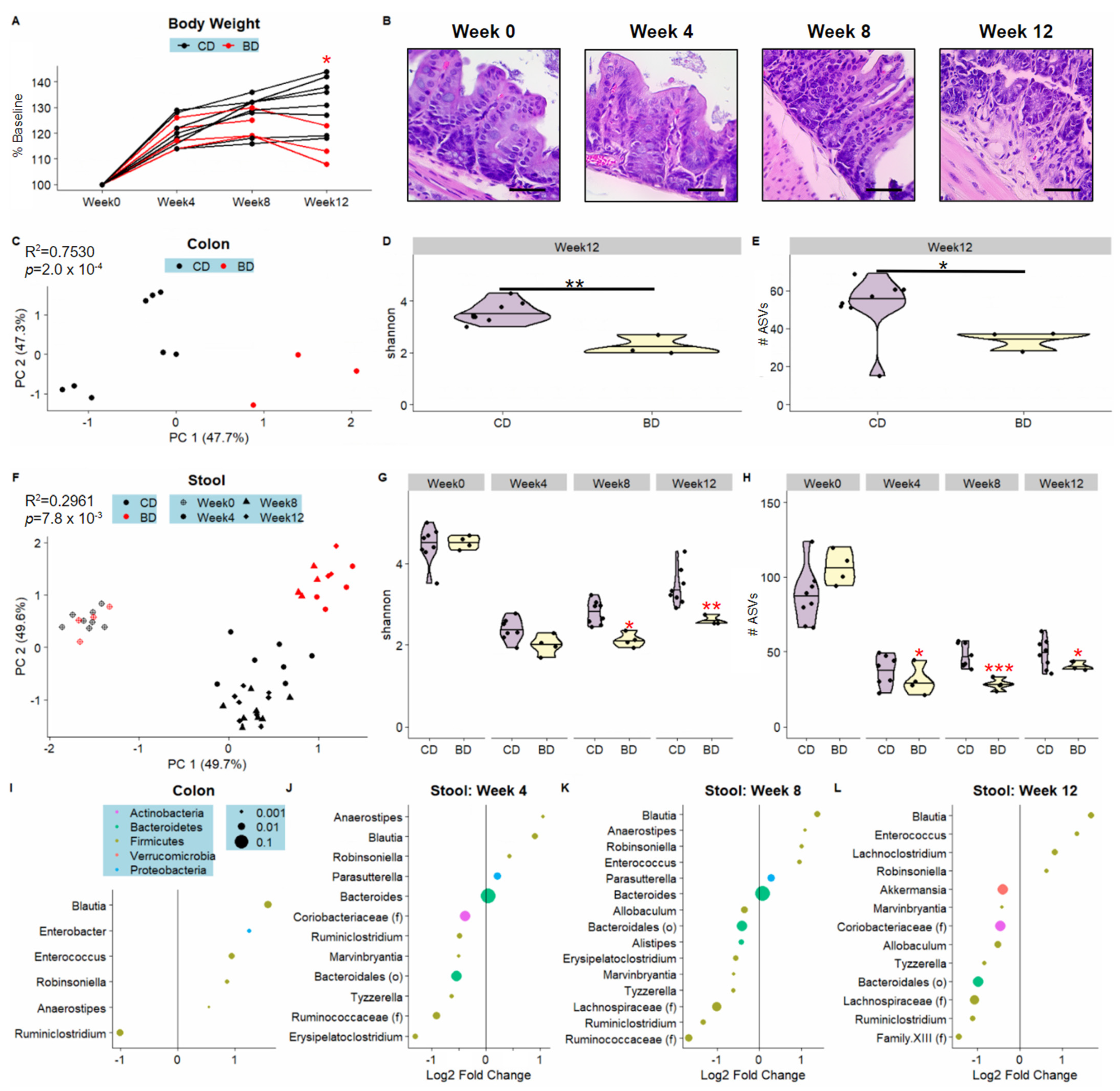
3.1. Dietary Biotin-Deficiency Is Associated with a Distinct Microbiome Composition along the Gastrointestinal Tract
3.2. Dietary Biotin-Deficiency Effects on the Microbiome Precede IBD-Linked Body Weight Loss and Colonic Inflammation
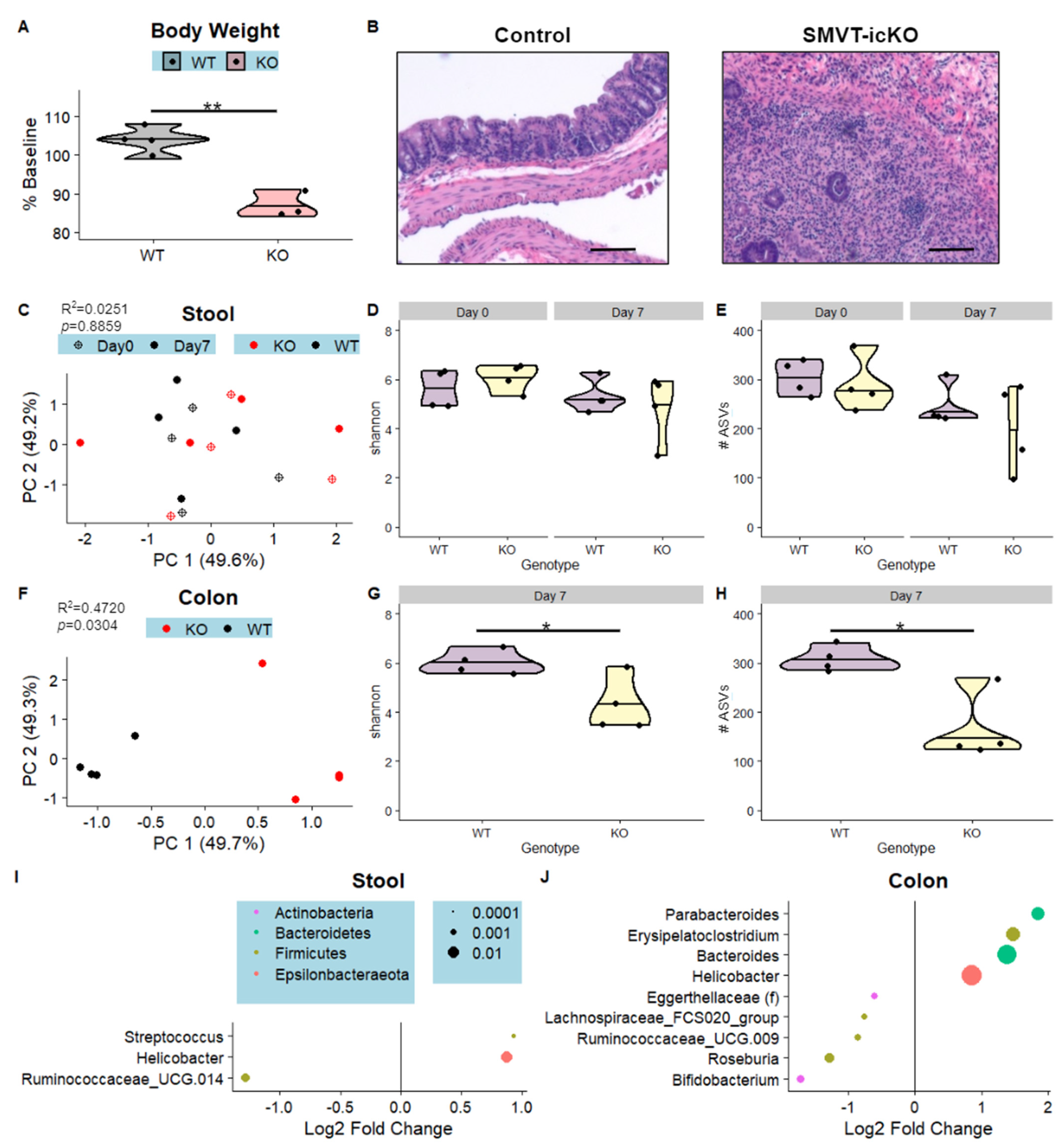
3.3. Intestine-Specific Deletion of Biotin Transporter Results in Perturbations to the Colonic and Fecal Microbiome
3.4. Microbes Associated with Biotin Deficiency Are Predicted to Exhibit Increased Biotin Biosynthetic Capabilities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Said, H.M. Biotin: Biochemical, physiological and clinical aspects. Subcell Biochem. 2012, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.T.; Sylvestersen, K.B.; Young, C.; Larsen, S.C.; Poulsen, J.W.; Andersen, M.A.; Palmqvist, E.A.; Hey-Mogensen, M.; Jensen, P.B.; Treebak, J.T. Biotin starvation causes mitochondrial protein hyperacetylation and partial rescue by the SIRT3-like deacetylase Hst4p. Nat. Commun. 2015, 6, 7726. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Y.I.; Zempleni, J. Epigenetic regulation of chromatin structure and gene function by biotin. J. Nutr. 2006, 136, 1763–1765. [Google Scholar] [CrossRef]
- Elahi, A.; Sabui, S.; Narasappa, N.N.; Agrawal, S.; Lambrecht, N.W.; Agrawal, A.; Said, H.M. Biotin Deficiency Induces Th1- and Th17-Mediated Proinflammatory Responses in Human CD4(+) T Lymphocytes via Activation of the mTOR Signaling Pathway. J. Immunol. 2018, 200, 2563–2570. [Google Scholar] [CrossRef]
- Kuroishi, T. Regulation of immunological and inflammatory functions by biotin. Can. J. Physiol. Pharmacol. 2015, 93, 1091–1096. [Google Scholar] [CrossRef]
- Bonjour, J.P. Vitamins and alcoholism. V. Riboflavin, VI. Niacin, VII. Pantothenic acid, and VIII. Biotin. Int. J. Vitam. Nutr. Res. Int. Z. Vitam.-Ernahr. J. Int. Vitaminol. Nutr. 1980, 50, 425–440. [Google Scholar]
- Fernandez-Banares, F.; Abad-Lacruz, A.; Xiol, X.; Gine, J.J.; Dolz, C.; Cabre, E.; Esteve, M.; Gonzalez-Huix, F.; Gassull, M.A. Vitamin status in patients with inflammatory bowel disease. Am. J. Gastroenterol. 1989, 84, 744–748. [Google Scholar]
