Role of Malnutrition in Atrial Fibrillation: A Prospective Study including Individuals ≥ 75 Years of Age
Abstract
1. Introduction
2. Materials and Methods
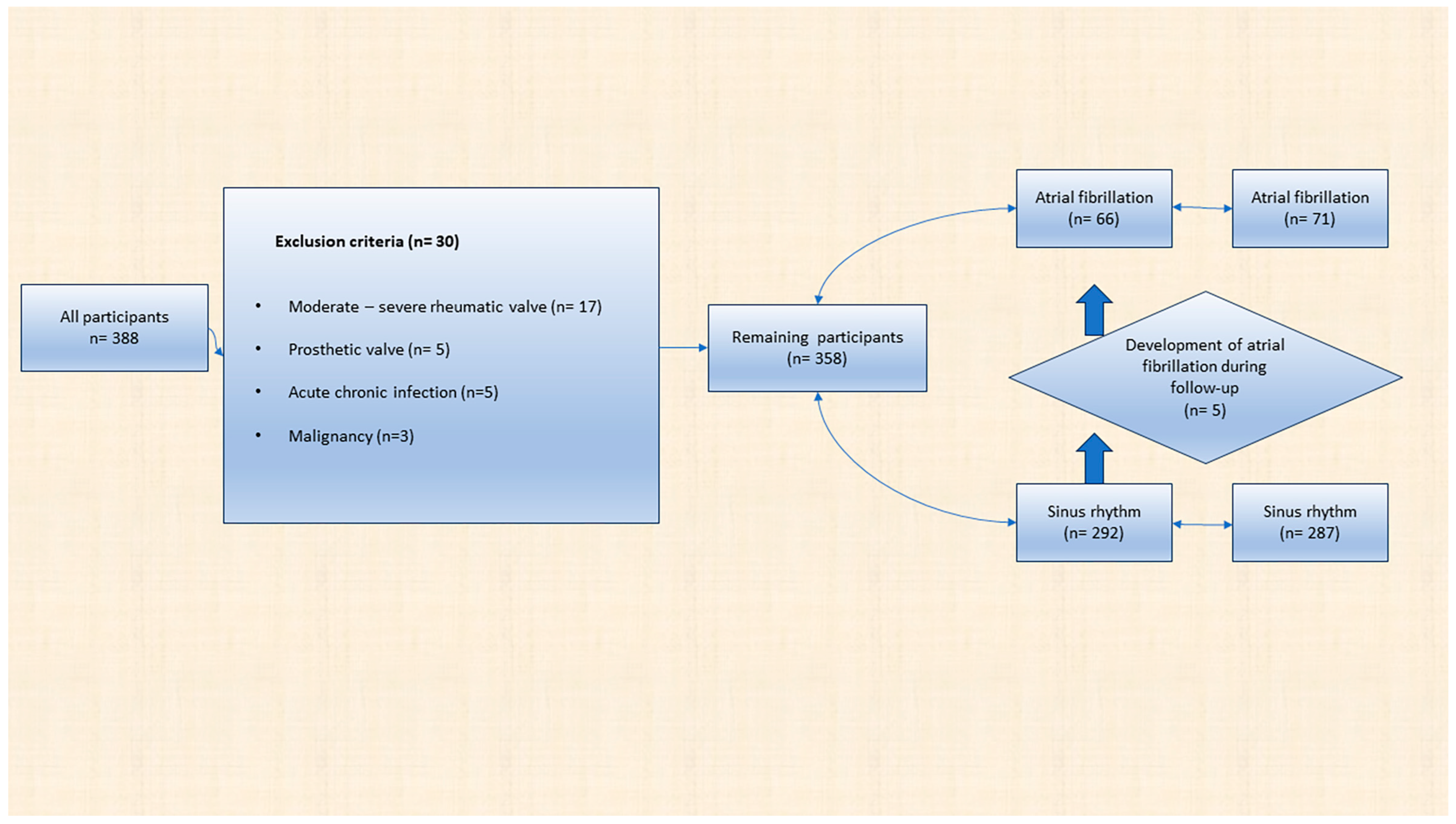
2.1. Study Design
Exclusion Criteria
2.2. Atrial Fibrillation Definition
2.3. Echocardiography
2.4. Controlling Nutritional Status
2.5. The Prognostic Nutritional Index
2.6. The Geriatric Nutrition Risk Index
2.7. Barthel Index
2.8. FRAIL Scale
2.9. Pfeiffer Questionnaire
2.10. Sarcopenia
2.11. Mini-Mental State Examination Score
2.12. Global Leadership Initiative on Malnutrition (GLIM)
2.13. Statistical Analyze
3. Results
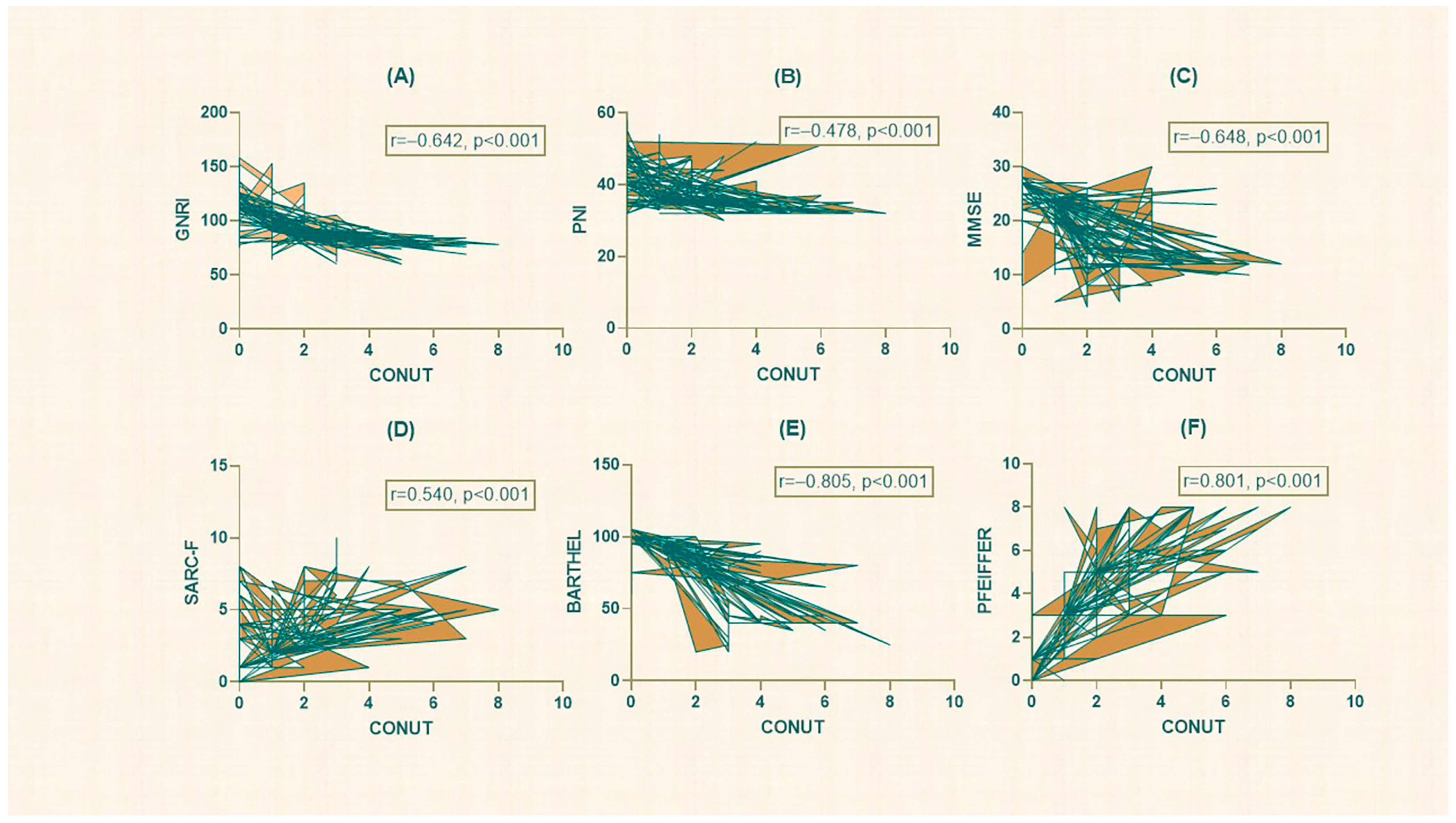
3.1. Evaluation of All Participants
3.2. Atrial Fibrillation and Sinus Rhythm Group
3.3. The Presence of GLIM Malnutrition and High CONUT Score
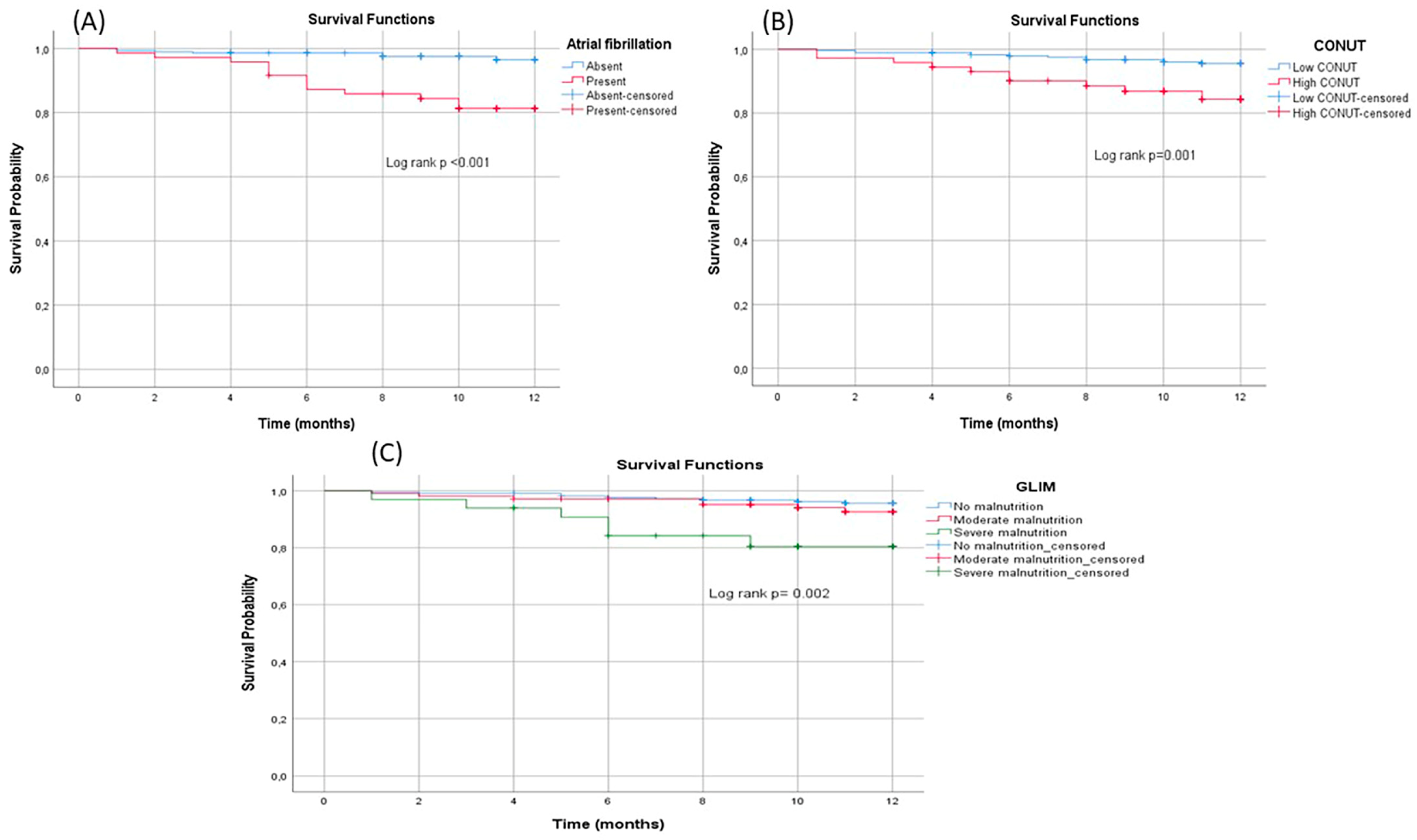
3.4. 1-Year Survival Analysis
3.5. Factors Affecting Atrial Fibrillation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bencivenga, L.; Komici, K.; Nocella, P.; Grieco, F.V.; Spezzano, A.; Puzone, B.; Cannavo, A.; Cittadini, A.; Corbi, G.; Ferrara, N.; et al. Atrial fibrillation in the elderly: A risk factor beyond stroke. Ageing Res. Rev. 2020, 61, 101092. [Google Scholar] [CrossRef] [PubMed]
- Dun, W.; Boyden, P.A. Aged atria: Electrical remodeling conducive to atrial fibrillation. J. Interv. Card. Electrophysiol. 2009, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, J.; van der Kuip, D.A.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.; Stijnen, T.; Lip, G.Y.; Witteman, J.C. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Singleton, M.J.; Imtiaz-Ahmad, M.; Kamel, H.; O’Neal, W.T.; Judd, S.E.; Howard, V.J.; Howard, G.; Soliman, E.Z.; Bhave, P.D. Association of Atrial Fibrillation Without Cardiovascular Comorbidities and Stroke Risk: From the REGARDS Study. J. Am. Heart Assoc. 2020, 9, e016380. [Google Scholar] [CrossRef]
- Castellano, J.M.; Chinitz, J.; Willner, J.; Fuster, V. Mechanisms of Stroke in Atrial Fibrillation. Card. Electrophysiol. Clin. 2014, 6, 5–15. [Google Scholar] [CrossRef]
- Aronow, W.S.; Banach, M. Atrial Fibrillation: The New Epidemic of the Ageing World. J. Atr. Fibrillation 2009, 1, 154. [Google Scholar] [CrossRef]
- Zikhathile, T.; Atagana, H. Challenges Facing Home-Based Caregivers in the Management of Health Care Risk Waste. Int. J. Environ. Res. Public Health 2018, 15, 2700. [Google Scholar] [CrossRef]
- Giacomello, E.; Toniolo, L. Nutrition, Diet and Healthy Aging. Nutrients 2021, 14, 190. [Google Scholar] [CrossRef]
