Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
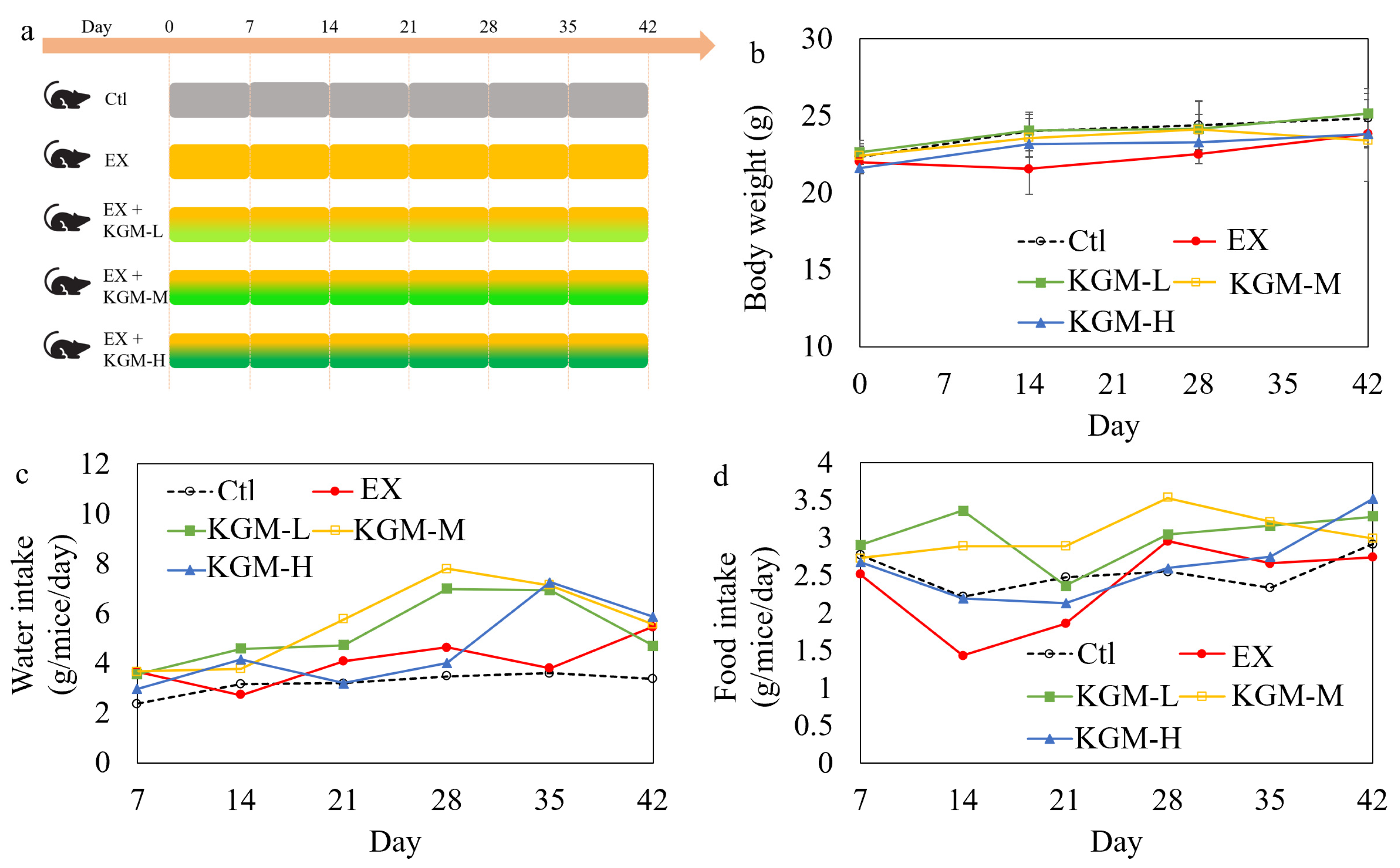
2.2. Animal Experiment Design
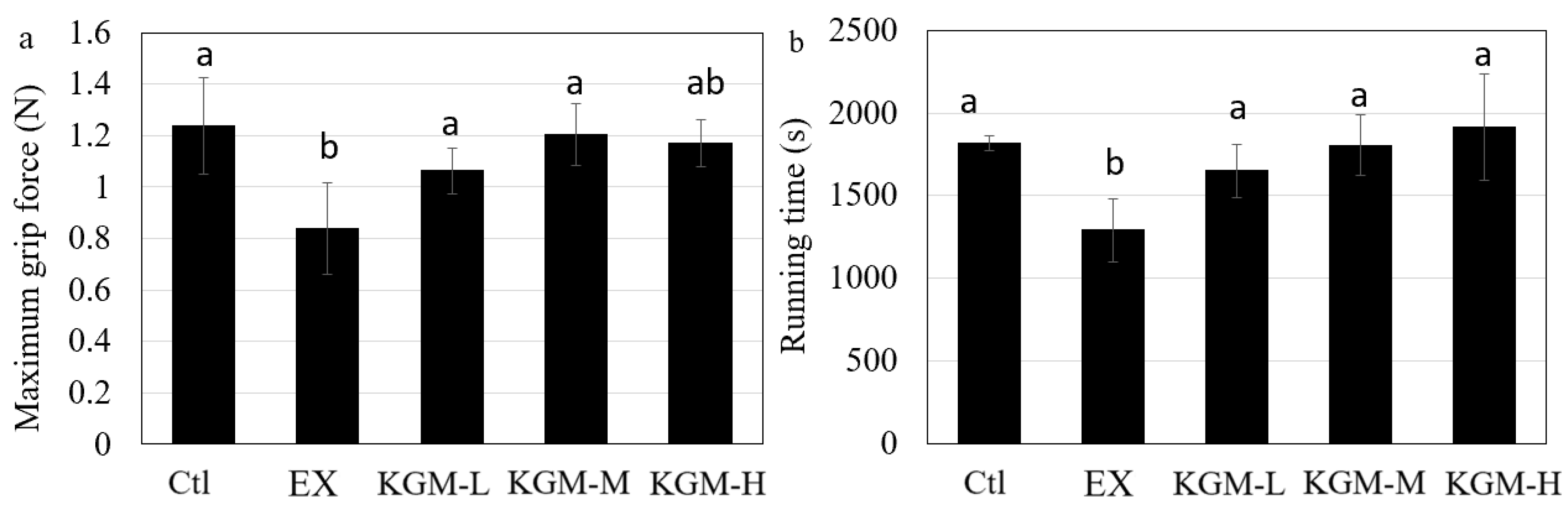
2.3. Maximum Grip Force Test
2.4. Endurance Test
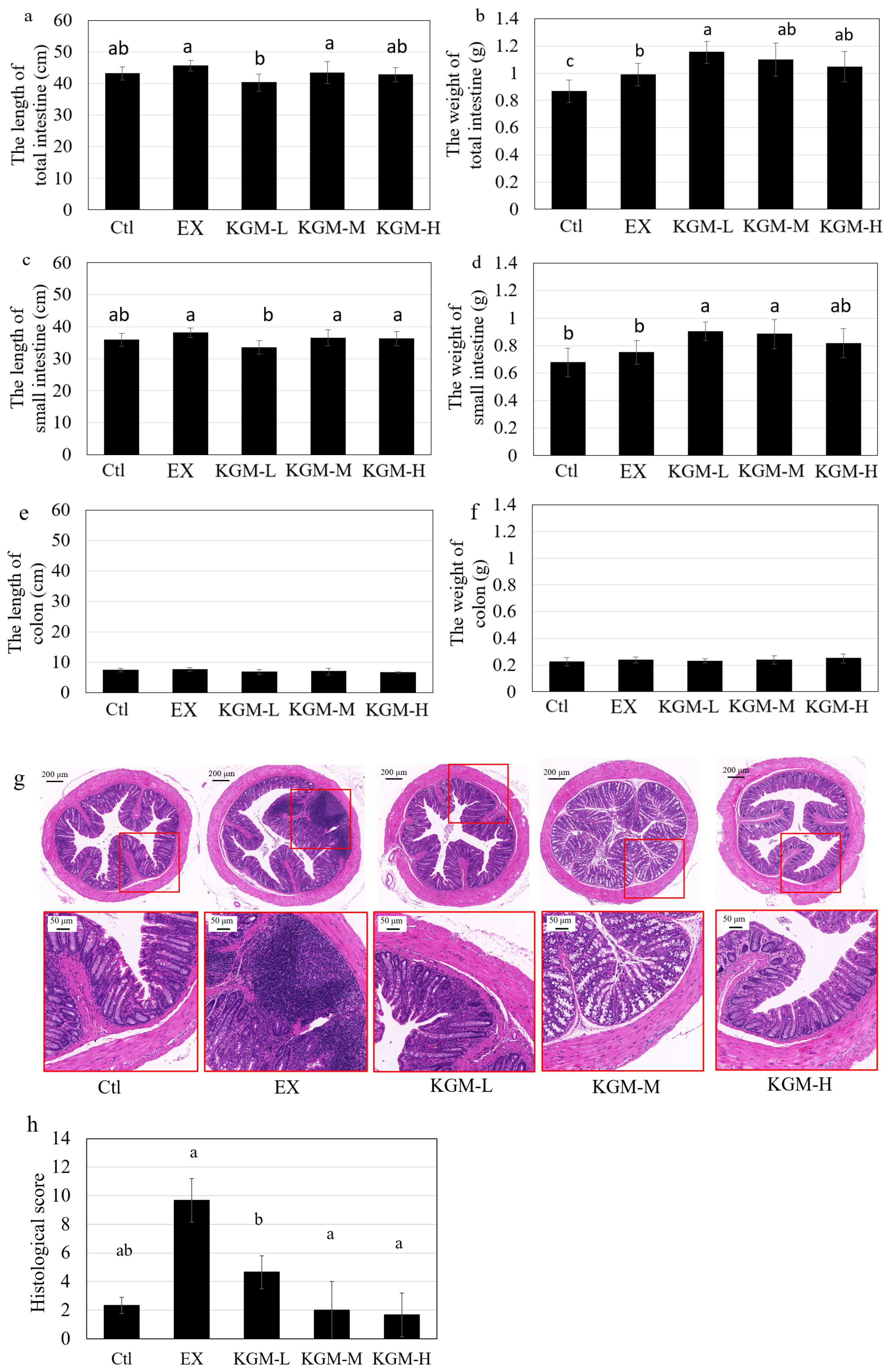
2.5. Intestinal Morphology
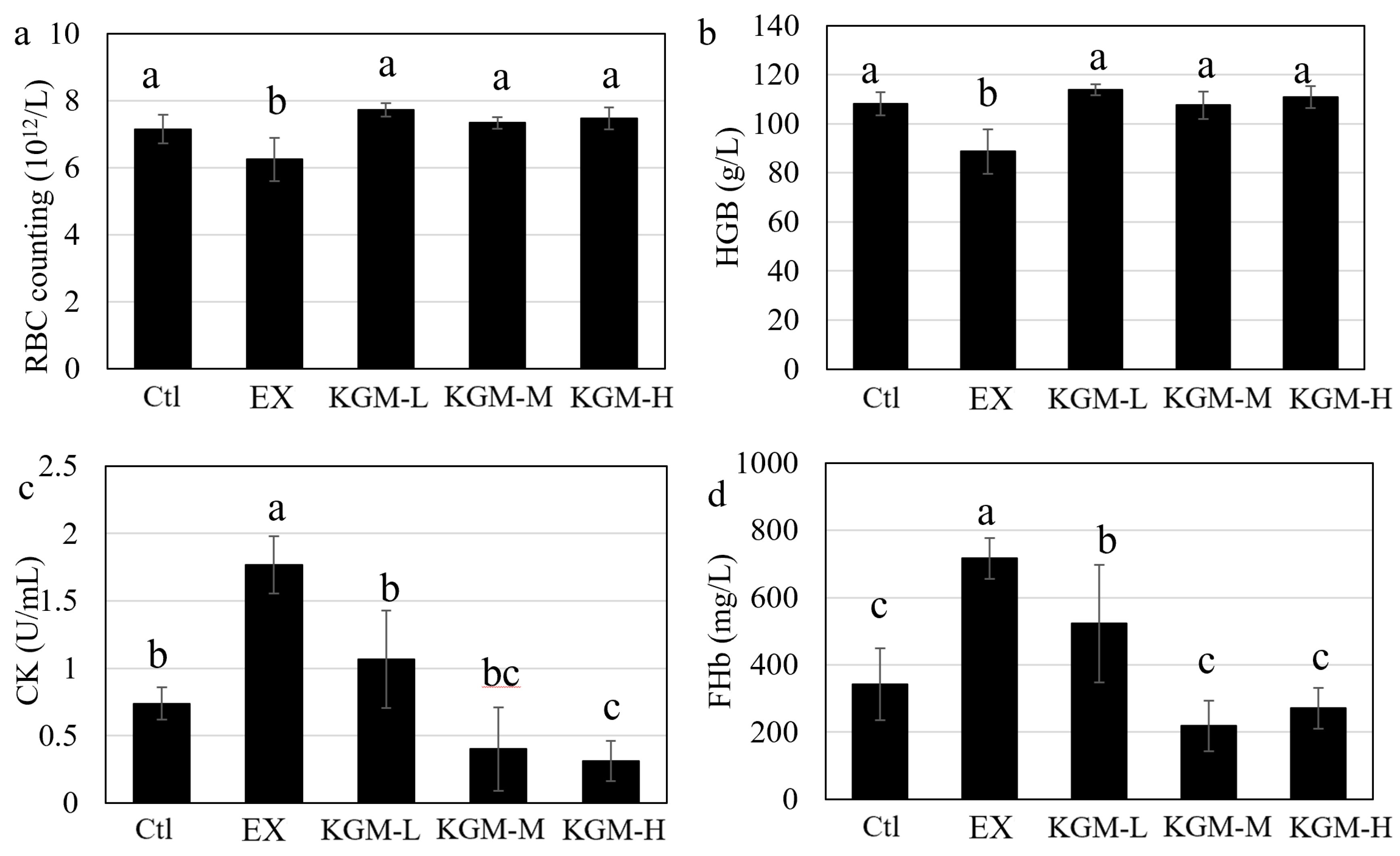
2.6. Determination of Fatigue-Associated Biomarkers in Plasma
2.7. Determination of Short-Chain Fatty Acids in Plasma and Feces
2.8. DNA Extraction and 16S rRNA Sequencing
2.9. Statistical Analysis
3. Results
3.1. The Influence of KGM on Body Weight, Water and Food Intake
3.2. The Influence of KGM on Sports Performance
3.3. The Influence of KGM on Blood Biochemical Indices
3.4. Intestinal Morphography
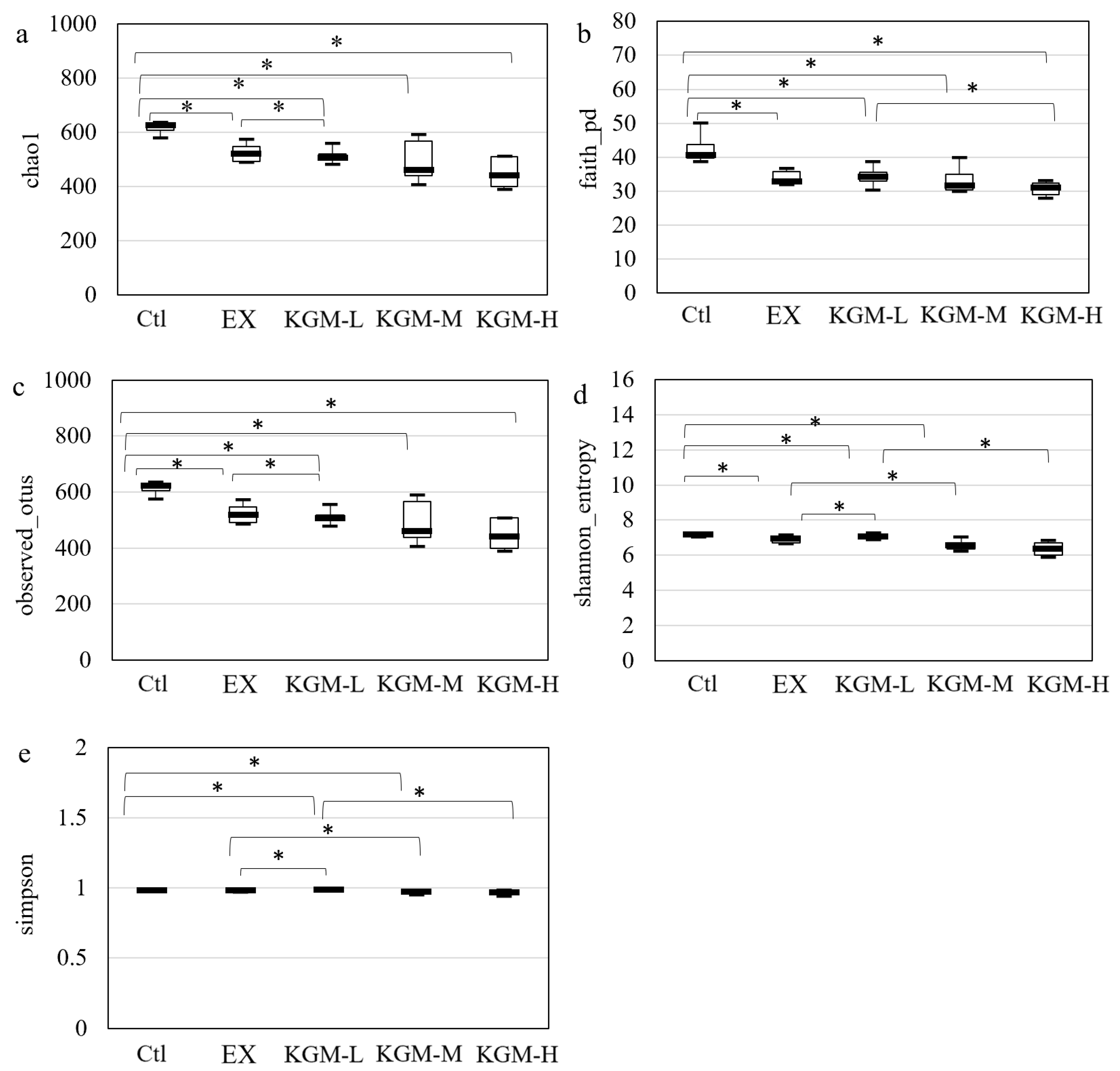
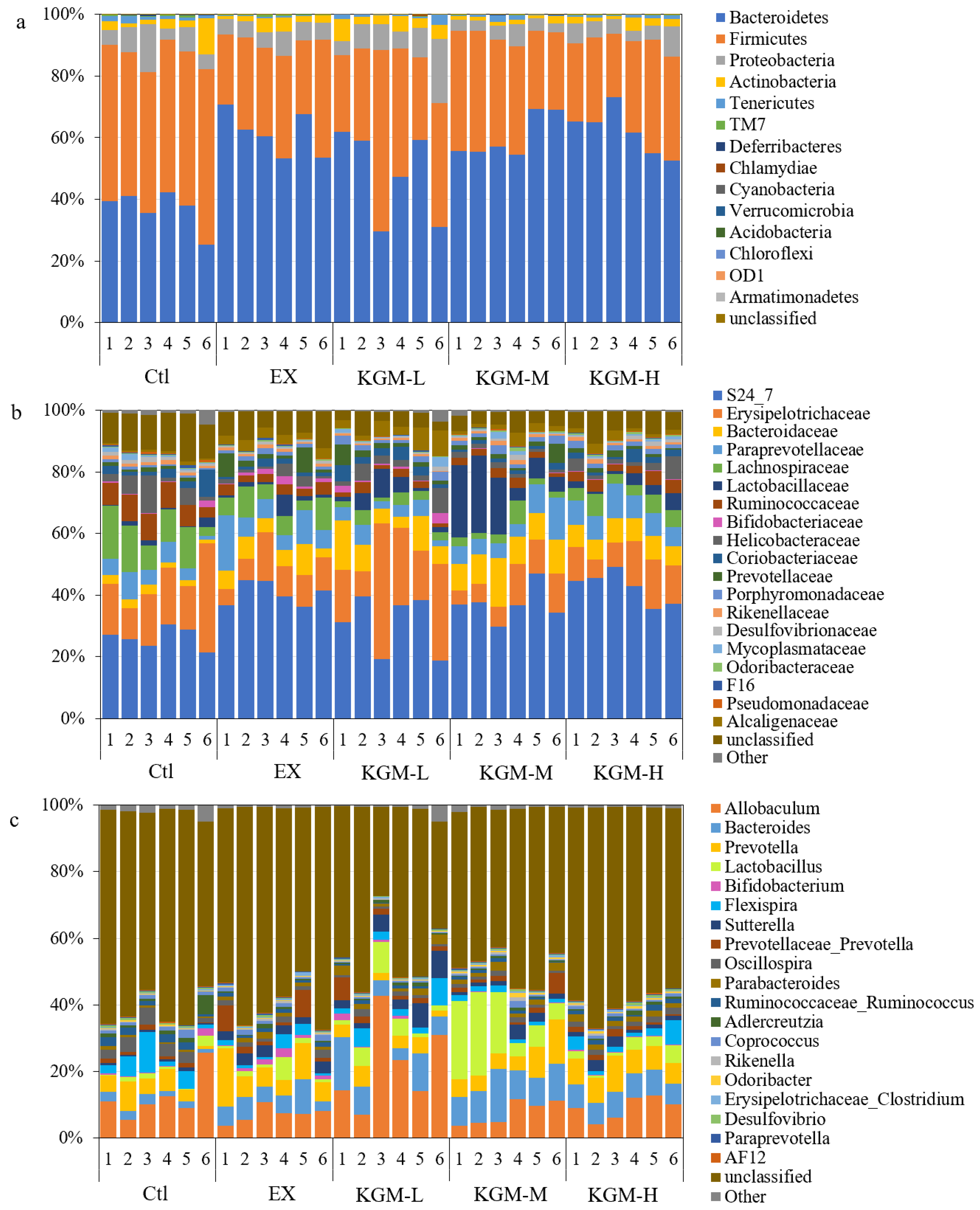
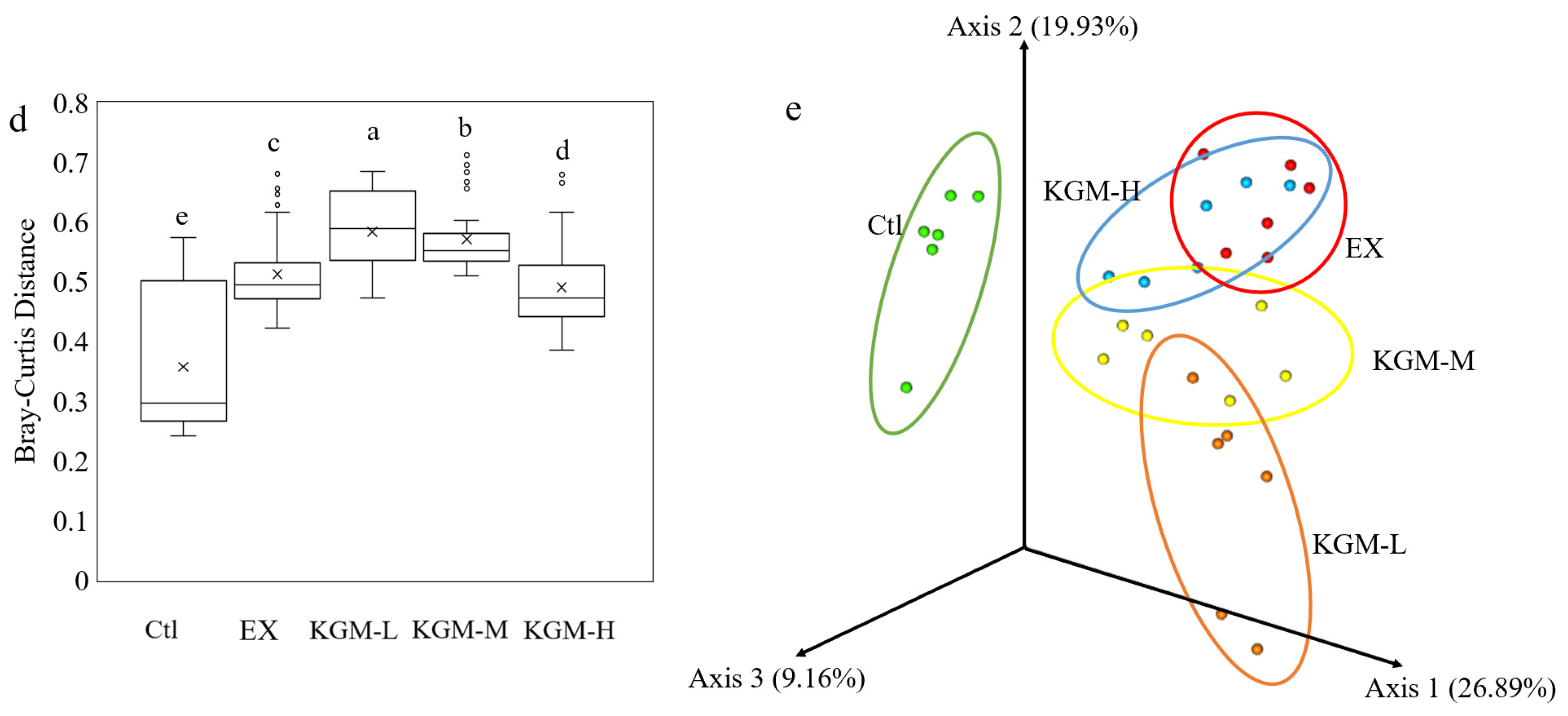
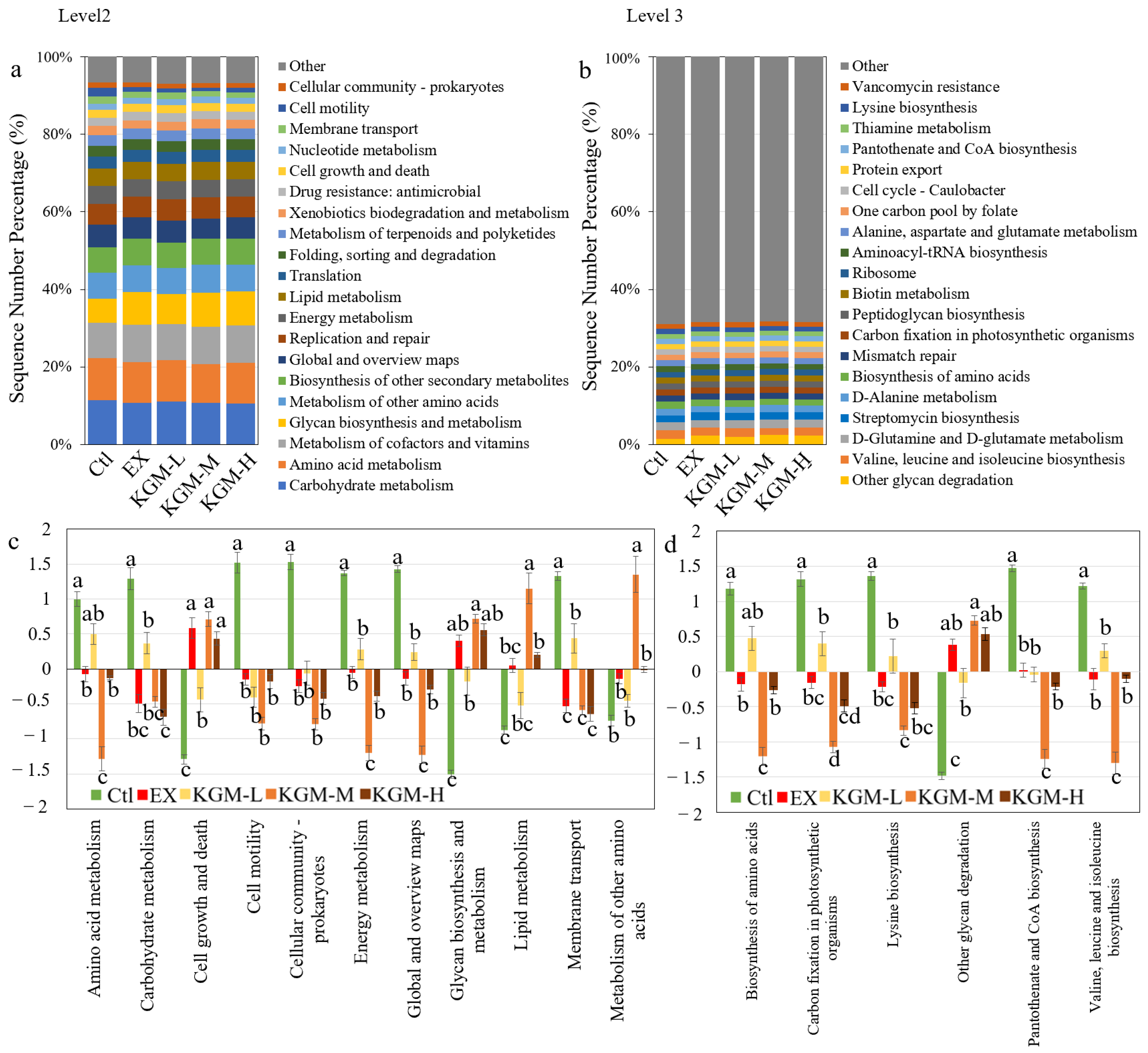
3.5. The Influence of KGM on Gut Microbiome
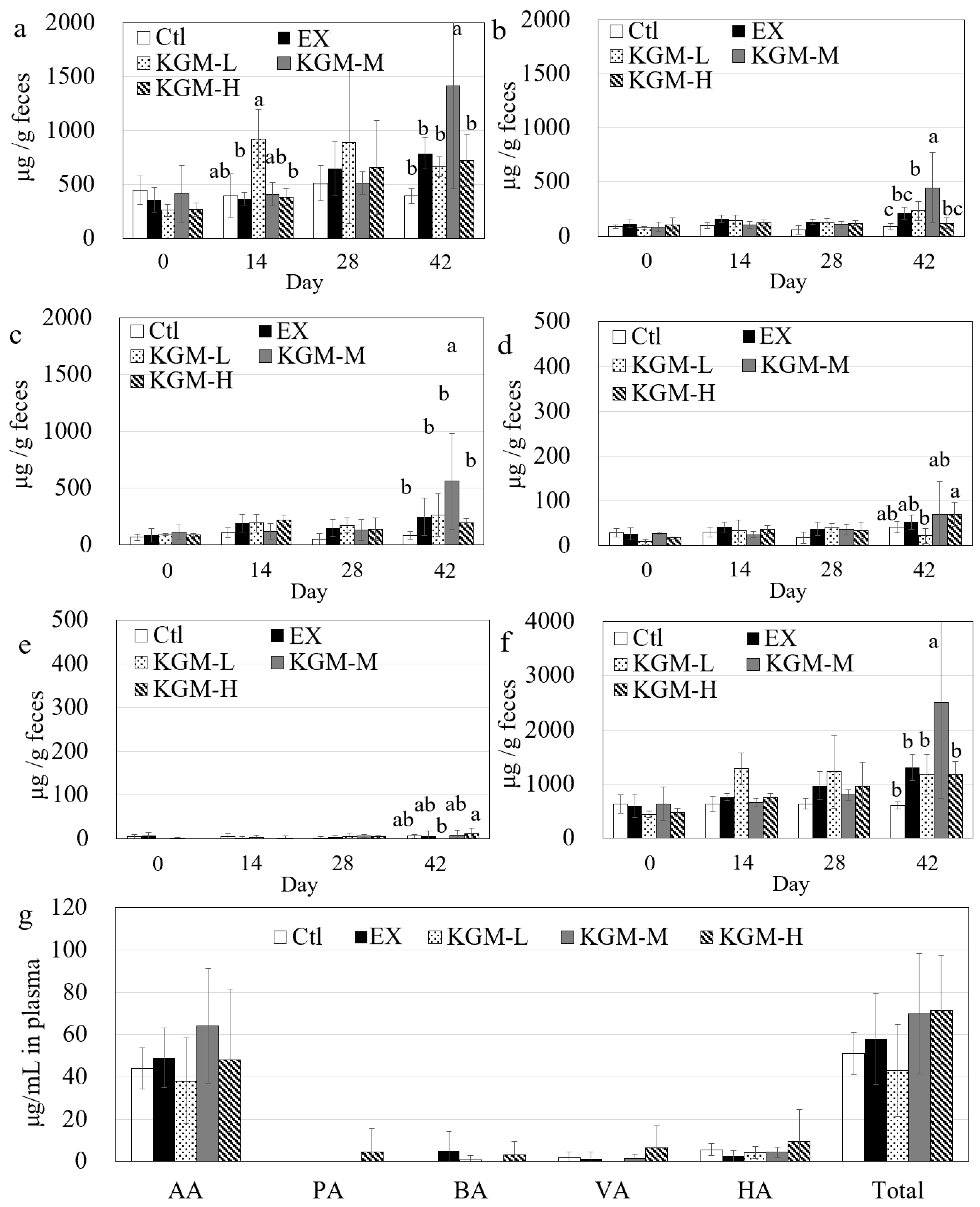
3.6. Short-Chain Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; Kubik, M.; et al. US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for Cardiovascular disease prevention in adults without cardiovascular disease risk factors: US preventive services task force recommendation statement. JAMA 2022, 328, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Weakley, J.; Halson, S.L.; Mujika, I. Overtraining syndrome symptoms and diagnosis in athletes: Where is the research? A sys tematic review. Int. J. Sports Physiol. Perform. 