Agaricus bisporus Extract Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese C57BL/6N Mice by Inhibiting Pancreatic Lipase-Mediated Fat Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ABE
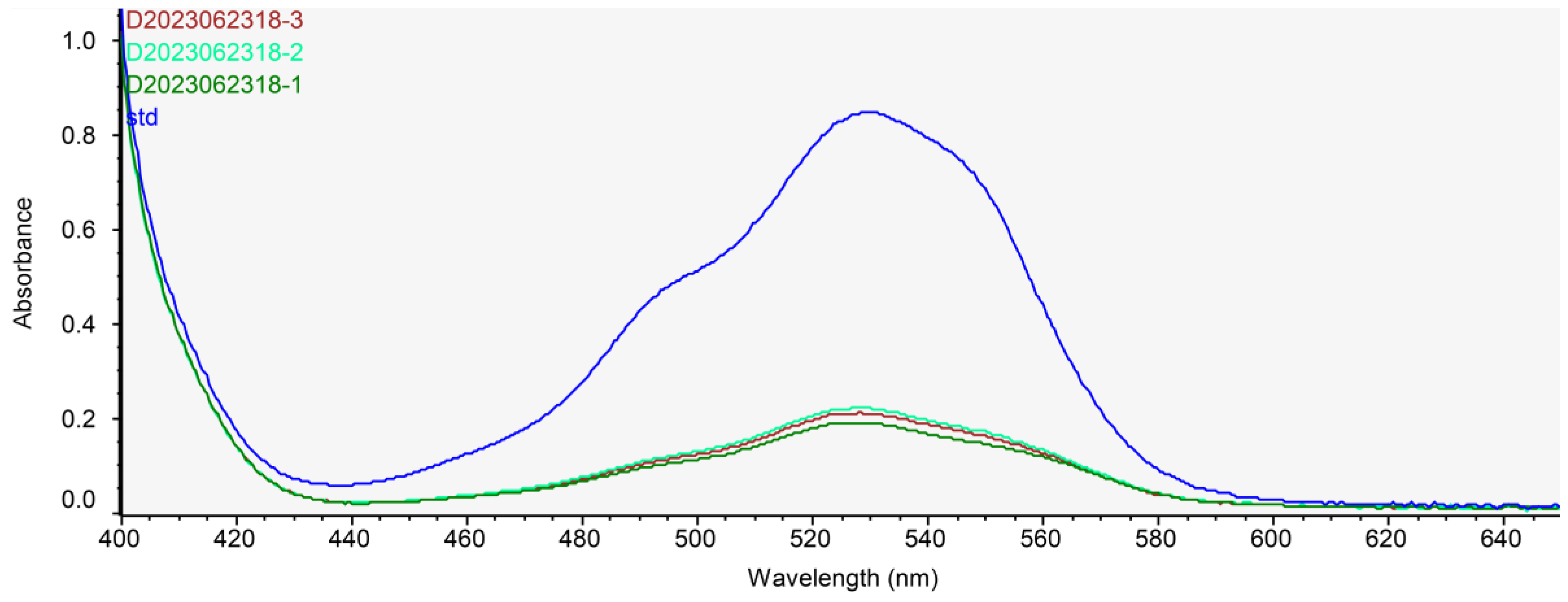
2.2. Determination of Chitosan Contents in ABE
2.3. In Vitro Pancreatic Lipase Activity Assay
2.4. In Vivo Oral Lipid Tolerance Test (OLTT)
2.5. Experimental Design in High-Fat Induced Obesity Animal Models
2.6. Body Composition Assessment
2.7. Serum Biochemical Analysis
2.8. Histological Analysis
2.9. Measurement of Lipids in Livers and Feces
2.10. Statistical Analysis
3. Results
3.1. Characterization of ABE
3.2. ABE Inhibits Pancreatic Lipase Activity In Vitro
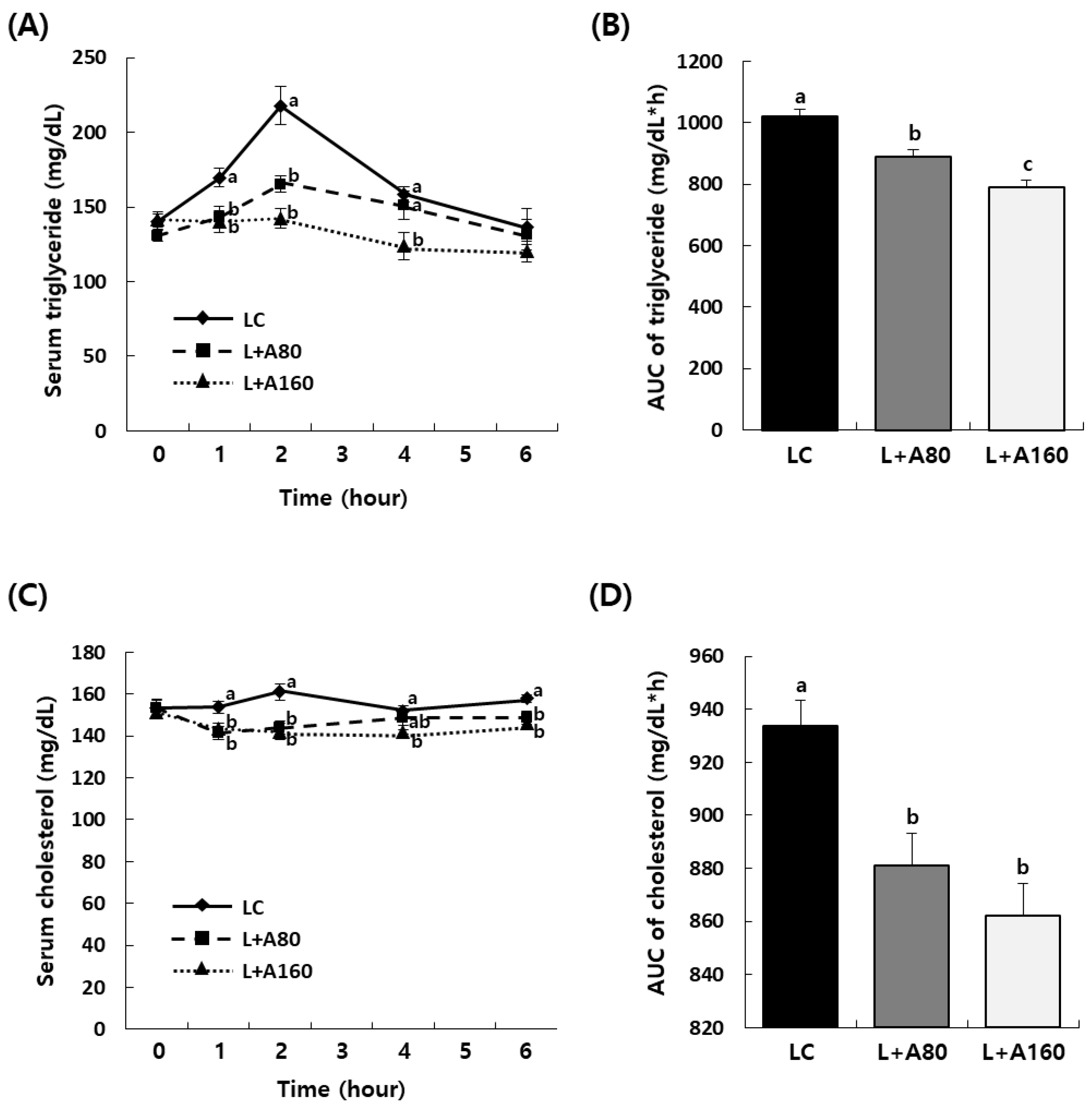
3.3. ABE Reduces Elevation of Serum Triglyceride and Cholesterol Levels after Oral Administration of Lipid Emulsion
3.4. ABE Reduces Body Weight Gain and Body Fat Deposition in HFD-Induced Obese C57BL/6N Mice
3.5. ABE Ameliorates Serum Glucose and Lipids Levels in HFD-Induced Obese C57BL/6N Mice
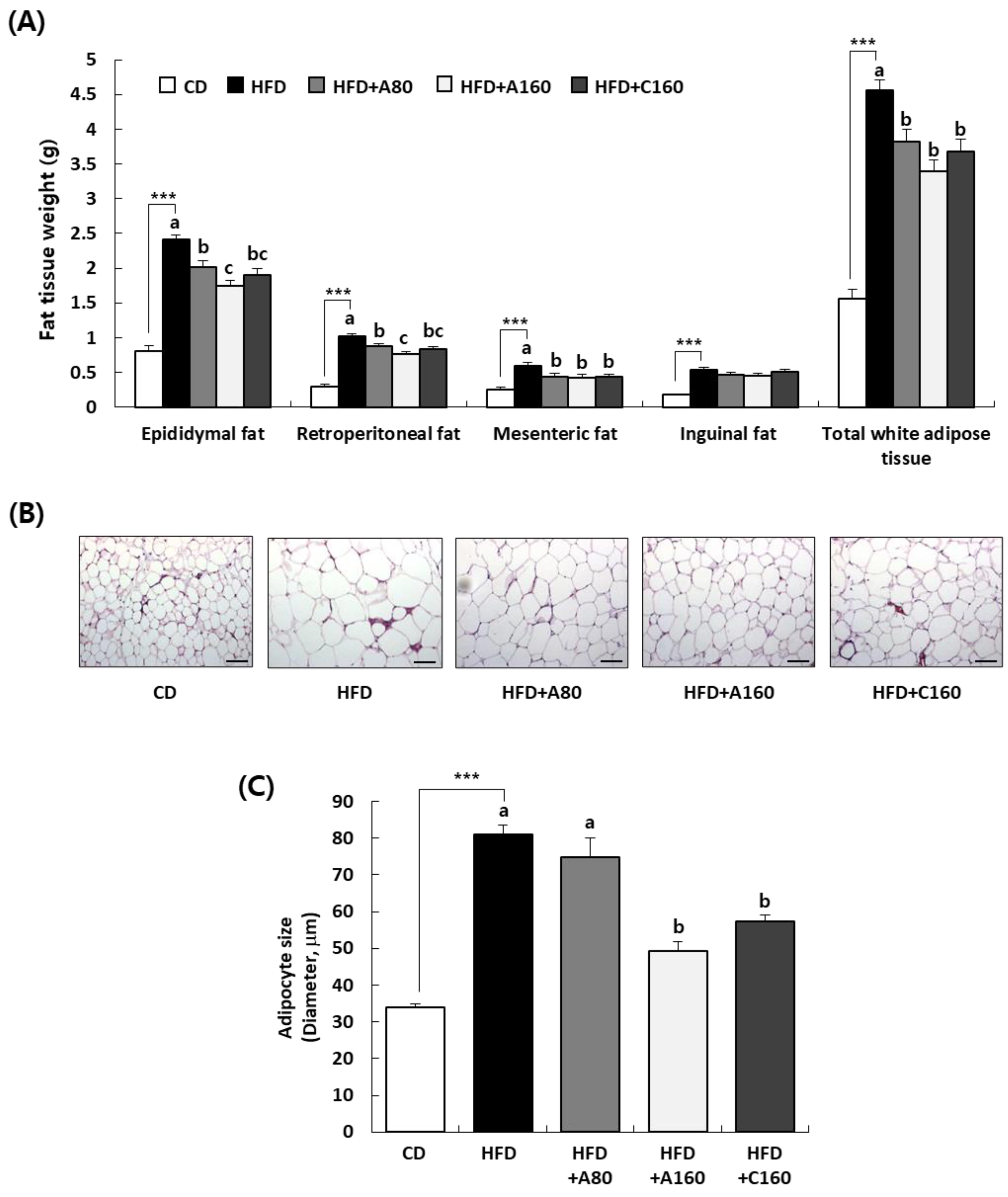
3.6. ABE Decreases White Adipose Tissue Deposition in HFD-Induced Obese C57BL/6N Mice
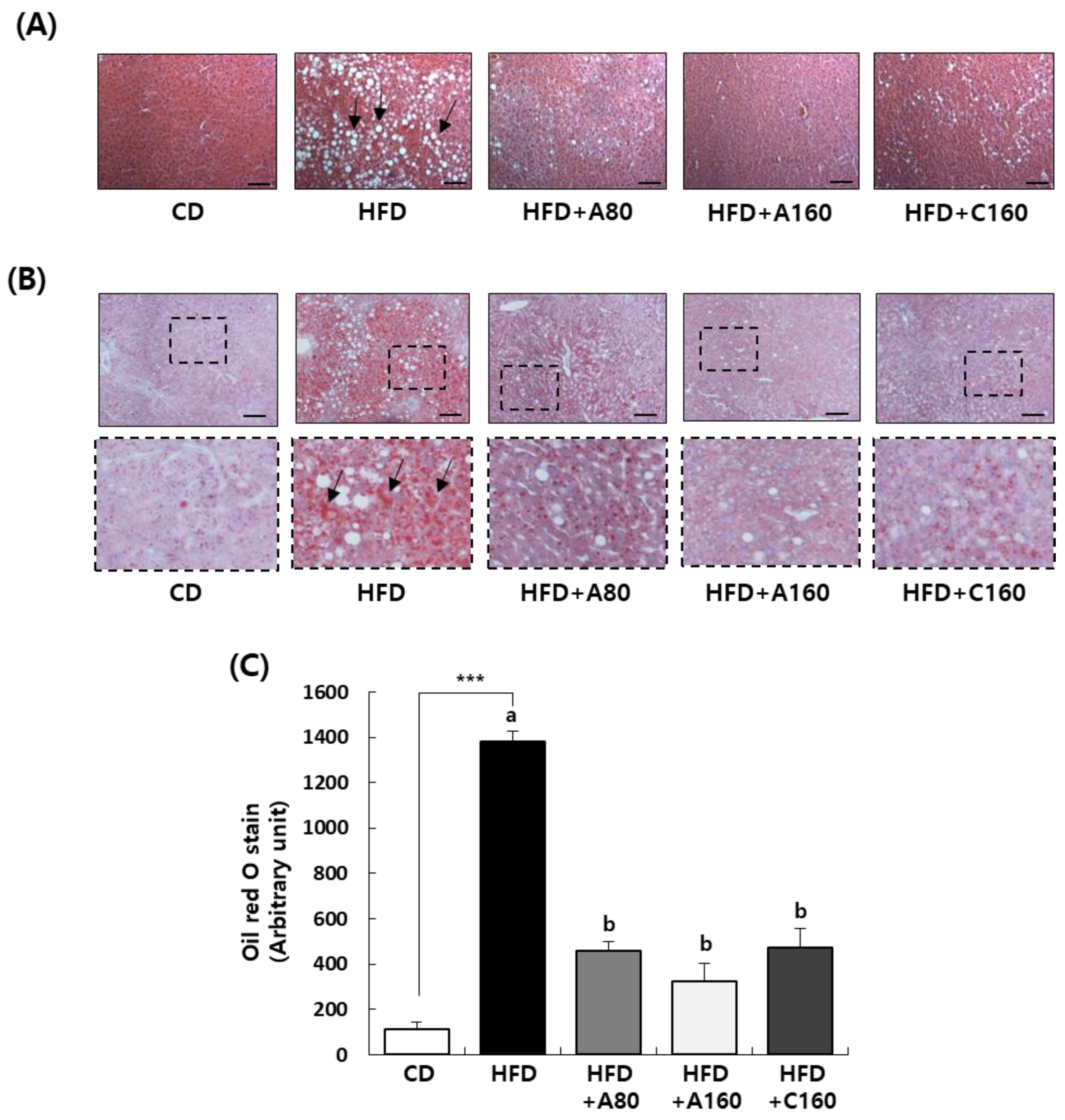
3.7. ABE Inhibits Fat Accumulation in Livers of HFD-Induced Obese C57BL/6N Mice
3.8. ABE Increases Fat Excretion in Feces of HFD-Induced Obese C57BL/6N Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gjermeni, E.; Kirstein, A.S.; Kolbig, F.; Kirchhof, M.; Bundalian, L.; Katzmann, J.L.; Laufs, U.; Blüher, M.; Garten, A.; Le Duc, D. Obesity—An Update on the Basic Pathophysiology and Review of Recent Therapeutic Advances. Biomolecules 2021, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef]
- Mahase, E. Global Cost of Overweight and Obesity Will Hit $4.32tn a Year by 2035, Report Warns. BMJ 2023, 380, 523. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H. World Obesity Federation Obesity: A Chronic Relapsing Progressive Disease Process. A Position Statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-Obesity Drug Discovery: Advances and Challenges. Nat. Rev. Drug. Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Shohag, S.; Hossain, M.E.; Rahaman, M.S.; Islam, F.; Ahmed, M.; Mitra, S.; Khandaker, M.U.; Idris, A.M.; et al. The Multifunctional Role of Herbal Products in the Management of Diabetes and Obesity: A Comprehensive Review. Molecules 2022, 27, 1713. [Google Scholar] [CrossRef]
- Yadav, R.P.; Mhatre, S.V.; Bhagit, A.A. Pancreatic Lipase Inhibitor from Food Plant: Potential Molecule for Development of Safe Anti-Obesity Drug. MGM J. Med. Sci. 2016, 3, 34–41. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural Inhibitors of Pancreatic Lipase as New Players in Obesity Treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef]
- Qi, X. Review of the Clinical Effect of Orlistat. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 301, 012063. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-Associated Adverse Effects and Drug Interactions: A Critical Review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Kanwar, S. Phytomolecules for Obesity and Body Weight Management. J. Biochem. Cell Biol. 2018, 1, 101. [Google Scholar]
- Gholamhose, A.; Shahouzehi, B.; Sharifi-far, F. Inhibitory Effect of Some Plant Extracts on Pancreatic Lipase. Int. J. Pharmacol. 2009, 6, 18–24. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and Biological Properties of Agaricus Bisporus Fruiting Bodies—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus Bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, J.; Wen, C.; Sedem Dzah, C.; Chidimma Juliet, I.; Duan, Y.; Zhang, H. Recent Advances in Agaricus Bisporus Polysaccharides: Extraction, Purification, Physicochemical Characterization and Bioactivities. Process Biochem. 2020, 94, 39–50. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Boufi, S. Chitin from Agaricus Bisporus: Extraction and Characterization. Int. J. Bioll Macromol. 2018, 117, 1334–1342. [Google Scholar] [CrossRef]