- van Bokhorst-de van der Schueren, M.A.; Lonterman-Monasch, S.; de Vries, O.J.; Danner, S.A.; Kramer, M.H.; Muller, M. Prevalence and determinants for malnutrition in geriatric outpatients. Clin. Nutr. 2013, 32, 1007–1011. [Google Scholar] [CrossRef]
- Kirkwood, T.B. A systematic look at an old problem. Nature 2008, 451, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Sergi, G.; Coin, A.; Enzi, G.; Volpato, S.; Inelmen, E.M.; Buttarello, M.; Peloso, M.; Mulone, S.; Marin, S.; Bonometto, P. Role of visceral proteins in detecting malnutrition in the elderly. Eur. J. Clin. Nutr. 2006, 60, 203–209. [Google Scholar] [CrossRef]
- Cox, N.J.; Morrison, L.; Ibrahim, K.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. New horizons in appetite and the anorexia of ageing. Age Ageing 2020, 49, 526–534. [Google Scholar] [CrossRef]
- Greco, E.A.; Pietschmann, P.; Migliaccio, S. Osteoporosis and Sarcopenia Increase Frailty Syndrome in the Elderly. Front. Endocrinol. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; Guia, M.C.; Vallecoccia, M.S.; Suarez, D.; Ibarz, M.; Irazabal, M.; Ferrer, R.; Artigas, A. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: A prospective, observational, multicenter cohort study. Ann. Intensive Care 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Hanon, O.; Assayag, P.; Belmin, J.; Collet, J.P.; Emeriau, J.P.; Fauchier, L.; Forette, F.; Friocourt, P.; Gentric, A.; Leclercq, C.; et al. Expert consensus of the French Society of Geriatrics and Gerontology and the French Society of Cardiology on the management of atrial fibrillation in elderly people. Arch. Cardiovasc. Dis. 2013, 106, 303–323. [Google Scholar] [CrossRef]
- Nigatu, Y.D.; Gebreyesus, S.H.; Allard, J.P.; Endris, B.S. The effect of malnutrition at admission on length of hospital stay among adult patients in developing country: A prospective cohort study. Clin. Nutr. ESPEN 2021, 41, 217–224. [Google Scholar] [CrossRef]
- Valentini, A.; Federici, M.; Cianfarani, M.A.; Tarantino, U.; Bertoli, A. Frailty and nutritional status in older people: The Mini Nutritional Assessment as a screening tool for the identification of frail subjects. Clin. Interv. Aging 2018, 13, 1237–1244. [Google Scholar] [CrossRef]
- Bolayir, B.; Arik, G.; Yeşil, Y.; Kuyumcu, M.E.; Varan, H.D.; Kara, Ö.; Güngör, A.E.; Yavuz, B.B.; Cankurtaran, M.; Halil, M.G. Validation of Nutritional Risk Screening-2002 in a Hospitalized Adult Population. Nutr. Clin. Pract. 2019, 34, 297–303. [Google Scholar] [CrossRef]