2022, 17, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; Heikura, I.A.; Meeusen, R.; Bermon, S.; Silier, S.; Mountjoy, M.L.; Burke, L.M. Overtraining syndrome (OTS) and relative energy deficiency in sport (RED-S): Shared pathways, symptoms and complexities. Sports Med. 2021, 51, 2251–2280. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Tsukahara, Y.; Kamada, H.; Torii, S.; Yamasawa, F. Association between self-reported overtraining syndrome and symptoms in high school track and field athletes. Int. J. Sports Med. 2023, 44, 138–144. [Google Scholar] [CrossRef]
- Keohane, D.M.; Woods, T.; O’Connor, P.; Underwood, S.; Cronin, O.; Whiston, R.; O’Sullivan, O.; Cotter, P.; Shanahan, F.; Molloy, M.G.M. Four men in a boat: Ultra-endurance exercise alters the gut microbiome. J. Sci. Med. Sport 2019, 22, 1059–1064. [Google Scholar] [CrossRef]
- Wegierska, A.E.; Charitos, I.A.; Topi, S.; Potenza, M.A.; Montagnani, M.; Santacroce, L. The connection between physical exercise and gut microbiota: Implications for competitive sports athletes. Sports Med. 2022, 52, 2355–2369. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Bergeron, M.F.; Lee, E.C.; Mershon, J.E.; Armstrong, E.M. Armstrong, overtraining syndrome as a complex systems phenomenon. Front. Netw. Physiol. 2022, 1, 20. [Google Scholar] [CrossRef]
