Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Protocol Registration
2.2. Selection Criteria
- (1)
- Population: Studies must involve adult patients with HNC. There are no restrictions regarding the stage of the tumor at diagnosis, allowing for a more inclusive and comprehensive analysis.
- (2)
- Intervention: The intervention of interest is aimed at assessing the impact of low GNRI on patient outcomes. The included studies should have evaluated the GNRI before the initiation of any type of anticancer therapy, such as surgical interventions or radiotherapy. This factor is crucial to obtaining an unaltered baseline that is not influenced by the effects or side effects of cancer treatments.
- (3)
- Comparison: Patients with normal/high GNRI served as the control group.
- (4)
- Outcome: The primary outcome of interest is overall survival. To be included, studies should either directly report HRs along with their 95% confidence intervals (CIs) for overall survival or provide sufficient data to allow for the calculation of these statistics.
- (1)
- Nature of Publication: Letters, editorials, reviews, case studies, or conference abstracts are excluded as these might not provide the comprehensive data required for our meta-analysis.
- (2)
- Insufficient Data: Any study that failed to provide the data to determine the correlation between pretreatment GNRI and overall survival is excluded.
- (3)
- Focus on Esophageal Cancer: Our research is explicitly concentrated on HNC. As a result, any study mainly targeting esophageal cancer is not considered.
2.3. Data Extraction
2.4. Outcomes and Definition
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Study Screening and Characteristics of Studies
3.2. Outcomes
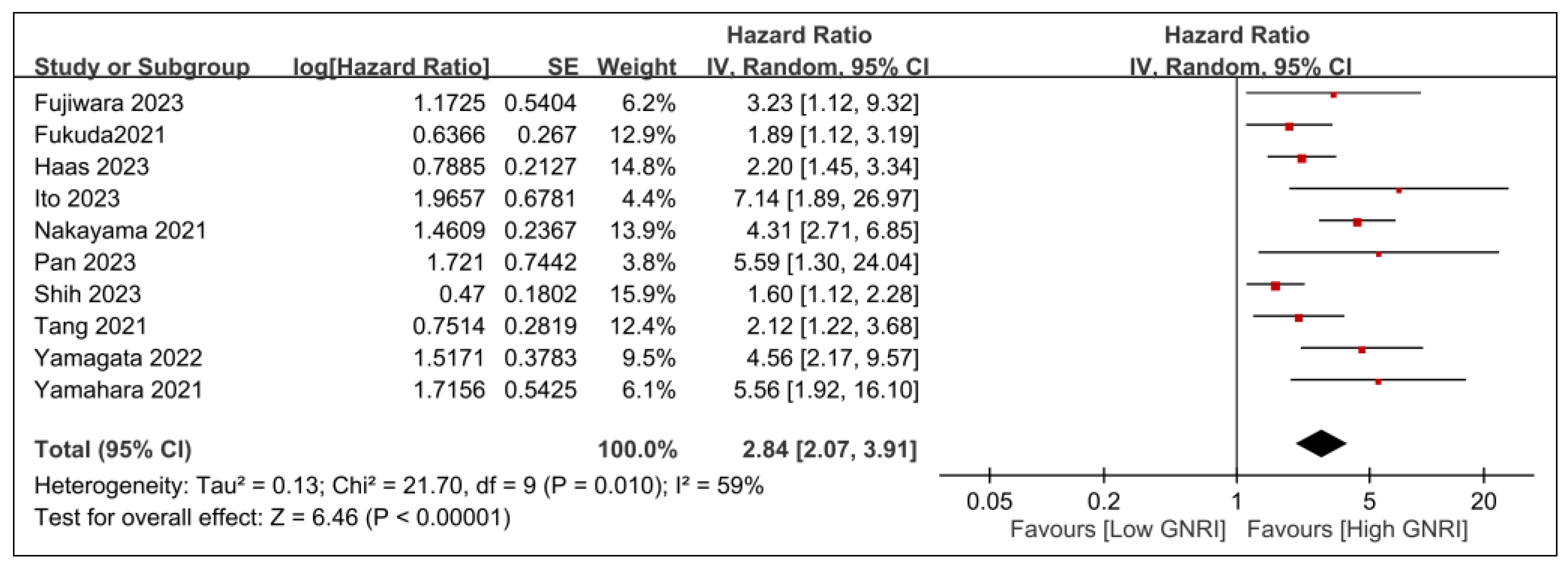
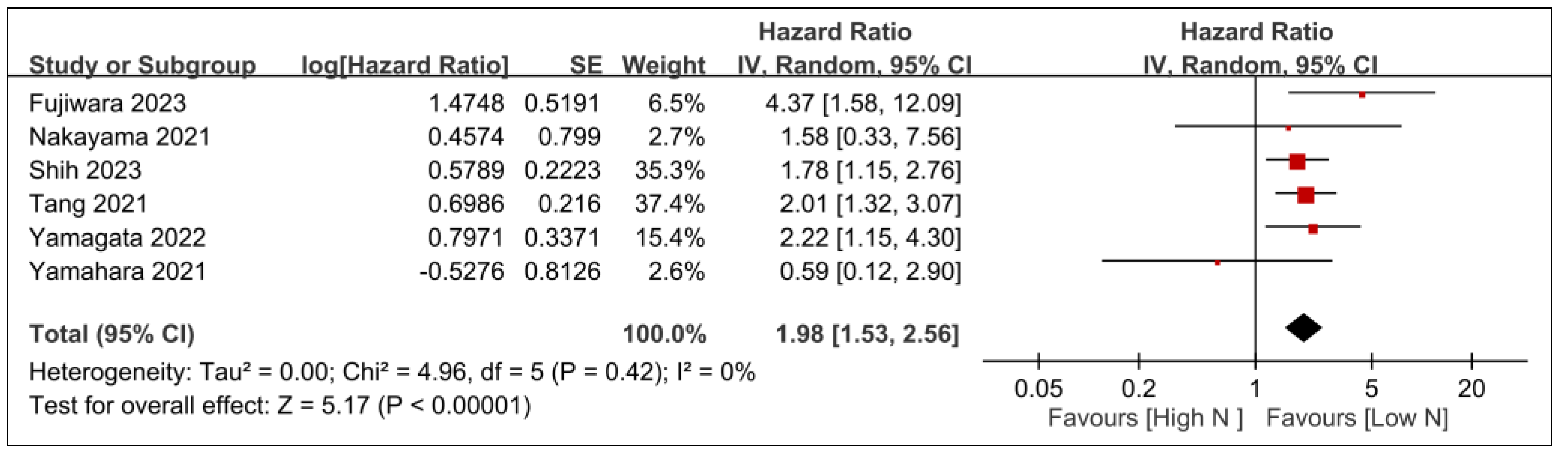
3.2.1. Primary Outcomes
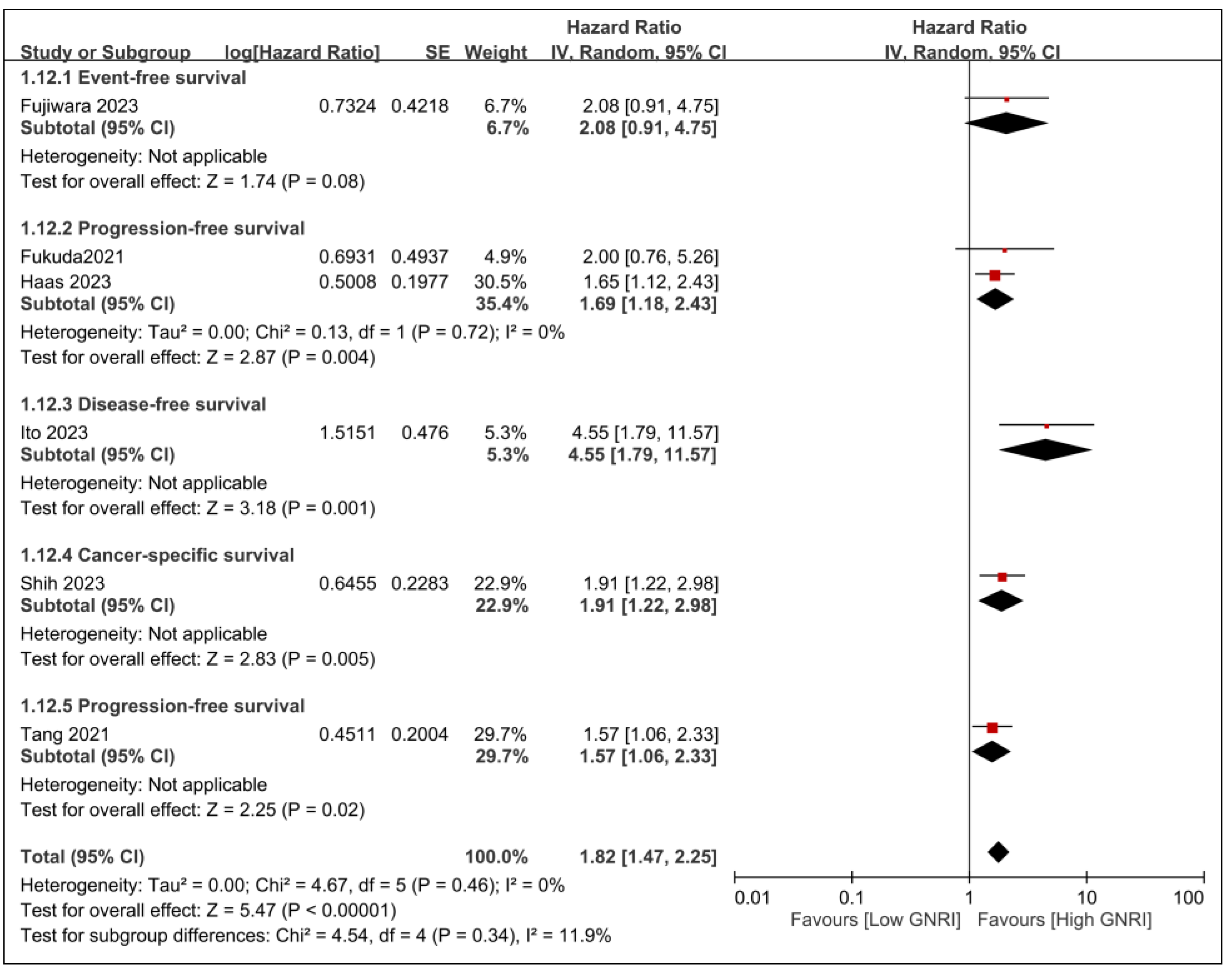