- Clemente-Ramos, G.; Benítez-Gil, C.; Lopez Diaz-Cano, G.; Esquinas-Trenas, E. H2Oslim®, the Water-Soluble Polysaccharides from Agaricus Bisporus, Significantly Reduces Body Weight and Improves Lipid Parameters in Overweight Humans in a Randomized Double-Blind vs Placebo Study. Am. J. Biomed. Sci. Res. 2020, 7, 479–482. [Google Scholar] [CrossRef]
- Li, X.; Xue, Y.; Pang, L.; Len, B.; Lin, Z.; Huang, J.; ShangGuan, Z.; Pan, Y. Agaricus Bisporus-Derived β-Glucan Prevents Obesity through PPAR γ Downregulation and Autophagy Induction in Zebrafish Fed by Chicken Egg Yolk. Int. J. Biol. Macromol. 2019, 125, 820–828. [Google Scholar] [CrossRef]
- Iñiguez, M.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Recio-Fernández, E.; Roncero-Ramos, I.; Pérez-Clavijo, M.; Oteo, J.-A. Agaricus Bisporus Supplementation Reduces High-Fat Diet-Induced Body Weight Gain and Fatty Liver Development. J. Physiol. Biochem. 2018, 74, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, J.; Bu, F.; Xia, W. Determination of Chitosan with a Modified Acid Hydrolysis and HPLC Method. Carbohydr. Res. 2013, 366, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hou, X.; Liu, M.; Han, Y.; Bao, W.; Wang, X.; Liu, H. Comparison of High Performance Liquid Chromatography and Spectrophotometry in the Determination of Chitosan Content in Water-Soluble Fertilizers. Adv. Anal. Sci. 2022, 3, 63–74. [Google Scholar]
- Kim, J.H.; Kim, H.J.; Kim, C.; Jung, H.; Kim, Y.O.; Ju, J.Y.; Shin, C.S. Development of Lipase Inhibitors from Various Derivatives of Monascus Pigment Produced by Monascus Fermentation. Food Chem. 2007, 101, 357–364. [Google Scholar] [CrossRef]
- Mopuri, R.; Kalyesubula, M.; Rosov, A.; Edery, N.; Moallem, U.; Dvir, H. Improved Folch Method for Liver-Fat Quantification. Front. Vet. Sci. 2020, 7, 594853. [Google Scholar] [CrossRef]
- Hassainia, A.; Satha, H.; Sami, B. Two Routes to Produce Chitosan from Agaricus Bisporus. J. Renew Mater. 2020, 8, 101–111. [Google Scholar] [CrossRef]