- Cataldi, S.; Poli, L.; Şahin, F.N.; Patti, A.; Santacroce, L.; Bianco, A.; Greco, G.; Ghinassi, B.; Di Baldassarre, A.; Fischetti, F. The effects of physical activity on the gut microbiota and the gut-brain axis in preclinical and human models: A narrative review. Nutrients 2022, 14, 3293. [Google Scholar] [CrossRef]
- Karl, J.P.; Margolis, L.M.; Madslien, E.H.; Murphy, N.E.; Castellani, J.W.; Gundersen, Y.; Hoke, A.V.; Levangie, M.W.; Kumar, R.; Chakraborty, N.; et al. Changes in intestinal microbiota composition and metabolism coincide with in creased intestinal permeability in young adults under prolonged physiological stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G559–G571. [Google Scholar] [CrossRef]
- Zhao, C.; Gong, Y.; Zheng, L.; Zhao, M. The degree of hydrolysis and peptide profile affect the anti-fatigue activities of whey protein hydrolysates in promoting energy metabolism in exercise mice. J. Agric. Food Chem. 2023, 71, 3010–3021. [Google Scholar] [CrossRef]
- Zhou, Y.; Cao, F.; Wu, Q.; Luo, Y.; Guo, T.; Han, S.; Huang, M.; Hu, Z.; Bai, J.; Luo, F.; et al. Dietary supplementation of octacosanol improves exercise-induced fatigue and its molecular mechanism. J. Agric. Food Chem. 2021, 69, 7603–7618. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Holscher, H.D. Fueling gut microbes: A review of the interaction between diet, exercise, and the gut microbiota in athletes. Adv. Nutr. 2021, 12, 2190–2215. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; Ray, R.C. Nutritional and potential health benefits of konjac glucomannan, a promising polysaccharide of elephant foot yam, Amorphophallus konjac K. Koch: A review. Food Rev. Int. 2017, 33, 22–43. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Vingren, J.L.; Silvestre, R.; Spiering, B.A.; Hatfield, D.L.; Ho, J.Y.; Fragala, M.S.; Maresh, C.M.; Volek, J.S. Effect of adding exercise to a diet containing glucomannan. Metabolism 2007, 56, 1149–1158. [Google Scholar] [CrossRef]
- Mao, Y.H.; Song, A.X.; Yao, Z.P.; Wu, J.Y. Protective effects of natural and partially degraded konjac glucomannan on Bifidobacteria against antibiotic damage. Carbohydr. Polym. 2018, 181, 368–375. [Google Scholar] [CrossRef]
- Mao, Y.H.; Xu, Y.; Song, F.; Wang, Z.M.; Li, Y.H.; Zhao, M.; He, F.; Tian, Z.; Yang, Y. Protective effects of konjac glucomannan on gut microbiome with antibiotic perturbation in mice. Carbohydr. Polym. 2022, 290, 119476. [Google Scholar] [CrossRef]
- Mao, Y.H.; Xu, Y.X.; Li, Y.H.; Cao, J.; Song, F.L.; Zhao, D.; Zhao, Y.; Wang, Z.M.; Yang, Y. Effects of konjac glucomannan with different molecular weights on gut microflora with antibiotic perturbance in in vitro fecal fermentation. Carbohydr. Polym. 2021, 273, 118546. [Google Scholar] [CrossRef]
- Pereira, B.C.; Filho, L.A.; Alves, G.F.; Pauli, J.R.; Ropelle, E.R.; Souza, C.T.; Cintra, D.E.; Saad, M.J.; Silva, A.S. A new overtraining protocol for mice based on downhill running sessions. Clin. Exp. Pharmacol. Physiol. 2012, 39, 793–798. [Google Scholar] [CrossRef]