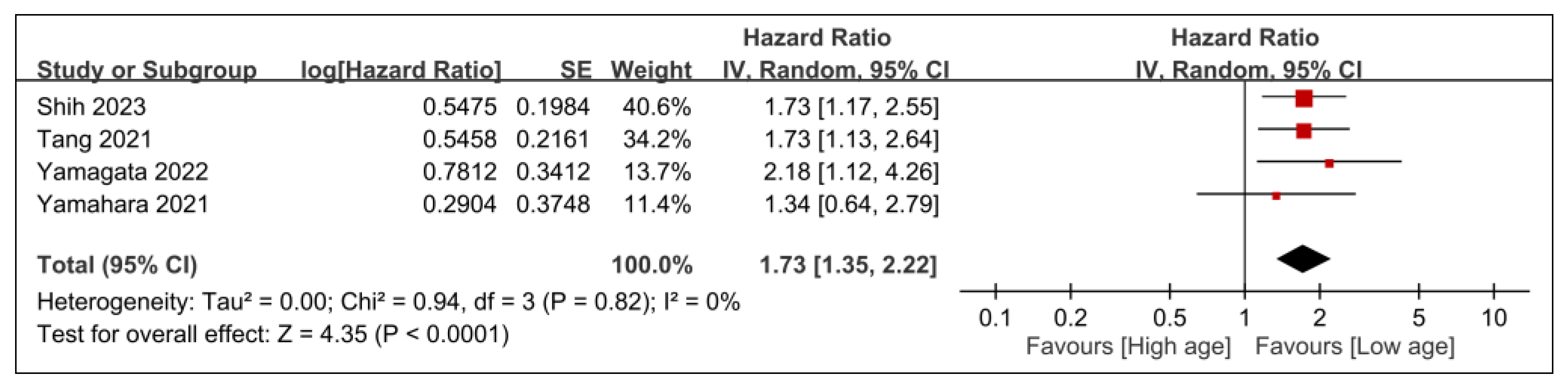
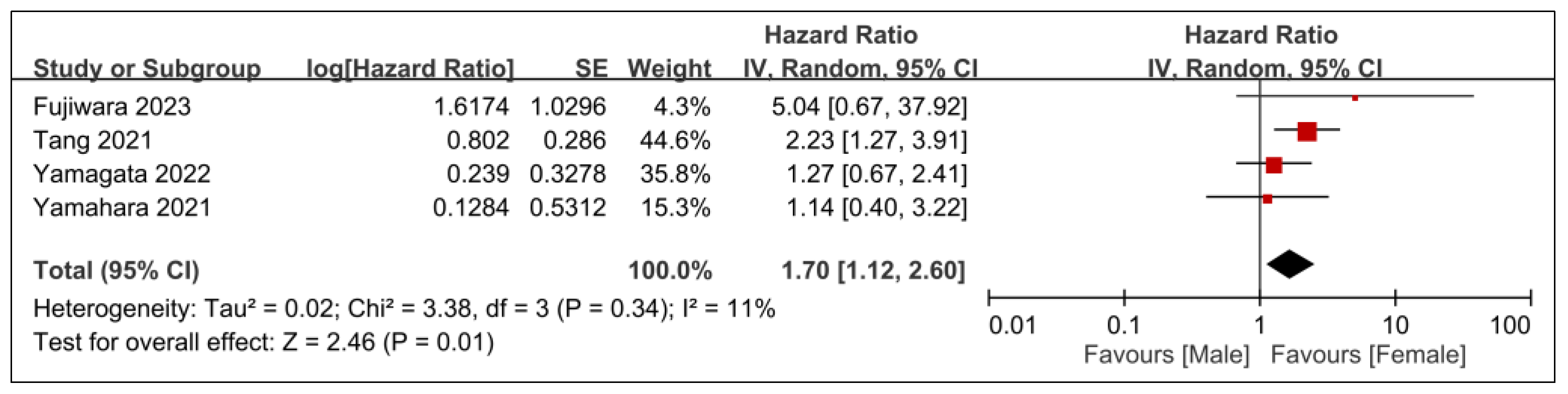
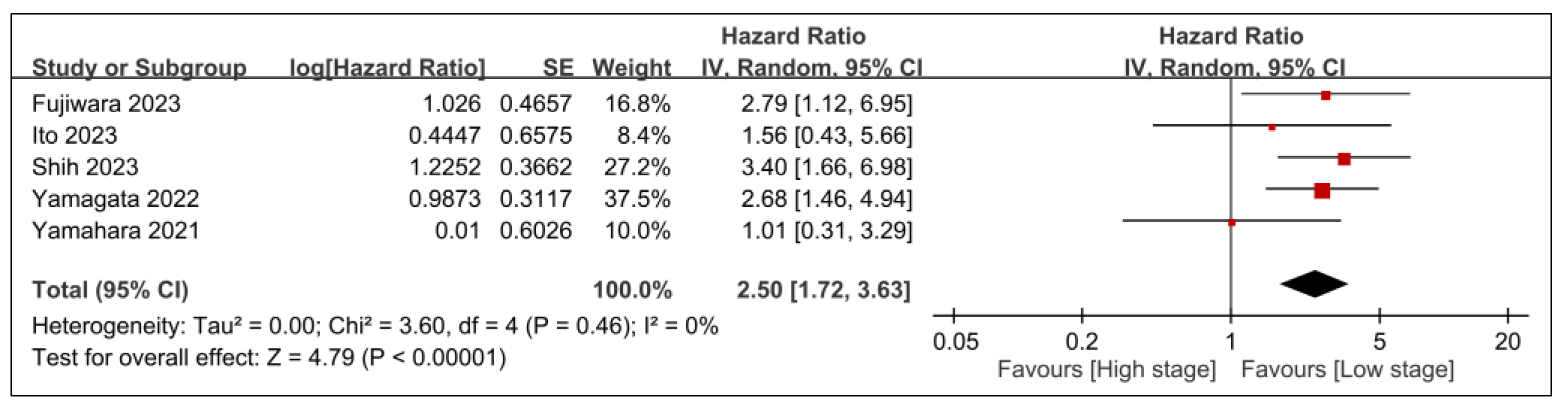
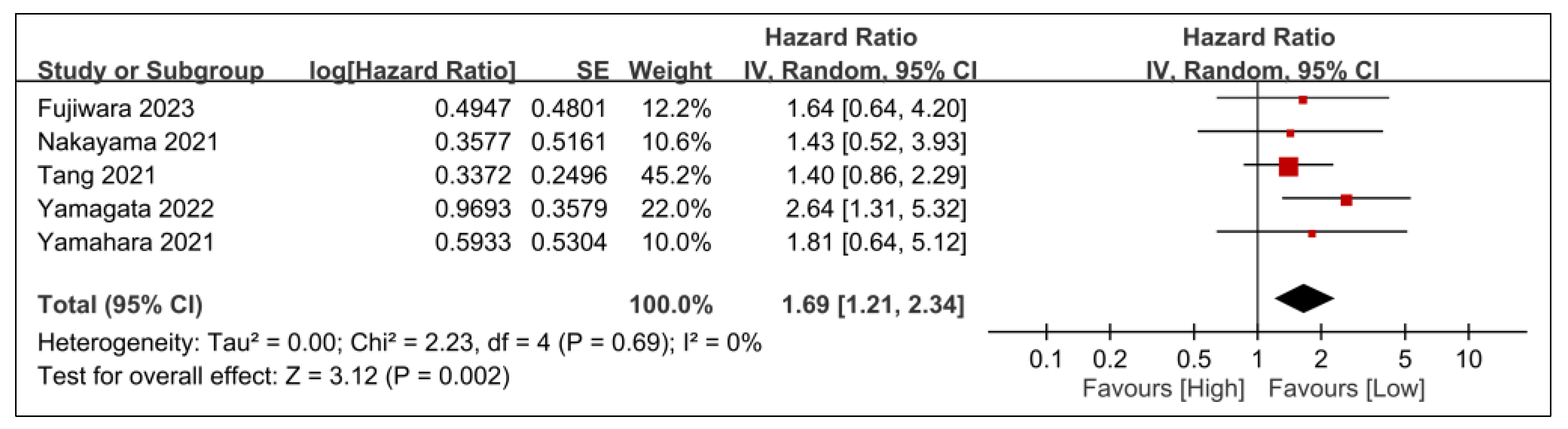
3.2.2. Secondary Outcomes
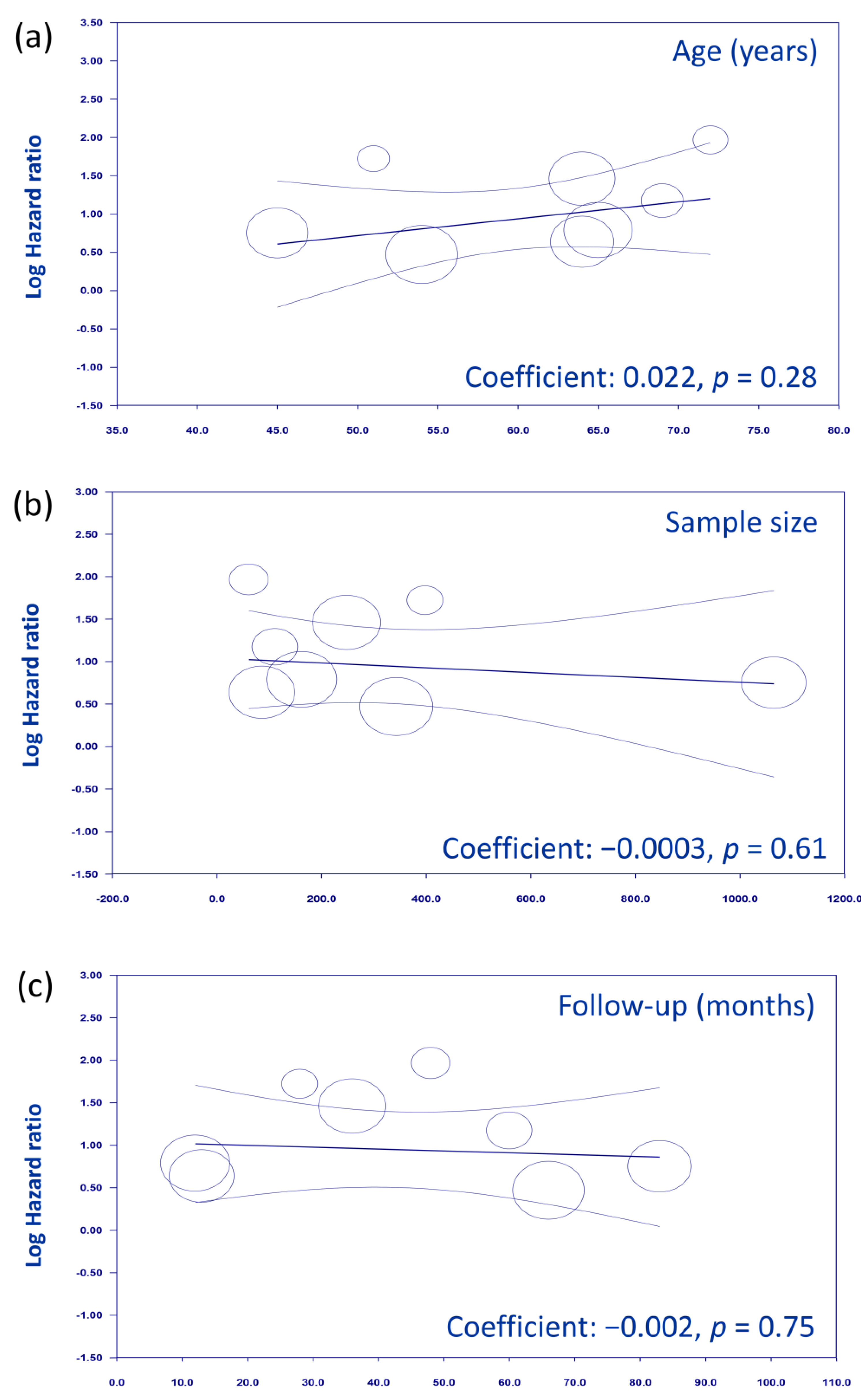
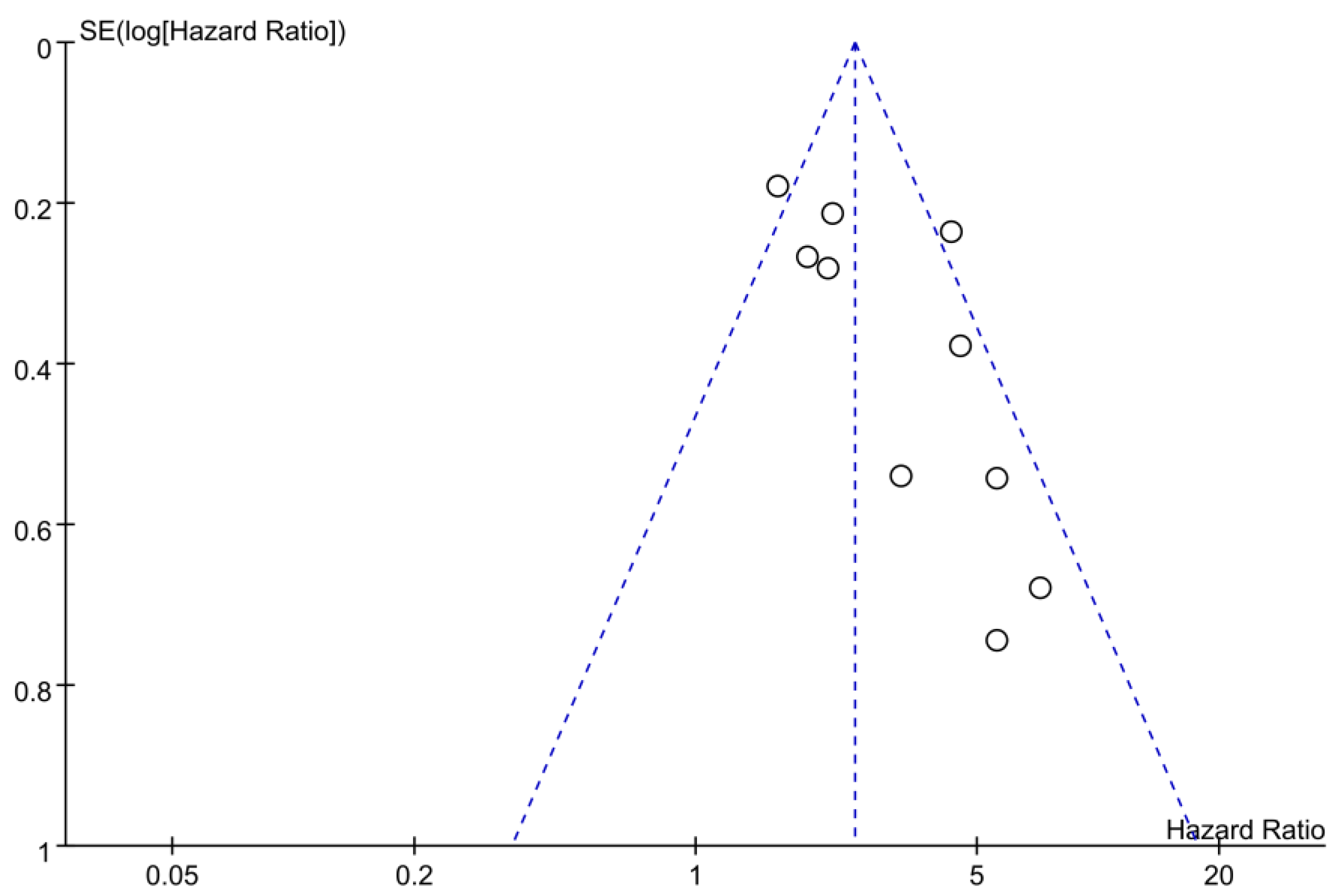
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—Systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef]
- Shield, K.D.; Ferlay, J.; Jemal, A.; Sankaranarayanan, R.; Chaturvedi, A.K.; Bray, F.; Soerjomataram, I. The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012. CA Cancer J. Clin. 2017, 67, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Bosetti, C.; Carioli, G.; Santucci, C.; Bertuccio, P.; Gallus, S.; Garavello, W.; Negri, A.; La Vecchia, C. Global trends in oral and pharyngeal cancer incidence and mortality. Int. J. Cancer 2020, 147, 1040–1049. [Google Scholar] [CrossRef]
- Imai, T.; Nakamura, K.; Morita, S.; Hasegawa, K.; Goto, T.; Katori, Y.; Asada, Y. Preoperative serum interleukin-6 level in head and neck cancer reflects systemic inflammatory response and is a predictor of postoperative prognosis. Jpn. J. Clin. Oncol. 2023, 53, 230–236. [Google Scholar] [CrossRef]
- Yu, B.; Ma, S.J.; Khan, M.; Gill, J.; Iovoli, A.; Fekrmandi, F.; Farrugia, M.K.; Wooten, K.; Gupta, V.; McSpadden, R.; et al. Association of pre-treatment lymphocyte-monocyte ratio with survival outcome in patients with head and neck cancer treated with chemoradiation. BMC Cancer 2023, 23, 572. [Google Scholar] [CrossRef]
- Silva, F.; Padín-Iruegas, M.E.; Caponio, V.C.A.; Lorenzo-Pouso, A.I.; Saavedra-Nieves, P.; Chamorro-Petronacci, C.M.; Saavedra-Nieves, J.; Pérez-Sayáns, M. Caspase 3 and Cleaved Caspase 3 Expression in Tumorogenesis and Its Correlations with Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 11937. [Google Scholar] [CrossRef]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- McMillan, D.C. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat. Rev. 2013, 39, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Sun, C.-K.; Chang, Y.-P.; Wu, J.-Y.; Huang, P.-Y.; Liu, T.-H.; Lin, C.-H.; Cheng, W.-J.; Chen, I.-W. Association of prognostic nutritional index with prognostic outcomes in patients with glioma: A meta-analysis and systematic review. Front. Oncol. 2023, 13, 1188292. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, W.; Wang, C.; Hu, Y. The Prognostic Value of Pretreatment Geriatric Nutritional Risk Index in Esophageal Cancer: A Meta-Analysis. Nutr. Cancer 2022, 74, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Russo, D.; Maisto, M.; Troiano, G.; Caponio, V.C.A.; Annunziata, M.; Laino, L. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2022, 51, 39–51. [Google Scholar] [CrossRef]
- Xu, J.; Sun, Y.; Gong, D.; Fan, Y. Predictive Value of Geriatric Nutritional Risk Index in Patients with Colorectal Cancer: A Meta-Analysis. Nutr. Cancer 2023, 75, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Ma, Y.; Guo, W.; Li, F. Prognostic Value of Geriatric Nutritional Risk Index for Patients with Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Lung 2022, 200, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Sato, Y.; Hayashi, N.; Fukuda, N.; Wang, X.; Nakano, K.; Ohmoto, A.; Urasaki, T.; Ono, M.; Tomomatsu, J.; et al. The Geriatric Nutritional Risk Index as a prognostic factor in older adult patients with locally advanced head and neck cancer receiving definitive chemoradiotherapy with tri-weekly cisplatin. J. Geriatr. Oncol. 2023, 14, 101523. [Google Scholar] [CrossRef]
- Fukuda, N.; Yunokawa, M.; Fujiwara, Y.; Wang, X.; Ohmoto, A.; Hayashi, N.; Urasaki, T.; Sato, Y.; Nakano, K.; Ono, M.; et al. Comparison of the efficacy and safety of the EXTREME regimen for treating recurrent or metastatic head and neck squamous cell carcinoma in older and younger adult patients. Cancer Rep. 2021, 4, e1322. [Google Scholar] [CrossRef]