- Said, H.M. Water-soluble vitamins. World Rev. Nutr. Diet 2015, 111, 30–37. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Kumar, C.K.; Ortiz, A.; Rubin, S.A.; Said, H.M. Molecular mechanism of the intestinal biotin transport process. Am. J. Physiol. 1999, 277, C605–C613. [Google Scholar] [CrossRef]
- Ghosal, A.; Subramanian, V.S.; Said, H.M. Role of the putative N-glycosylation and PKC-phosphorylation sites of the human sodium-dependent multivitamin transporter (hSMVT) in function and regulation. Biochim. Biophys. Acta. 2011, 1808, 2073–2080. [Google Scholar] [CrossRef]
- Reidling, J.C.; Said, H.M. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: Confirmation of promoter activity in vivo. Am. J. Physiol. Cell Physiol. 2007, 292, C1305–C1312. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.S.; Marchant, J.S.; Boulware, M.J.; Ma, T.Y.; Said, H.M. Membrane targeting and intracellular trafficking of the human sodium-dependent multivitamin transporter in polarized epithelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C663–C671. [Google Scholar] [CrossRef]
- Ghosal, A.; Lambrecht, N.; Subramanya, S.B.; Kapadia, R.; Said, H.M. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G64–G71. [Google Scholar] [CrossRef] [PubMed]
- Sabui, S.; Skupsky, J.; Kapadia, R.; Cogburn, K.; Lambrecht, N.W.; Agrawal, A.; Said, H.M. Tamoxifen-induced, intestinal-specific deletion of Slc5a6 in adult mice leads to spontaneous inflammation: Involvement of NF-κB, NLRP3, and gut microbiota. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G518–G530. [Google Scholar] [CrossRef] [PubMed]
- Skupsky, J.; Sabui, S.; Hwang, M.; Nakasaki, M.; Cahalan, M.D.; Said, H.M. Biotin Supplementation Ameliorates Murine Colitis by Preventing NF-κB Activation. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Uno, Y.; Hamada, K.; Kawabe, H.; Sakakibara, B. Effect of Primary Biotin Deficiency on the Skin of Germ-Free and Conventional Mice Fed a Purified Biotin-Deficient Diet without Supplementation with Egg White. J. Clin. Biochem. Nutr. 1997, 22, 63–72. [Google Scholar] [CrossRef][Green Version]