- Nickel, K.; Zhu, L.; Mangalindan, R.; Snyder, J.M.; Tucker, M.; Whitson, J.; Sweetwyne, M.; Valencia, A.P.; Klug, J.; Jiang, Z.; et al. Long-term treatment with elamipretide enhances healthy aging phenotypes in mice. Aging Pathobiol. Ther. 2022, 4, 76–83. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jung, J.I.; Jeon, Y.E.; Kim, S.M.; Hong, S.H.; Kim, T.Y.; Kim, E.J. Gynostemma pentaphyllum extract and its active component gypenoside L improve the exercise performance of treadmill-trained mice. Nutr. Res. Pract. 2022, 16, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Hoving, L.R.; Heijink, M.; van Harmelen, V.; van Dijk, K.W.; Giera, M. GC-MS analysis of short-chain fatty acids in feces, cecum content, and blood samples. Methods Mol. Biol. 2018, 1730, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yacov, O.; Godneva, A.; Rein, M.; Shilo, S.; Kolobkov, D.; Koren, N.; Cohen Dolev, N.; Travinsky Shmul, T.; Wolf, B.C.; Kosower, N.; et al. Personalized postprandial glucose response-targeting diet versus mediterranean diet for glycemic control in prediabetes. Diabetes Care 2021, 44, 1980–1991. [Google Scholar] [CrossRef] [PubMed]