- Haas, M.; Lein, A.; Fuereder, T.; Brkic, F.F.; Schnoell, J.; Liu, D.T.; Kadletz-Wanke, L.; Heiduschka, G.; Jank, B.J. The Geriatric Nutritional Risk Index (GNRI) as a Prognostic Biomarker for Immune Checkpoint Inhibitor Response in Recurrent and/or Metastatic Head and Neck Cancer. Nutrients 2023, 15, 880. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.-C.; Chang, Y.-J.; Chang, Y.-P.; Ho, C.-N.; Lan, K.-M.; Chen, J.-Y.; Wang, L.-K.; Huang, P.-W.; Sun, C.-K. The impact of esophageal device insertion on cuff pressure of endotracheal tube: A literature review and meta-analysis. Sci. Rep. 2022, 12, 18192. [Google Scholar] [CrossRef]
- Hung, K.-C.; Wu, J.-Y.; Illias, A.M.; Chiu, C.-C.; Chang, Y.-J.; Liao, S.-W.; Wang, K.-F.; Chen, I.-W.; Sun, C.-K. Association of a low vitamin D status with risk of post-stroke depression: A meta-analysis and systematic review. Front. Nutr. 2023, 10, 1142035. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-L.; Chuang, H.-C.; Lin, Y.-T.; Chien, C.-Y.; Yang, C.-H.; Lai, C.-C.; Su, Y.-Y.; Tsai, Y.-T.; Lu, H.; Tsai, M.-H. The prognostic utility of preoperative geriatric nutritional risk index on survival outcomes of locally advanced oral cancer. J. Formos. Med. Assoc. Taiwan Yi Zhi 2023, in press. [Google Scholar] [CrossRef]
- Pan, D.; Shen, Q.; Li, Y.; Rong, X.; Li, H.; Xu, Y.; He, B.; Zuo, X.; Deng, Z.; Tang, Y. Prognostic Value of Nutritional Assessments on Overall Survival in Head and Neck Cancer Survivors with Radiation-Induced Brain Necrosis. Nutrients 2023, 15, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Abe, A.; Hayashi, H.; Momokita, M.; Furuta, H. Prognostic impact of preoperative Geriatric Nutritional Risk Index in oral squamous cell carcinoma. Oral Dis. 2023, 29, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Fukuzawa, S.; Uchida, F.; Terada, K.; Ishibashi-Kanno, N.; Bukawa, H. Does the geriatric nutrition risk index predict the prognosis of patients with oral squamous cell carcinoma? Br. J. Oral Maxillofac. Surg. 2022, 60, 475–481. [Google Scholar] [CrossRef]