- Bouillanne, O.; Morineau, G.; Dupont, C.; Coulombel, I.; Vincent, J.P.; Nicolis, I.; Benazeth, S.; Cynober, L.; Aussel, C. Geriatric Nutritional Risk Index: A new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005, 82, 777–783. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.F.; Wong, K.; Clark, A.L. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure: A Comparison with Body Mass Index. JACC Heart Fail. 2018, 6, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Watanabe, T.; Otaki, Y.; Watanabe, K.; Toshima, T.; Sugai, T.; Takahashi, T.; Kinoshita, D.; Tamura, H.; Nishiyama, S.; et al. Impact of Objective Malnutrition Status on the Clinical Outcomes in Patients With Peripheral Artery Disease Following Endovascular Therapy. Circ. J. 2018, 82, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Flachskampf, F.; Lancellotti, P.; Badano, L.; Aguilar, R.; Monaghan, M.; Zamorano, J.; Nihoyannopoulos, P. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur. J. Echocardiogr. 2008, 9, 438–448. [Google Scholar] [CrossRef]
- Akkuzu, M.Z.; Altıntaş, E.; Yaraş, S.; Sezgin, O.; Ateş, F.; Üçbilek, E.; Özdoğan, O. Controlling Nutritional Status (CONUT) Score and Prognostic Nutritional Index (PNI) Are Good Candidates for Prognostic Markers for Acute Pancreatitis. Medicina 2023, 59, 70. [Google Scholar] [CrossRef]
- Basta, G.; Chatzianagnostou, K.; Paradossi, U.; Botto, N.; Del Turco, S.; Taddei, A.; Berti, S.; Mazzone, A. The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int. J. Cardiol. 2016, 221, 987–992. [Google Scholar] [CrossRef]
- Sasaki, M.; Miyoshi, N.; Fujino, S.; Ogino, T.; Takahashi, H.; Uemura, M.; Matsuda, C.; Yamamoto, H.; Mizushima, T.; Mori, M.; et al. The Geriatric Nutritional Risk Index predicts postoperative complications and prognosis in elderly patients with colorectal cancer after curative surgery. Sci. Rep. 2020, 10, 10744. [Google Scholar] [CrossRef]
- Katano, S.; Yano, T.; Ohori, K.; Kouzu, H.; Nagaoka, R.; Honma, S.; Shimomura, K.; Inoue, T.; Takamura, Y.; Ishigo, T.; et al. Barthel Index Score Predicts Mortality in Elderly Heart Failure—A Goal of Comprehensive Cardiac Rehabilitation. Circ. J. 2021, 86, 70–78. [Google Scholar] [CrossRef]
- Seccia, R.; Boresta, M.; Fusco, F.; Tronci, E.; Di Gemma, E.; Palagi, L.; Mangone, M.; Agostini, F.; Bernetti, A.; Santilli, V.; et al. Data of patients undergoing rehabilitation programs. Data Brief 2020, 30, 105419. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, E.M.S.; Pereira, A.G.; Mori, V.; da Silva Neves, R.; Vieira, N.M.; Silva, M.Z.C.; Seki, M.M.; Rodrigues, H.C.N.; Costa, N.A.; Ponce, D.; et al. Comparison between FRAIL Scale and Clinical Frailty Scale in predicting hospitalization in hemodialysis patients. J. Nephrol. 