The Effect of a High-Protein Diet Supplemented with Blackthorn Flower Extract on Polyphenol Bioavailability and Antioxidant Status in the Organs of C57BL/6 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blackthorn Flower Aqueous Extract and Whey Proteins
2.2. Animals
2.3. Experimental Design and Doses
2.4. Determination of the Bioavailability of the Blackthorn Flower Aqueous Extract Polyphenols in Mouse Organs
2.5. Markers of Oxidative Stress
2.6. Statistical Analysis
3. Results
3.1. Bioaccumulation of Polyphenols in the Small Intestine after 28 Days of Treatment with Extract P. spinosa with a Whey-Protein-Enriched Diet
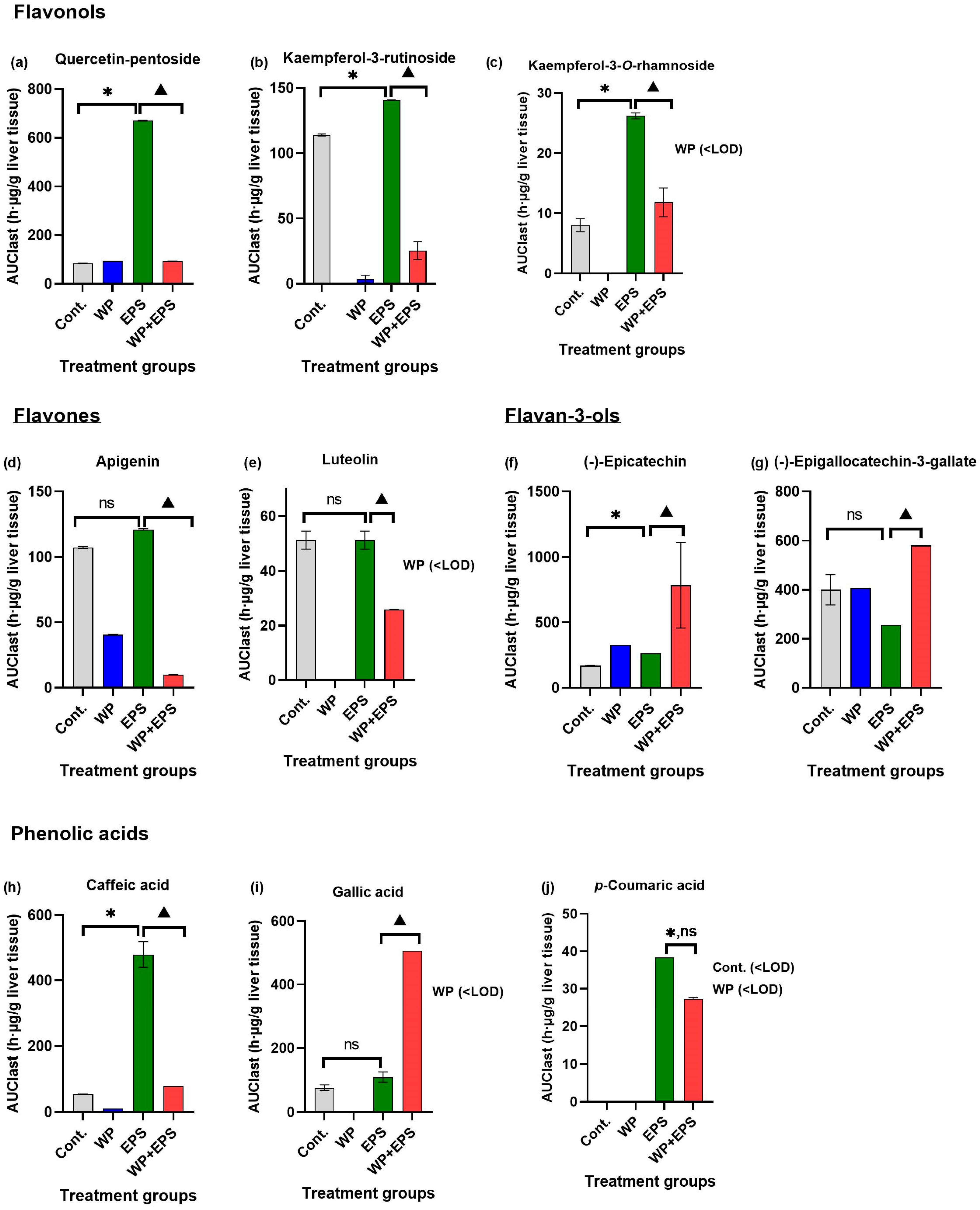