- Gunning, M.N.; Sir Petermann, T.; Crisosto, N.; van Rijn, B.B.; de Wilde, M.A.; Christ, J.P.; Uiterwaal, C.S.P.M.; de Jager, W.; Eijkemans, M.J.C.; Kunselman, A.R.; et al. Cardiometabolic health in offspring of women with PCOS compared to healthy controls: A systematic review and individual participant data meta-analysis. Hum. Reprod. Update 2020, 26, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Zhang, B.; Huang, R.; Zhang, J.; Chen, Z.; Shang, X.; Li, X.; Nie, X. Intestinal flora disruption and novel biomarkers associated with nasopharyngeal carcinoma. Front. Oncol. 2019, 9, 1346. [Google Scholar] [CrossRef]
- Winsley, R.; Matos, N. Overtraining and elite young athletes. Med. Sport Sci. 2011, 56, 97–105. [Google Scholar] [CrossRef]
- Stensel, D. Exercise, appetite and appetite-regulating hormones: Implications for food intake and weight contro. Ann. Nutr. Metab. 2010, 57 (Suppl. S2), 36–42. [Google Scholar] [CrossRef]
- Arakawa, K.; Hosono, A.; Shibata, K.; Ghadimi, R.; Fuku, M.; Goto, C.; Imaeda, N.; Tokudome, Y.; Hoshino, H.; Marumoto, M.; et al. Changes in blood biochemical markers before, during, and after a 2-day ultramara thon. Open Access J. Sports Med. 2016, 7, 43–50. [Google Scholar] [CrossRef]
- Taylor, S.R.; Ramsamooj, S.; Liang, R.J.; Katti, A.; Pozovskiy, R.; Vasan, N.; Hwang, S.K.; Nahiyaan, N.; Francoeur, N.J.; Schatoff, E.M.; et al. Dietary fructose improves intestinal cell survival and nutrient absorption. Nature 2021, 597, 263–267. [Google Scholar] [CrossRef]
- Increasing Fiber Intake. 2023. Available online: www.ucsfhealth.org/education/increasing-fiber-intake (accessed on 31 August 2023).