2023, 36, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Kojaie-Bidgoli, A.; Fadayevatan, R.; Sharifi, F.; Alizadeh-Khoei, M.; Vahabi, Z.; Aminalroaya, R. Applicability of SPMSQ in illiterate outpatients in clinics: The validity and reliability of the Short Portable Mental Status Questionnaire. Appl. Neuropsychol. Adult 2022, 29, 591–597. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Takeuchi, T.; Goto, M.; Ogura, T.; Nakamura, S.; Kakimoto, K.; Miyazaki, T.; Nishiguchi, S.; et al. Screening Tools for Sarcopenia. Vivo 2021, 35, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Qian, M.; Liu, Y.; Graham, S.; Mann, D.L.; Nakanishi, K.; Teerlink, J.R.; Lip, G.Y.H.; Freudenberger, R.S.; Sacco, R.L.; et al. Cognitive Decline over Time in Patients with Systolic Heart Failure: Insights from WARCEF. JACC Heart Fail. 2019, 7, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.L.; Cederholm, T.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; de Baptista, G.A.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM Criteria for the Diagnosis of Malnutrition: A Consensus Report From the Global Clinical Nutrition Community. J. Parenter. Enter. Nutr. 2019, 43, 32–40. [Google Scholar] [CrossRef]
- Pang, H.; Zhu, X.; Cheang, I.; Zhang, H.; Zhou, Y.; Liao, S.; Li, X. CHA2DS2-VASc score for in-hospital recurrence risk stratification in patients with myocardial infarction. Front. Cardiovasc. Med. 2022, 9, 925932. [Google Scholar] [CrossRef]
- Benitez-Lugo, M.L.; Suárez-Serrano, C.; Galvao-Carmona, A.; Vazquez-Marrufo, M.; Chamorro-Moriana, G. Effectiveness of feedback-based technology on physical and cognitive abilities in the elderly. Front. Aging Neurosci. 2022, 14, 1050518. [Google Scholar] [CrossRef]
- Rasheed, S.; Woods, R.T. Malnutrition and quality of life in older people: A systematic review and meta-analysis. Ageing Res. Rev. 2013, 12, 561–566. [Google Scholar] [CrossRef]
- Covinsky, K.E. Malnutrition and bad outcomes. J. Gen. Intern. Med. 2002, 17, 956–957. [Google Scholar] [CrossRef][Green Version]
- Harris, D.; Haboubi, N. Malnutrition screening in the elderly population. J. R. Soc. Med. 2005, 98, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Milne, A.C.; Avenell, A.; Potter, J. Meta-analysis: Protein and energy supplementation in older people. Ann. Intern. Med. 2006, 144, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.M.; Tang, W.H.; Woo, J. Predictors of in-hospital mortality of older patients admitted for community-acquired pneumonia. Age Ageing 2011, 40, 736–741. [Google Scholar] [CrossRef]