- Yamahara, K.; Mizukoshi, A.; Lee, K.; Ikegami, S. Pretherapeutic nutritional/inflammatory factors as predictors for survival of both early and advanced staged head and neck cancer patients. Auris Nasus Larynx 2021, 48, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Gosho, M.; Adachi, M.; Ii, R.; Matsumoto, S.; Miyamoto, H.; Hirose, Y.; Nishimura, B.; Tanaka, S.; Wada, T.; et al. The geriatric nutritional risk index as a prognostic factor in patients with advanced head and neck cancer. Laryngoscope 2021, 131, E151–E156. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.-N.; Qiu, H.-Z.; Sun, X.-Q.; Guo, S.-S.; Liu, L.-T.; Wen, Y.-F.; Liu, S.-L.; Xie, H.-J.; Liang, Y.-J.; Sun, X.-S.; et al. Geriatric nutritional risk index as an independent prognostic factor in locally advanced nasopharyngeal carcinoma treated using radical concurrent chemoradiotherapy: A retrospective cohort study. Ann. Transl. Med. 2021, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Chen, Y.Y.; Chen, W.C.; Hsieh, C.C. The relationship of nutritional status with anticancer immunity and its prognostic value for head and neck cancer. Mol. Carcinog. 2023, 62, 1388–1398. [Google Scholar] [CrossRef]
- Findlay, M.; White, K.; Brown, C.; Bauer, J.D. Nutritional status and skeletal muscle status in patients with head and neck cancer: Impact on outcomes. J. Cachexia Sarcopenia Muscle 2021, 12, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.; Brown, C.; De Abreu Lourenço, R.; White, K.; Bauer, J. Sarcopenia and myosteatosis in patients undergoing curative radiotherapy for head and neck cancer: Impact on survival, treatment completion, hospital admission and cost. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2020, 33, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef]