- Ejeda-Manzanera, J.M.; Rodrigo-Vega, M. Eating habits and diet quality in university students ofteaching in relation to their adherence to the mediterranean diet. Rev. Esp. Salud Publica 2021, 17, e202102028. [Google Scholar]
- King, D.E.; Mainous, A.G., 3rd; Lambourne, C.A. Trends in dietary fiber intake in the United States, 1999–2008. J. Acad. Nutr. Diet 2012, 112, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Gouma, E.; Simos, Y.; Verginadis, I.; Lykoudis, E.; Evangelou, A.; Karkabounas, S. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Lab. Anim. 2012, 46, 40–45. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Sun, H.; Wang, Y.; Yang, Z.; Zhang, G.; Yang, W. Immunomodulatory, antioxidant and intestinal morphology-regulating activities of alfalfa poly saccharides in mice. Int. J. Biol. Macromol. 2019, 133, 1107–1114. [Google Scholar] [CrossRef]
- Xu, C.; Yu, C.; Yang, S.; Deng, L.; Zhang, C.; Xiang, J.; Shang, L. Effects of physical properties of konjac glucomannan on appetite response of rats. Foods 2023, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Tan, N.; Zhu, H.; Zeng, G.; He, W.; Yu, B.; Chen, X. Anti-sports anaemia effects of verbascoside and martynoside in mice. Int. J. Sports Med. 2010, 31, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Sharma, A.P.; Stellingwerff, T. Contemporary periodization of altitude training for elite endurance athletes: A narrative review. Sports Med. 2019, 49, 1651–1669. [Google Scholar] [CrossRef]
- Borren, N.Z.; Plichta, D.; Joshi, A.D.; Bonilla, G.; Peng, V.; Colizzo, F.P.; Luther, J.; Khalili, H.; Garber, J.J.; Janneke van der Woude, C.; et al. Alterations in fecal microbiomes and serum metabolomes of fatigued patients with quiescent inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 519–527.e5. [Google Scholar] [CrossRef]
- Henriques, S.F.; Dhakan, D.B.; Serra, L.; Francisco, A.P.; Carvalho-Santos, Z.; Baltazar, C.; Elias, A.P.; Anjos, M.; Zhang, T.; Maddocks, O.D.K.; et al. Metabolic cross-feeding in imbalanced diets allows gut microbes to improve reproduction and alter host behaviour. Nat. Commun. 2020, 11, 4236. [Google Scholar] [CrossRef]
- Ni, Y.; Lin, W.; Mu, R.; Wu, C.; Lin, Z.; Chen, S.; Pang, J. Facile fabrication of novel konjac glucomannan films with antibacterial properties via microfluidic spinning strategy. Carbohydr. Polym. 2019, 208, 469–476. [Google Scholar] [CrossRef]
- McKee, L.S.; La Rosa, S.L.; Westereng, B.; Eijsink, V.G.; Pope, P.B.; Larsbrink, J. Polysaccharide degradation by the Bacteroidetes: Mechanisms and nomenclature. Environ. Microbiol. Rep. 2021, 13, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise rraining modulates gut microbiota profile and improves endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Hiipplala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef]
- Zheng, Z.; Lyu, W.; Ren, Y.; Li, X.; Zhao, S.; Yang, H.; Xiao, Y. Allobaculum involves in the modulation of intestinal ANGPTLT4 expression in mice treated by high-fat Diet. Front. Nutr. 2021, 8, 690138. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E. Gut microbiota in 2015: Prevotella in the gut: Choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef]
- Yang, R.; Shan, S.; Shi, J.; Li, H.; An, N.; Li, S.; Cui, K.; Guo, H.; Li, Z. Coprococcus eutactus, a potent probiotic, alleviates Colitis via acetate-mediated IgA response and microbiota restoration. J. Agric. Food Chem. 2023, 71, 3273–3284. [Google Scholar] [CrossRef]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFA: What are the potential underlying mech anisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef]
- Bongiovanni, T.; Yin, M.O.L.; Heaney, L.M. The athlete and gut microbiome: Short-chain fatty acids as potential ergogenic aids for exercise and training. Int. J. Sports Med. 2021, 42, 1143–1158. [Google Scholar] [CrossRef]
- Pan, J.H.; Kim, J.H.; Kim, H.M.; Lee, E.S.; Shin, D.H.; Kim, S.; Shin, M.; Kim, S.H.; Lee, J.H.; Kim, Y.J. Acetic acid enhances endurance capacity of exercise-trained mice by increasing skeletal muscle oxidative properties. Biosci. Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef]
- Walsh, M.E.; Bhattacharya, A.; Sataranatarajan, K.; Qaisar, R.; Sloane, L.; Rahman, M.M.; Kinter, M.; Van Remmen, H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell 2015, 14, 957–970. [Google Scholar] [CrossRef]