- Yeo, H.J.; Byun, K.S.; Han, J.; Kim, J.H.; Lee, S.E.; Yoon, S.H.; Jeon, D.; Kim, Y.S.; Cho, W.H. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: A propensity score matched analysis. Korean J. Intern. Med. 2019, 34, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, H.; Lin, Z.; Li, X.; Kong, X.; Sun, G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail. Rev. 2016, 21, 549–565. [Google Scholar] [CrossRef]
- Yin, L.; Lin, X.; Li, N.; Zhang, M.; He, X.; Liu, J.; Kang, J.; Chen, X.; Wang, C.; Wang, X.; et al. Evaluation of the Global Leadership Initiative on Malnutrition Criteria Using Different Muscle Mass Indices for Diagnosing Malnutrition and Predicting Survival in Lung Cancer Patients. J. Parenter. Enter. Nutr. 2021, 45, 607–617. [Google Scholar] [CrossRef]
- Einarsson, S.; Laurell, G.; Tiblom Ehrsson, Y. Mapping the frequency of malnutrition in patients with head and neck cancer using the GLIM Criteria for the Diagnosis of Malnutrition. Clin. Nutr. ESPEN 2020, 37, 100–106. [Google Scholar] [CrossRef]
- Sun, X.; Luo, L.; Zhao, X.; Ye, P. Controlling Nutritional Status (CONUT) score as a predictor of all-cause mortality in elderly hypertensive patients: A prospective follow-up study. BMJ Open 2017, 7, e015649. [Google Scholar] [CrossRef]
- Cheng, N.; Dang, A.; Lv, N.; He, Y.; Wang, X. Malnutrition status in patients of very advanced age with nonvalvular atrial fibrillation and its impact on clinical outcomes. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1101–1109. [Google Scholar] [CrossRef]
- Ogawa, M.; Izawa, K.P.; Satomi-Kobayashi, S.; Tsuboi, Y.; Komaki, K.; Gotake, Y.; Yoshida, N.; Wakida, K.; Uchida, J.; Sakai, Y.; et al. Effects of postoperative dietary intake on functional recovery of patients undergoing cardiac surgery. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 90–96. [Google Scholar] [CrossRef]
- Belardinelli, R.; Muçaj, A.; Lacalaprice, F.; Solenghi, M.; Seddaiu, G.; Principi, F.; Tiano, L.; Littarru, G.P. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006, 27, 2675–2681. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Melita, H.; Mikhailidis, D.P.; Manolis, A.S. Low serum albumin: A neglected predictor in patients with cardiovascular disease. Eur. J. Intern. Med. 2022, 102, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Cumming, R.G.; Hilmer, S.N. The Impact of Frailty on Mortality, Length of Stay and Re-hospitalisation in Older Patients with Atrial Fibrillation. Heart Lung Circ. 2016, 25, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Agra Bermejo, R.M.; González Ferreiro, R.; Varela Román, A.; Gómez Otero, I.; Kreidieh, O.; Conde Sabarís, P.; Rodríguez-Mañero, M.; Moure González, M.; Seoane Blanco, A.; Virgós Lamela, A.; et al. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int. J. Cardiol. 2017, 230, 108–114. [Google Scholar] [CrossRef]
- Takele, Y.; Adem, E.; Getahun, M.; Tajebe, F.; Kiflie, A.; Hailu, A.; Raynes, J.; Mengesha, B.; Ayele, T.A.; Shkedy, Z.; et al. Malnutrition in Healthy Individuals Results in Increased Mixed Cytokine Profiles, Altered Neutrophil Subsets and Function. PLoS ONE 2016, 11, e0157919. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Chen, S.; Ying, M.; Chen, G.; Liu, L.; Lun, Z.; Li, H.; Huang, H.; Li, Q.; et al. Malnutrition affects cholesterol paradox in coronary artery disease: A 41,229 Chinese cohort study. Lipids Health Dis. 2021, 20, 36. [Google Scholar] [CrossRef]
| Score | Serum Albumin (g/dL) | Total Lymphocyte Rate | Total Cholesterol (mg/dL) |
|---|---|---|---|
| 0 | ≥3.5 | 1600 | ≥180 |
| 1 | 1200−1599 | 140−179 | |
| 2 | 3.0−3.4 | 800−199 | 100−139 |
| 3 | <800 | <100 | |
| 4 | 2.5–2.9 | ||
| 6 | <2.5 |
| Presence of AF N = 71 | Sinus Rhythm N = 287 | p-Value | |
|---|---|---|---|
| Age, year | 83.05 ± 5.89 | 82.63 ± 5.83 | 0.583 |
| Male, n (%) | 45 (63.4) | 142 (49.5) | 0.036 |
| Current smoker, n (%) | 9 (12.7) | 20 (7.0) | 0.115 |
| BMI, kg/m² | 24.36 ± 3.38 | 24.25 ± 3.21 | 0.806 |
| GFR, mL/min/1.73 m2 | 67.49 ± 15.59 | 66.74 ± 16.75 | 0.733 |
| Hypertension, n (%) | 7 (9.9) | 47 (16.4) | 0.169 |
| Diabetes mellitus, n (%) | 3 (4.2) | 25 (8.7) | 0.208 |
| CAD, n (%) | 9 (12.7) | 46 (16.0) | 0.483 |
| COPD, n (%) | 11 (15.5) | 33 (11.5) | 0.359 |
| Stroke, n (%) | 5 (7.0) | 16 (5.6) | 0.638 |
| Heart failure, n (%) | 6 (8.5) | 16 (5.6) | 0.366 |
| NYHA classification | |||
| I | 11 (15.5) | 60 (20.9) | 0.358 |
| II | 42 (59.2) | 150 (52.3) | |
| III | 13 (18.3) | 66 (23.0) | |
| IV | 5 (7.0) | 11 (3.8) | |
| Usage drug | |||
| ACE-inhibitors, n (%) | 4 (5.6) | 21 (7.3) | 0.618 |
| B-blockers, n (%) | 18 (25.4) | 77 (26.8) | 0.801 |
| Spironolactone, n (%) | 1 (1.4) | 11 (3.8) | 0.310 |
| Ca-channel blockers, n (%) | 5 (7.0) | 31 (10.8) | 0.346 |
| Furosemide, n (%) | 5 (7.0) | 23 (8.0) | 0.785 |
| ARB, n (%) | 3 (4.2) | 26 (9.1) | 0.181 |
| Statin, n (%) | 8 (11.3) | 40 (13.9) | 0.554 |
| Digoxin, n (%) | 13 (18.3) | 7 (2.4) | <0.001 |