- Hayashi, A.; Mikami, Y.; Miyamoto, K.; Kamada, N.; Sato, T.; Mizuno, S.; Naganuma, M.; Teratani, T.; Aoki, R.; Fukuda, S.; et al. Intestinal Dysbiosis and Biotin Deprivation Induce Alopecia through Overgrowth of Lactobacillus murinus in Mice. Cell Rep. 2017, 20, 1513–1524. [Google Scholar] [CrossRef]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr. Protoc. Immunol. 2014, 107, 7.41.1–7.41.11. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S rRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, 15. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Arze, C.; Ananthakrishnan, A.N.; Schirmer, M.; Avila-Pacheco, J.; Poon, T.W.; Andrews, E.; Ajami, N.J.; Bonham, K.S.; Brislawn, C.J.; et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019, 569, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.H.; Dulai, P.S.; Vázquez-Baeza, Y.; Sauceda, C.; Daniel, N.; Gerner, R.R.; Batachari, L.E.; Malfavon, M.; Zhu, Q.; Weldon, K.; et al. Multi-omics analyses of the ulcerative colitis gut microbiome link Bacteroides vulgatus proteases with disease severity. Nat. Microbiol. 2022, 7, 262–276. [Google Scholar] [CrossRef]
- Sellon, R.K.; Tonkonogy, S.; Schultz, M.; Dieleman, L.A.; Grenther, W.; Balish, E.D.; Rennick, D.M.; Sartor, R.B. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998, 66, 5224–5231. [Google Scholar] [CrossRef]
- Jacob, N.; Jacobs, J.P.; Kumagai, K.; Ha, C.W.; Kanazawa, Y.; Lagishetty, V.; Altmayer, K.; Hamill, A.M.; Von Arx, A.; Sartor, R.B.; et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal. Immunol. 2018, 11, 1466–1476. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Kozik, A.J.; Nakatsu, C.H.; Chun, H.; Jones-Hall, Y.L. Comparison of the fecal, cecal, and mucus microbiome in male and female mice after TNBS-induced colitis. PloS ONE 2019, 14, e0225079. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host. Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Bell, A.; Juge, N. Mucosal glycan degradation of the host by the gut microbiota. Glycobiology 2021, 31, 691–696. [Google Scholar] [CrossRef]