- Alimi, B.A.; Pathania, S.; Wilson, J.; Duffy, B.; Frias, J.M.C. Extraction, Quantification, Characterization, and Application in Food Packaging of Chitin and Chitosan from Mushrooms: A Review. Int. J. Biol. Macromol. 2023, 237, 124195. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Jull, A.B.; Ni Mhurchu, C.; Bennett, D.A.; Dunshea-Mooij, C.A.; Rodgers, A. Chitosan for Overweight or Obesity. Cochrane Database Syst. Rev. 2008, 16, CD003892. [Google Scholar] [CrossRef]
- Mhurchu, C.N.; Poppitt, S.D.; McGill, A.-T.; Leahy, F.E.; Bennett, D.A.; Lin, R.B.; Ormrod, D.; Ward, L.; Strik, C.; Rodgers, A. The Effect of the Dietary Supplement, Chitosan, on Body Weight: A Randomised Controlled Trial in 250 Overweight and Obese Adults. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Marañón, J.A.; Plaza, D.M.; Lonzano, C. Natural Approaches for Reducing Risk Markers of Cardiovascular Diseases Related to Obesity. Am. J. Biomed. Sci. Res. 2019, 5, 325–328. [Google Scholar] [CrossRef]
- Lankalapalli, S.; Kolapalli, V.R.M. Polyelectrolyte Complexes: A Review of Their Applicability in Drug Delivery Technology. Indian J. Pharm. Sci. 2009, 71, 481–487. [Google Scholar] [CrossRef]
- Sugiyama, H.; Akazome, Y.; Shoji, T.; Yamaguchi, A.; Yasue, M.; Kanda, T.; Ohtake, Y. Oligomeric Procyanidins in Apple Polyphenol Are Main Active Components for Inhibition of Pancreatic Lipase and Triglyceride Absorption. J. Agric. Food Chem. 2007, 55, 4604–4609. [Google Scholar] [CrossRef]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a New Lipase Inhibitor for the Management of Obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Pérez, L.G.; Piñón-Simental, J.S.; Magaña-Rodríguez, O.R.; Lopéz-Mejía, A.; Ayala-Ruiz, L.A.; García-Calderón, A.J.; Godínez-Hernández, D.; Rios-Chavez, P. Evaluation of the toxicology, anti-lipase, and antioxidant effects of Callistemon citrinus in rats fed with a high fat-fructose diet. Pharm. Biol. 2022, 60, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, M. Evaluating the Appropriate Oral Lipid Tolerance Test Model for Investigating Plasma Triglyceride Elevation in Mice. PLoS ONE 2020, 15, e0235875. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High Fat Diet Induced Obesity Model Using Four Strains of Mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Blake, G.M.; Fogelman, L. Technical principles of dual energy x-ray absorptiometry. Semin. Nucl. Med. 1997, 27, 210–228. [Google Scholar] [CrossRef]
- Marko, L.; Deike, H.; Nancy, N.; Stephan, S.; Petra, W.; Annette, S. Non-Invasive Quantification of White and Brown Adipose Tissues and Liver Fat Content by Computed Tomography in Mice. PLoS ONE 2012, 7, e37026. [Google Scholar] [CrossRef]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef] [PubMed]
- Natalia, S.; Camila, A.; Amanda, D.; Gonzalo, C. White Adipose Tissue Dysfunction: Pathophysiology and Emergent Measurements. Nutrients 2023, 31, 1722. [Google Scholar] [CrossRef]
- Poddar, K.H.; Ames, M.; Hsin-Jen, C.; Feeney, M.J.; Wang, Y.; Cheskin, L.J. Positive Effect of Mushrooms Substituted for Meat on Body Weight, Body Composition, and Health Parameters. A 1-Year Randomized Clinical Trial. Appetite 2013, 71, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Cheskin, L.J.; Davis, L.M.; Lipsky, L.M.; Mitola, A.H.; Lycan, T.; Mitchell, V.; Mickle, B.; Adkins, E. Lack of energy compensation over 4 days when white button mushrooms are substituted for beef. Appetite 2008, 51, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Lindqvist, A.; Krabisch, L.; Rehfeld, J.F.; Erlanson-Albertsson, C. Appetite suppression through delayed fat digestion. Physiol. Behav. 2006, 89, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.-Å.; Köhnke, R.; Emek, S.C.; Mei, J.; Rehfeld, J.F.; Åkerlund, H.-E.; Erlanson-Albertsson, C. Chloroplast membranes retard fat digestion and induce satiety: Effect of biological membranes on pancreatic lipase/co-lipase. Biochem. J. 2007, 401, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-Alcoholic Fatty Liver Disease (NAFLD): A Review of Pathophysiology, Clinical Management and Effects of Weight Loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Goodman, Z.D. The Impact of Obesity on Liver Histology. Clin. Liver Dis. 2014, 18, 33–40. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver Lipid Metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Lunagariya, N.A.; Patel, N.K.; Jagtap, S.C.; Bhutani, K.K. Inhibitors of pancreatic lipase: State of the art and clinical perspectives. EXCLI J. 2014, 13, 897–921. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Kimura, Y. Low molecular weight chitosan inhibits obesity induced by feeding a high-fat diet long-term in mice. J. Pharm. Pharmacol. 2006, 58, 201–207. [Google Scholar] [CrossRef]
- Teh, L.B.; Stopard, M.; Anderson, S.; Grant, A.; Quantrill, D.; Wilkinson, R.H.; Jewell, D.P. Assessment of Fat Malabsorption. J. Clin. Pathol. 1983, 36, 1362–1366. [Google Scholar] [CrossRef] [PubMed]
| Class Description | Ingredients | Control Diet (CD) | High-Fat Diet (HFD) |
|---|---|---|---|
| Protein | Casein, latic | 200.00 g | 200.00 g |
| Protein | Cystine, L | 3.00 g | 3.00 g |
| Carbohydrate | Sucrose | 354.00 g | 72.80 g |
| Carbohydrate | Starch, corn | 315.00 g | - |
| Carbohydrate | Maltodextrin | 35.00 g | 125.00 g |
| Fiber | Cellulose | 50.00 g | 50.00 g |
| Fat | Lard | 20.00 g | 245.00 g |
| Fat | Soybean oil | 25.00 g | 25.00 g |
| Mineral | Mineral mixture (1) | 50.00 g | 50.00 g |
| Vitamin | Choline bitartrate | 2.00 g | 2.00 g |
| Vitamin | Vitamin mixture (2) | 1.00 g | 1.00 g |
| Dye | Dye | 0.05 g | 0.05 g |
| Total: 1055.05 g | Total: 773.85 g |
| Inhibition of Pancreatic Lipase Activity (%) | ||
|---|---|---|
| (mg/mL) | ABE | Chitosan |
| 0 | 0 d | 0 e |
| 0.01 | 21.4 ± 2.0 c | 16.8 ± 2.2 d |
| 0.05 | 29.1 ± 2.2 b | 23.4 ± 1.9 cd |
| 0.1 | 32.2 ± 2.4 ab | 23.9 ± 1.7 bcd |
| 0.5 | 33.2 ± 2.1 ab | 25.4 ± 3.7 bc |
| 1.0 | 36.9 ± 1.7 a | 26.3 ± 2.7 abc |
| 1.5 | 35.1 ± 2.0 a | 29.1 ± 2.4 abc |
| 2.0 | 34.5 ± 1.7 ab | 31.5 ± 2.1 ab |
| 3.0 | 32.3 ± 1.7 ab | 33.4 ± 2.1 a |
| CD | HFD | HFD + A80 | HFD + A160 | HFD + C160 | |
|---|---|---|---|---|---|
| Initial body weight (g) | 19.2 ± 0.7 | 20.7 ± 0.4 | 21.1 ± 0.2 | 20.7 ± 0.5 | 21.1 ± 0.2 |