| Presence of AF N = 71 | Sinus Rhythm N = 287 | p-Value | |
|---|---|---|---|
| Hemoglobin, g/dL | 11.85 ± 1.37 | 11.74 ± 1.61 | 0.582 |
| Neutrophil, 103/µL | 9.23 ± 3.59 | 9.62 ± 3.45 | 0.396 |
| Lymphocyte, 103/µL | 2.55 ± 0.80 | 2.60 ± 0.90 | 0.633 |
| Platelet, 103/µL | 280.18 ± 104.29 | 279.72 ± 99.07 | 0.972 |
| Creatinine, mg/dL | 1.07 ± 0.25 | 1.12 ± 0.26 | 0.221 |
| Calcium, mg/dL | 8.51 ± 0.43 | 8.57 ± 0.45 | 0.286 |
| Magnesium, mg/dL | 1.72 ± 0.22 | 1.73 ± 0.18 | 0.801 |
| Albumin, g/dL | 3.54 ± 0.91 | 3.87 ± 0.95 | 0.010 |
| Total protein, g/dL | 6.61 ± 0.79 | 6.77 ±0.78 | 0.128 |
| CRP, mg/L | 20.43 ± 9.59 | 19.86 ± 8.99 | 0.640 |
| Total cholesterol, mg/dL | 149.71 ± 46.63 | 164.33 ± 44.59 | 0.015 |
| Triglyceride, mg/dL | 164.21 ± 69.17 | 148.25 ± 71.96 | 0.093 |
| HDL, mg/dL | 41.33 ± 11.96 | 41.95 ± 13.24 | 0.722 |
| LDL, mg/dL | 101.30 ± 38.71 | 94.72 ± 38.23 | 0.195 |
| Sodium, mEq/L | 137.84 ± 5.69 | 138.26 ± 5.93 | 0.591 |
| Potassium, mEq/L | 4.40 ± 0.84 | 4.36 ± 0.87 | 0.698 |
| AST, U/L | 28.91 ± 15.80 | 31.23 ± 16.92 | 0.295 |
| ALT, U/L | 19.50 ± 9.62 | 21.42 ± 12.06 | 0.214 |
| Uric aside, mg/dL | 4.32 ± 1.05 | 4.23 ± 1.21 | 0.587 |
| BNP, pg/mL | 410–535 | 410–400 | 0.268 |
| LVEF, % | 53.73 ± 6.10 | 55.35 ± 3.83 | 0.036 |
| LVEDD, mm | 51.11 ± 4.26 | 49.31 ± 3.93 | 0.001 |
| LVESD, mm | 33.94 ± 3.41 | 33.51 ± 3.34 | 0.337 |
| IVST, mm | 11.76 ± 1.81 | 11.48 ± 1.74 | 0.243 |
| PWT, mm | 10.60 ± 1.55 | 10.51 ± 1.48 | 0.638 |
| sPAP, mm Hg | 32.50 ± 13.61 | 30.40 ± 10.98 | 0.171 |
| Mitral e, cm/s | 7.16 ± 1.01 | 7.22 ± 0.959 | 0.649 |
| Presence of AF N = 71 | Sinus Rhythm N = 287 | p-Value | |
|---|---|---|---|
| CONUT score | 2.15 ± 1.68 | 1.39 ± 1.52 | <0.001 |
| Presence of GLIM malnutrition, n (%) | 41 (57.7) | 98 (34.1) | <0.001 |
| GNRI | 90.67 ± 14.71 | 96.58 ± 16.72 | 0.007 |
| PNI | 37.02 ± 5.51 | 38.09 ± 5.10 | 0.122 |
| Barthel index | 75.98 ± 24.90 | 87.02 ± 18.68 | <0.001 |
| FRAIL scale | 1.61 ± 1.07 | 1.09 ± 0.97 | <0.001 |
| Pfeiffer Questionnaire | 4.14 ± 2.35 | 3.18 ± 2.25 | 0.002 |
| SARC-F score | 3.56 ± 2.02 | 2.75 ±1.81 | 0.001 |
| MMSE score | 18.43 ± 6.09 | 20.70 ± 5.98 | 0.005 |
| GLIM (+) N = 139 | GLIM (−) N = 219 | p-Value | Low CONUT N = 72 | High CONUT N = 286 | p-Value | |
|---|---|---|---|---|---|---|
| Presence of AF, n (%) | 41 (29.5) | 30 (13.7) | <0.001 | 29 (40.3) | 42 (14.7) | <0.001 |
| LVEF | 55.12 ± 4.30 | 54.96 ± 4.49 | 0.737 | 55.11 ± 4.28 | 54.70 ± 4.93 | 0.489 |
| Age, year | 83.94 ± 6.55 | 81.93 ± 5.19 | 0.002 | 82.28 ± 5.38 | 84.41 ± 7.16 | 0.020 |