- Majid, H.A.; Cole, J.; Emery, P.W.; Whelan, K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: A randomized controlled trial. Clin. Nutr. 2014, 33, 966–972. [Google Scholar] [CrossRef] [PubMed]
| Week | Intensity (% EV) | Duration (min) | Sessions/Day | Recovery between Sessions (h) | Treadmill Grade (up, %) |
|---|---|---|---|---|---|
| 1 | 60 | 30 | 1 | 24 | 0 |
| 2 | 60 | 45 | 1 | 24 | 0 |
| 3 | 60 | 60 | 1 | 24 | 14 |
| 4 | 70 | 60 | 1 | 24 | 14 |
| 5 | 75 | 75 | 1 | 24 | 14 |
| 6 | 75 | 75 | 2 | 4 | 14 |
| Ctl | EX | KGM-L | KGM-M | KGM-H | |
|---|---|---|---|---|---|
| Phylum | |||||
| Bacteroidetes | 36.92 ± 6.16 b | 61.34 ± 7.15 a | 48.06 ± 14.59 ab | 60.14 ± 7.03 a | 62.07 ± 7.54 a |
| Firmicutes | 49.91 ± 3.89 a | 29.57 ± 5.82 b | 37.08 ± 12.69 b | 33.16 ± 6.39 b | 28.94 ± 5.88 b |
| Proteobacteria | 7.47 ± 4.42 ab | 5.71 ± 1.10 ab | 9.33 ± 5.87 a | 4.29 ± 1.59 b | 5.74 ± 2.27 ab |
| Actinobacteria | 3.79 ± 3.91 ab | 2.64 ± 1.43 ab | 4.26 ± 1.78 a | 1.34 ± 0.53 b | 2.25 ± 1.24 ab |
| Cyanobacteria | 0.01 ± 0.01 ab | 0.00 ± 0.00 b | 0.01 ± 0.00 a | 0.02 ± 0.03 ab | 0.02 ± 0.01 ab |
| unclassified | 0.02 ± 0.01 a | 0.01 ± 0.01 bc | 0.00 ± 0.00 c | 0.01 ± 0.01 ab | 0.01 ± 0.01 bc |
| Family | |||||
| S24_7 | 26.21 ± 3.36 c | 40.58 ± 3.76 a | 30.64 ± 9.47 bc | 37.06 ± 5.62 ab | 42.48 ± 5.22 a |
| unclassified | 12.02 ± 1.98 a | 8.13 ± 2.29 b | 4.44 ± 1.97 b | 4.80 ± 1.09 b | 6.83 ± 1.88 b |
| Bacteroidaceae | 2.22 ± 0.77 b | 5.97 ± 2.50 a | 8.24 ± 4.71 ab | 10.41 ± 2.88 a | 7.13 ± 0.66 a |
| Paraprevotellaceae | 5.12 ± 2.65 ab | 8.69 ± 5.05 a | 3.98 ± 1.65 b | 7.08 ± 3.68 ab | 7.96 ± 1.69 ab |
| Lactobacillaceae | 1.17 ± 1.09 b | 2.03 ± 2.36 b | 3.97 ± 3.27 b | 13.79 ± 9.69 a | 2.69 ± 1.80 b |
| Ruminococcaceae | 7.14 ± 2.02 a | 3.02 ± 0.83 b | 2.09 ± 0.96 b | 2.37 ± 0.84 b | 3.40 ± 1.03 b |
| Alcaligenaceae | 0.68 ± 0.23 b | 3.01 ± 0.62 a | 4.55 ± 2.75 ab | 2.04 ± 1.33 ab | 1.87 ± 1.07 ab |
| Prevotellaceae | 1.01 ± 0.49 b | 3.51 ± 3.46 a | 2.44 ± 2.14 ab | 2.06 ± 2.10 ab | 1.24 ± 0.64 ab |
| Porphyromonadaceae | 0.67 ± 0.21 b | 1.29 ± 0.37 ab | 1.84 ± 0.84 ab | 1.76 ± 0.73 ab | 1.67 ± 0.38 a |
| Rikenellaceae | 1.04 ± 0.20 a | 0.88 ± 0.22 ab | 0.54 ± 0.11 b | 1.02 ± 0.40 ab | 0.94 ± 0.27 ab |
| Desulfovibrionaceae | 1.08 ± 0.41 a | 0.61 ± 0.28 ab | 0.50 ± 0.39 b | 0.60 ± 0.50 ab | 0.77 ± 0.39 ab |
| Bifidobacteriaceae | 0.53 ± 0.76 ab | 1.26 ± 0.89 ab | 1.22 ± 1.25 ac | 0.07 ± 0.07 b | 0.22 ± 0.07 b |
| Genus | |||||
| unclassified | 59.56 ± 6.53 ab | 58.68 ± 7.26 ab | 43.57 ± 11.57 ab | 47.87 ± 5.45 b | 58.80 ± 4.25 a |
| Bacteroides | 2.22 ± 0.77 b | 5.97 ± 2.50 ab | 8.24 ± 4.71 ab | 10.41 ± 2.88 a | 7.13 ± 0.66 a |
| Lactobacillus | 1.17 ± 1.09 b | 2.03 ± 2.36 b | 3.97 ± 3.27 b | 13.79 ± 9.68 a | 2.69 ± 1.80 b |
| Sutterella | 0.67 ± 0.23 b | 3.01 ± 0.62 a | 4.55 ± 2.75 ab | 2.04 ± 1.33 ab | 1.87 ± 1.07 ab |
| Parabacteroides | 0.67 ± 0.21 b | 1.29 ± 0.37 ab | 1.84 ± 0.84 ab | 1.76 ± 0.73 ab | 1.67 ± 0.38 a |
| Ruminococcaceae _Ruminococcus | 1.95 ± 0.77 a | 0.98 ± 0.24 bc | 0.60 ± 0.35 c | 0.73 ± 0.26 bc | 1.17 ± 0.42 b |
| Coprococcus | 1.23 ± 0.59 a | 1.11 ± 0.44 a | 0.33 ± 0.15 b | 0.69 ± 0.69 ab | 0.70 ± 0.20 ab |
| Bifidobacterium | 0.53 ± 0.76 ab | 1.26 ± 0.89 a | 0.66 ± 0.70 ab | 0.07 ± 0.07 b | 0.22 ± 0.07 b |
| AF12 | 0.28 ± 0.10 ab | 0.23 ± 0.07 a | 0.10 ± 0.03 b | 0.19 ± 0.03 a | 0.21 ± 0.04 a |
| Other | 2.13 ± 1.40 a | 0.75 ± 0.21 b | 1.36 ± 1.78 ab | 1.06 ± 0.62 ab | 0.70 ± 0.19 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.-H.; Wang, M.; Yuan, Y.; Yan, J.-K.; Peng, Y.; Xu, G.; Weng, X. Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model. Nutrients 2023, 15, 4206. https://doi.org/10.3390/nu15194206
Mao Y-H, Wang M, Yuan Y, Yan J-K, Peng Y, Xu G, Weng X. Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model. Nutrients. 2023; 15(19):4206. https://doi.org/10.3390/nu15194206
Chicago/Turabian StyleMao, Yu-Heng, Minghan Wang, Yu Yuan, Jing-Kun Yan, Yanqun Peng, Guoqin Xu, and Xiquan Weng. 2023. "Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model" Nutrients 15, no. 19: 4206. https://doi.org/10.3390/nu15194206
APA StyleMao, Y.-H., Wang, M., Yuan, Y., Yan, J.-K., Peng, Y., Xu, G., & Weng, X. (2023). Konjac Glucomannan Counteracted the Side Effects of Excessive Exercise on Gut Microbiome, Endurance, and Strength in an Overtraining Mice Model. Nutrients, 15(19), 4206. https://doi.org/10.3390/nu15194206