| Final body weight (g) | 29.4 ± 0.4 | 40.6 ± 0.7 ***,a | 34.8 ± 0.5 b | 33.5 ± 0.9 b | 35.5 ± 1.0 b |
| Body weight gain (g) | 10.2 ± 0.9 | 20.0 ± 0.6 ***,a | 13.7 ± 0.4 b | 12.8 ± 1.2 b | 14.4 ± 0.9 b |
| Lean mass percentage (%) | 75.8 ± 0.8 | 60.4 ±± 0.7 ***,b | 62.4 ± 0.8 ab | 64.1 ± 1.0 a | 63.2 ± 0.8 a |
| Fat mass percentage (%) | 24.2 ± 0.9 | 39.6 ± 0.7 ***,a | 37.6 ± 0.8 ab | 35.9 ± 1.0 b | 36.8 ± 0.8 b |
| Food intake (g/day) | 2.85 ± 0.04 | 2.46 ± 0.04 ***,a | 2.18 ± 0.02 c | 2.24 ± 0.02 c | 2.32 ± 0.01 b |
| Food efficiency ratio (1) | 0.065 ± 0.006 | 0.148 ± 0.005 ***,a | 0.115 ± 0.004 b | 0.104 ± 0.010 b | 0.112 ± 0.007 b |
| CD | HFD | HFD + A80 | HFD + A160 | HFD + C160 | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | 197.2 ± 6.6 | 222.6 ± 8.4 *,a | 186.3 ± 6.5 b | 178.0 ± 10.8 b | 195.6 ± 13.0 ab |
| Triglycerides (mg/dL) | 38.0 ± 2.5 | 54.5 ± 2.8 ***,a | 47.2 ± 3.2 ab | 41.1 ± 2.7 b | 39.0 ± 2.3 b |
| Total cholesterol (mg/dL) | 145.4 ± 9.2 | 176.2 ± 3.8 ** | 171.2 ± 5.6 | 166.4 ± 9.5 | 160.0 ± 4.6 |
| ALT (U/L) | 51.4 ± 7.4 | 122.1 ± 21.6 **,a | 70.4 ± 8.9 b | 55.9 ± 5.6 b | 90.2 ± 22.4 ab |
| AST (U/L) | 118.8 ± 12.1 | 192.1 ± 19.6 **,a | 138.3 ± 9.5 b | 139.2 ± 11.1 b | 169.3 ± 17.8 ab |
| CD | HFD | HFD + A80 | HFD + A160 | HFD + C160 | |
|---|---|---|---|---|---|
| Liver weight (g) | 0.98 ± 0.02 | 1.14 ± 0.05 **,a | 0.95 ± 0.05 b | 0.89 ± 0.03 b | 0.91 ± 0.03 b |
| Lipids (mg/g liver) | 84.0 ± 2.9 | 121.6 ± 7.4 ***,a | 99.2 ± 3.1 ab | 89.6 ± 3.4 b | 90.3 ± 4.0 b |
| Total lipids in liver (mg/liver) | 82.5 ± 7.0 | 139.5 ± 14.2 **,a | 96.2 ± 10.6 b | 83.6 ± 5.7 b | 82.1 ± 6.4 b |
| Triglycerides (μmol/g liver) | 19.8 ± 0.7 | 24.6 ± 1.0 ** | 23.6 ± 1.1 | 22.9 ± 0.8 | 23.8 ± 1.3 |
| Total triglycerides in liver (μmol/liver) | 19.7 ± 1.2 | 26.6 ± 2.1 *,a | 23.5 ± 2.0 ab | 20.8 ± 1.2 b | 21.5 ± 1.3 b |
| Cholesterol (μmol/g liver) | 5.4 ± 0.3 | 7.4 ± 0.5 ** | 7.3 ± 0.4 | 6.7 ± 0.4 | 6.8 ± 0.4 |