- Xiaogang, H.; Sharma, M.; Saif, I.; Ali, G.; Li, X.; Salama, E.S. The role of nutrition in harnessing the immune system: A potential approach to prevent cancer. Med. Oncol. 2022, 39, 245. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. The Potential Role of Exercise and Nutrition in Harnessing the Immune System to Improve Colorectal Cancer Survival. Gastroenterology 2018, 155, 596–600. [Google Scholar] [CrossRef]
- Arihara, Y.; Takada, K.; Murase, K.; Kawamura, K.; Kakiuchi, A.; Kurose, M.; Sasaki, T.; Ogi, K.; Yamazaki, M.; Miyazaki, A.; et al. Inflammation and malnutrition as markers of poor outcomes in head and neck cancer patients treated with nivolumab. Acta Oto-Laryngol. 2023, 143, 714–720. [Google Scholar] [CrossRef]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef] [PubMed]
- Pressoir, M.; Desné, S.; Berchery, D.; Rossignol, G.; Poiree, B.; Meslier, M.; Traversier, S.; Vittot, M.; Simon, M.; Gekiere, J.P.; et al. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Cancer 2010, 102, 966–971. [Google Scholar] [CrossRef]
- Luan, C.-W.; Tsai, Y.-T.; Yang, H.-Y.; Chen, K.-Y.; Chen, P.-H.; Chou, H.-H. Pretreatment prognostic nutritional index as a prognostic marker in head and neck cancer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 17117. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Kuo, L.-T.; Lai, C.-H.; Tsai, Y.-H.; Lee, Y.-C.; Hsu, C.-M.; Liao, C.-T.; Kang, C.-J.; Huang, E.I.; Tsai, M.-S.; et al. Low Pretreatment Albumin-to-Globulin Ratios Predict Poor Survival Outcomes in Patients with Head and Neck Cancer: A Systematic Review and Meta-analysis. J. Cancer 2023, 14, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Xu, S.; Wang, D.; Qin, G. Prognostic significance of systemic immune-inflammation index in patients with nasopharyngeal carcinoma: A meta-analysis. Syst. Rev. 2022, 11, 247. [Google Scholar] [CrossRef]