| Male, n (%) | 72 (51.8) | 115 (52.5) | 0.895 | 147 (51.4) | 40 (55.6) | 0.528 |
| Barthel index | 68.30 ± 22.64 | 95.31 ± 8.94 | <0.001 | 93.12 ± 9.15 | 51.87 ± 20.02 | <0.001 |
| FRAIL scale | 2.08 ± 0.81 | 0.63 ± 0.67 | <0.001 | 0.87 ± 0.78 | 2.50 ± 0.73 | <0.001 |
| Pfeiffer Questionnaire | 5.32 ± 1.94 | 2.13 ± 1.52 | <0.001 | 2.61 ± 1.73 | 6.38 ± 1.75 | <0.001 |
| SARC-F score | 4.10 ± 1.73 | 2.16 ± 1.57 | <0.001 | 2.40 ± 1.57 | 4.93 ± 1.65 | <0.001 |
| MMSE score | 15.11 ± 5.10 | 23.51 ± 4.04 | <0.001 | 21.75 ± 5.35 | 14.26 ± 4.94 | <0.001 |
| Dead N = 22 | Survivors N = 336 | p-Value | |
|---|---|---|---|
| GLIM, n (%) | 13 (59.1) | 126 (37.5) | 0.044 |
| CONUT score | 2.00–3.25 | 1.00–2.00 | 0.018 |
| GNRI | 86.00–18.00 | 93.00–20.00 | 0.062 |
| PNI | 35.50–4.00 | 36.00–6.00 | 0.061 |
| Barthel index | 80.00–55.00 | 95.00–20.00 | 0.002 |
| FRAIL scale | 2.00–1.25 | 1.00–2.00 | 0.032 |
| Pfeiffer Questionnaire | 3.50–5.00 | 3.00–4.00 | 0.057 |
| SARC-F score | 3.00–3.00 | 2.00–2.00 | 0.233 |
| MMSE score | 16.50–11.25 | 23.00–10.00 | 0.012 |
| Presence of AF, n (%) | 12 (54.5) | 59 (17.6) | <0.001 |
| LVEF | 51.50–7.25 | 55.00–6.00 | 0.001 |
| B | OR | p | 95%Cl | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| GFR | 0.015 | 1.015 | 0.089 | 0.998 | 1.033 |
| LVEF | −0.107 | 0.899 | 0.002 | 0.842 | 0.960 |
| Presence of GLIM malnutrition | 1.036 | 2.819 | <0.001 | 1.613 | 4.927 |
| Diabetes Mellitus | −0.843 | 0.430 | 0.324 | 0.080 | 2.301 |
| Hypertension | −0.455 | 0.634 | 0.424 | 0.208 | 1.938 |
| COPD | 0.473 | 1.605 | 0.241 | 0.728 | 3.540 |
| Albumin | −0.294 | 0.745 | 0.061 | 0.548 | 1.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göçer, K.; Öztürk, B. Role of Malnutrition in Atrial Fibrillation: A Prospective Study including Individuals ≥ 75 Years of Age. Nutrients 2023, 15, 4195. https://doi.org/10.3390/nu15194195
Göçer K, Öztürk B. Role of Malnutrition in Atrial Fibrillation: A Prospective Study including Individuals ≥ 75 Years of Age. Nutrients. 2023; 15(19):4195. https://doi.org/10.3390/nu15194195
Chicago/Turabian StyleGöçer, Kemal, and Bayram Öztürk. 2023. "Role of Malnutrition in Atrial Fibrillation: A Prospective Study including Individuals ≥ 75 Years of Age" Nutrients 15, no. 19: 4195. https://doi.org/10.3390/nu15194195
APA StyleGöçer, K., & Öztürk, B. (2023). Role of Malnutrition in Atrial Fibrillation: A Prospective Study including Individuals ≥ 75 Years of Age. Nutrients, 15(19), 4195. https://doi.org/10.3390/nu15194195