- Nava, G.M.; Friedrichsen, H.J.; Stappenbeck, T.S. Spatial organization of intestinal microbiota in the mouse ascending colon. ISME J. 2011, 5, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Gustafsson, J.K.; Sjöberg, K.E.; Petersson, J.; Holm, L.; Sjövall, H.; Hansson, G.C. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PloS ONE 2010, 5, e12238. [Google Scholar] [CrossRef]
- Salaemae, W.; Booker, G.W.; Polyak, S.W. The Role of Biotin in Bacterial Physiology and Virulence: A Novel Antibiotic Target for Mycobacterium tuberculosis. Microbiol. Spectr. 2016, 4, 4.2.14. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hanson, R.E.; Cronan, J.E. Biotin synthesis begins by hijacking the fatty acid synthetic pathway. Nat. Chem. Biol. 2010, 6, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Shang, F.; Liu, W.; Lan, J.; Gao, P.; Ha, N.C.; Nam, K.H.; Dong, Y.; Quan, C.; et al. Structural insight into the carboxylesterase BioH from Klebsiella pneumoniae. Biochem. Biophys. Res. Commun. 2019, 520, 538–543. [Google Scholar] [CrossRef]
- Waller, J.R.; Lichstein, H.C. Biotin Transport and Accumulation by Cells of Lactobacillus plantarum II. Kinetics of the System. J. Bacteriol. 1965, 90, 853–856. [Google Scholar] [CrossRef]
- Novichkov, P.S.; Kazakov, A.E.; Ravcheev, D.A.; Leyn, S.A.; Kovaleva, G.Y.; Sutormin, R.A.; Kazanov, M.D.; Riehl, W.; Arkin, A.P.; Dubchak, I.; et al. RegPrecise 3.0--a resource for genome-scale exploration of transcriptional regulation in bacteria. BMC Genom. 2013, 14, 745. [Google Scholar] [CrossRef]
- Peralta, D.R.; Adler, C.; Corbalán, N.S.; Paz García, E.C.; Pomares, M.F.; Vincent, P.A. Enterobactin as Part of the Oxidative Stress Response Repertoire. PloS ONE 2016, 11, e0157799. [Google Scholar] [CrossRef] [PubMed]