- Findlay, M.; White, K.; Stapleton, N.; Bauer, J. Is sarcopenia a predictor of prognosis for patients undergoing radiotherapy for head and neck cancer? A meta-analysis. Clin. Nutr. 2021, 40, 1711–1718. [Google Scholar] [CrossRef]
- Zhou, J.; Fang, P.; Li, X.; Luan, S.; Xiao, X.; Gu, Y.; Shang, Q.; Zhang, H.; Yang, Y.; Zeng, X.; et al. Prognostic Value of Geriatric Nutritional Risk Index in Esophageal Carcinoma: A Systematic Review and Meta-Analysis. Front Nutr. 2022, 9, 831283. [Google Scholar] [CrossRef]
- Li, L.; He, J. Prognostic Role of Geriatric Nutritional Risk Index in Patients with Pancreatic Cancer: A Meta-Analysis. Nutr. Cancer 2023, 75, 1531–1540. [Google Scholar] [CrossRef] [PubMed]

| 1 | ((“gingival” or “Lip” or “Palatal” or “Salivary Gland” or “Tongue” or “Otorhinolaryngologic” or “Ear” or “Laryngeal” or “Nose” or “Pharyngeal” or “Parathyroid” or “Squamous Cell Carcinoma of Head and Neck” or “Thyroid” or “Thyroid” or “Tracheal” or “oral” or “larynx” or “pharynx”) adj3 (Neoplasm or tumor or cancer)).mp. |
| 2 | exp “Head and Neck Neoplasms”/ |
| 3 | (“Geriatric Nutritional Risk Index” or GNRI).mp. |
| 4 | (“Overall survival” or “Prognosis” or “Mortality” or “Disease-Free Survival” or “Progression-Free Survival”).mp. |
| 5 | exp “Survival”/or exp “Mortality”/or exp “Disease-Free Survival”/or exp “Progression-Free Survival”/ |
| 6 | (1 or 2) and 3 and (4 or 5) |
| Age (Years) | Male (%) | n | Tumor Stage | Treatment | GNRI Cutoff Values | Follow-Up | Country | NOS | |
|---|---|---|---|---|---|---|---|---|---|
| Fujiwara 2023 [20] | 69 (67–71) | 79 | 111 | I–IV | CRT | 98 | 5 yrs | Japan | 7 |
| Fukuda 2021 [21] | 64 (32–77) | 87.2 | 86 | NA | Platinum + fluorouracil + cetuximab | 98 | 13.2 m | Japan | 5 |