| Total cholesterol in liver (μmol/liver) | 5.3 ± 0.3 | 8.5 ± 1.1 **,a | 7.0 ± 0.7 ab | 6.0 ± 0.4 b | 6.2 ± 0.4 b |
| CD | HFD | HFD + A80 | HFD + A160 | HFD + C160 | |
|---|---|---|---|---|---|
| Lipids (mg/g feces) | 23.41 ± 1.68 | 35.40 ± 2.53 *** | 37.48 ± 1.84 | 40.76 ± 2.86 | 39.70 ± 2.14 |
| Total lipids in feces (mg/day) | 6.49 ± 1.05 | 12.64 ± 0.89 ***,b | 12.47 ± 0.86 b | 18.32 ± 1.93 a | 15.50 ± 1.88 ab |
| Triglycerides (μmol/g feces) | 14.86 ± 0.93 | 17.08 ± 1.12 | 18.68 ± 1.18 | 19.22 ± 1.73 | 17.95 ± 1.07 |
| Total triglycerides in feces (μmol/day) | 4.89 ± 0.61 | 5.30 ± 0.38 b | 5.77 ± 0.42 b | 7.70 ± 0.67 a | 6.90 ± 0.83 ab |
| Cholesterol (μmol/g feces) | 4.81 ± 0.42 | 5.40 ± 0.40 | 5.76 ± 0.31 | 6.05 ± 0.47 | 5.92 ± 0.29 |
| Total cholesterol in feces (μmol/day) | 1.46 ± 0.24 | 1.88 ± 0.15 | 1.88 ± 0.17 | 2.40 ± 0.16 | 2.42 ± 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jeon, Y.-E.; Kim, S.-M.; Jung, J.-I.; Ko, D.; Kim, E.-J. Agaricus bisporus Extract Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese C57BL/6N Mice by Inhibiting Pancreatic Lipase-Mediated Fat Absorption. Nutrients 2023, 15, 4225. https://doi.org/10.3390/nu15194225
Kim H, Jeon Y-E, Kim S-M, Jung J-I, Ko D, Kim E-J. Agaricus bisporus Extract Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese C57BL/6N Mice by Inhibiting Pancreatic Lipase-Mediated Fat Absorption. Nutrients. 2023; 15(19):4225. https://doi.org/10.3390/nu15194225
Chicago/Turabian StyleKim, Hyungkeun, Young-Eun Jeon, So-Mi Kim, Jae-In Jung, Donghyeon Ko, and Eun-Ji Kim. 2023. "Agaricus bisporus Extract Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese C57BL/6N Mice by Inhibiting Pancreatic Lipase-Mediated Fat Absorption" Nutrients 15, no. 19: 4225. https://doi.org/10.3390/nu15194225
APA StyleKim, H., Jeon, Y.-E., Kim, S.-M., Jung, J.-I., Ko, D., & Kim, E.-J. (2023). Agaricus bisporus Extract Exerts an Anti-Obesity Effect in High-Fat Diet-Induced Obese C57BL/6N Mice by Inhibiting Pancreatic Lipase-Mediated Fat Absorption. Nutrients, 15(19), 4225. https://doi.org/10.3390/nu15194225