- Karatan, E.; Duncan, T.R.; Watnick, P.I. NspS, a predicted polyamine sensor, mediates activation of Vibrio cholerae biofilm formation by norspermidine. J. Bacteriol. 2005, 187, 7434–7443. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhya, I.; Hansen, R.; El-Omar, E.M.; Hold, G.L. IBD—What role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Zhu, L.; Jia, J.; Cronan, J.E. A Biotin Biosynthesis Gene Restricted to Helicobacter. Sci. Rep. 2016, 6, 21162. [Google Scholar] [CrossRef]
- Shomer, N.H.; Dangler, C.A.; Schrenzel, M.D.; Fox, J.G. Helicobacter bilis-induced inflammatory bowel disease in scid mice with defined flora. Infect. Immun. 1997, 65, 4858–4864. [Google Scholar] [CrossRef]
- Ito, T.; Kobayashi, D.; Uchida, K.; Takemura, T.; Nagaoka, S.; Kobayashi, I.; Yokoyama, T.; Ishige, I.; Ishige, Y.; Ishida, N.; et al. Helicobacter pylori invades the gastric mucosa and translocates to the gastric lymph nodes. Lab. Investig. 2008, 88, 664–681. [Google Scholar] [CrossRef]
- Dupraz, L.; Magniez, A.; Rolhion, N.; Richard, M.L.; Da Costa, G.; Touch, S.; Mayeur, C.; Planchais, J.; Agus, A.; Danne, C.; et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021, 36, 109332. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.C.; Jacobs, J.P.; Hwang, M.; Sabui, S.; Liang, F.; Said, H.M.; Skupsky, J. Biotin Deficiency Induces Intestinal Dysbiosis Associated with an Inflammatory Bowel Disease-like Phenotype. Nutrients 2023, 15, 264. https://doi.org/10.3390/nu15020264
Yang JC, Jacobs JP, Hwang M, Sabui S, Liang F, Said HM, Skupsky J. Biotin Deficiency Induces Intestinal Dysbiosis Associated with an Inflammatory Bowel Disease-like Phenotype. Nutrients. 2023; 15(2):264. https://doi.org/10.3390/nu15020264
Chicago/Turabian StyleYang, Julianne C., Jonathan P. Jacobs, Michael Hwang, Subrata Sabui, Fengting Liang, Hamid M. Said, and Jonathan Skupsky. 2023. "Biotin Deficiency Induces Intestinal Dysbiosis Associated with an Inflammatory Bowel Disease-like Phenotype" Nutrients 15, no. 2: 264. https://doi.org/10.3390/nu15020264
APA StyleYang, J. C., Jacobs, J. P., Hwang, M., Sabui, S., Liang, F., Said, H. M., & Skupsky, J. (2023). Biotin Deficiency Induces Intestinal Dysbiosis Associated with an Inflammatory Bowel Disease-like Phenotype. Nutrients, 15(2), 264. https://doi.org/10.3390/nu15020264