| Haas 2023 [22] | 65 (28–85) | 71 | 162 | NA | Immune checkpoint inhibitors | 92 | 12 m | Austria | 8 |
| Ito 2023 [27] | 72.1 ± 5.4 | 70.5 | 61 | I–IV | Radical surgery | 93.7 | 48.3 m | Japan | 6 |
| Nakayama 2021 [30] | 64 (29–85) | 85.9 | 248 | III–IV | RT/surgery/CRT | 92 vs. 98 | 36 m | Japan | 5 |
| Pan 2023 [26] | 50.9 (44.5–57.0) | 73.1 | 398 | I–IV | Radiotherapy | 82 vs. 98 | 2.3 yrs | China | 8 |
| Shih 2023 [25] | 54 (30–59) | 91 | 343 | III–IV | Radical surgery | 97.8 | 66.5 m | Taiwan | 7 |
| Tang 2021 [31] | 45 (38–52) | 72.40 | 1065 | II–III | IMRT/CRT | 107.7 | 83 m | China | 7 |
| Yamagata 2022 [28] | 49.7% (>70 years) | 61.30 | 155 | I–IV | NA | 98 | 36 m | Japan | 6 |
| Yamahara 2021 [29] | 76.2% (>65 years) | 87.20 | 164 | I–IV | RT/surgery | 82 vs. 98 | 53 | Japan | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yiu, C.-Y.; Liu, C.-C.; Wu, J.-Y.; Tsai, W.-W.; Liu, P.-H.; Cheng, W.-J.; Chen, J.-Y.; Hung, K.-C. Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis. Nutrients 2023, 15, 4348. https://doi.org/10.3390/nu15204348
Yiu C-Y, Liu C-C, Wu J-Y, Tsai W-W, Liu P-H, Cheng W-J, Chen J-Y, Hung K-C. Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis. Nutrients. 2023; 15(20):4348. https://doi.org/10.3390/nu15204348
Chicago/Turabian StyleYiu, Ching-Yi, Chien-Cheng Liu, Jheng-Yan Wu, Wen-Wen Tsai, Ping-Hsin Liu, Wan-Jung Cheng, Jen-Yin Chen, and Kuo-Chuan Hung. 2023. "Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis" Nutrients 15, no. 20: 4348. https://doi.org/10.3390/nu15204348
APA StyleYiu, C.-Y., Liu, C.-C., Wu, J.-Y., Tsai, W.-W., Liu, P.-H., Cheng, W.-J., Chen, J.-Y., & Hung, K.-C. (2023). Efficacy of the Geriatric Nutritional Risk Index for Predicting Overall Survival in Patients with Head and Neck Cancer: A Meta-Analysis. Nutrients, 15(20), 4348. https://doi.org/10.3390/nu15204348