3.2. Bioaccumulation of Polyphenols in the Liver after 28 Days of Treatment with Extract P. spinosa with a Whey-Protein-Enriched Diet
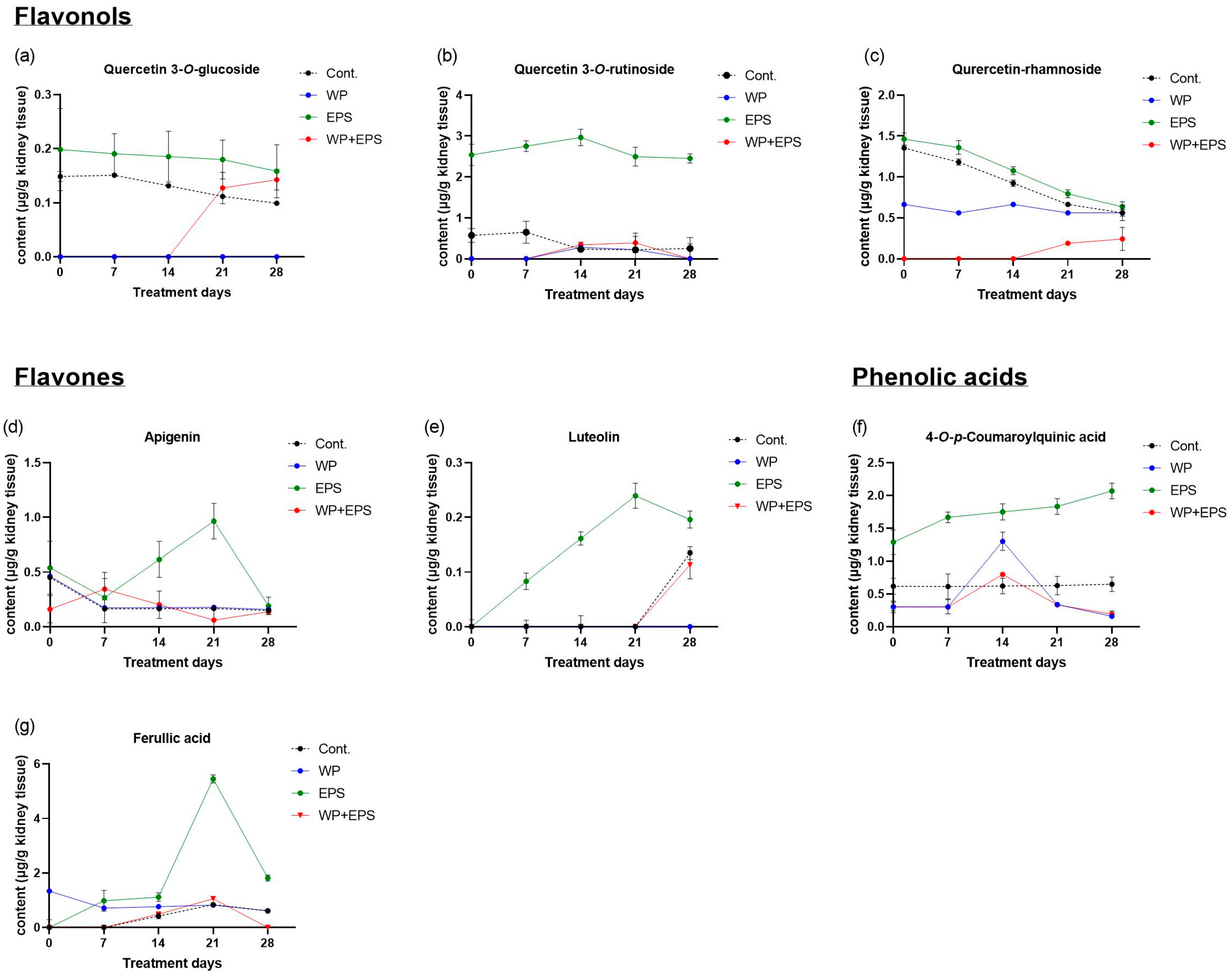
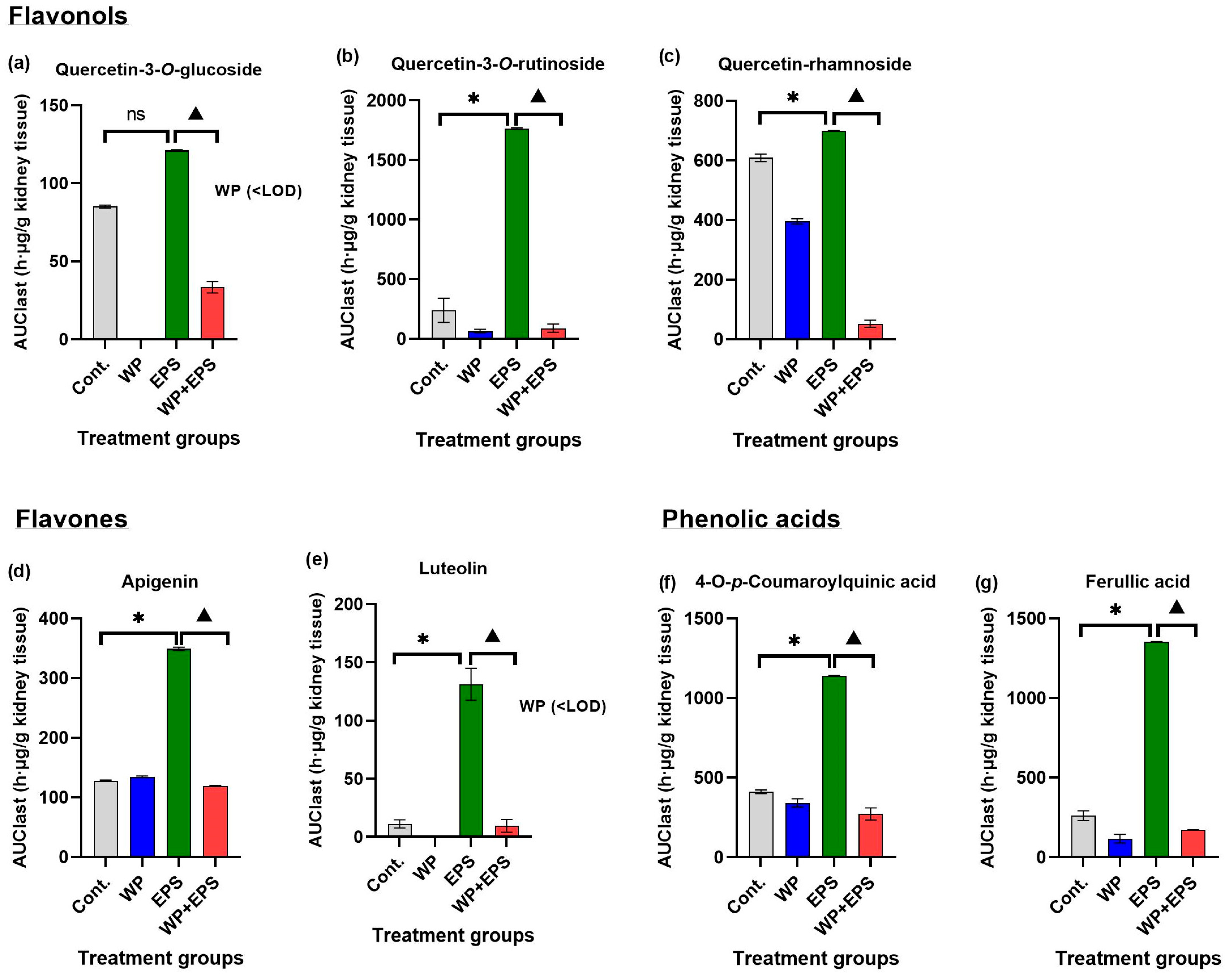
3.3. Bioaccumulation of Polyphenols in the Kidneys after 28 Days of Treatment with Extract P. spinosa with a Whey-Protein-Enriched Diet
3.4. Oxidative Stress Parameters in Small Intestine, Liver, and Kidney of Mice after 28 Days of Treatment with Extract P. spinosa with a Whey-Protein-Enriched Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of blackthorn flower polyphenols isolated by ultrasound-assisted extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Popescu, I.; Caudullo, G. Prunus spinosa in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; no. 145. [Google Scholar]
- Pinacho, R.; Cavero, R.; Astiasarán, I.; Ansorena, D.; Calvo, M. Phenolic compounds of blackthorn (Prunus spinosa L.) and influence of in vitro digestion on their antioxidant capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Alarcόn, R.; Pardo-de-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 24, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Meschini, S.; Pellegrini, E.; Condello, M.; Occhionero, G.; Delfine, S.; Condello, G.; Mastrodonato, F. Cytotoxic and apoptotic activities of Prunus spinosa Trigno ecotype extract on human cancer cells. Molecules 2017, 22, 1578. [Google Scholar] [CrossRef]
- Negrean, O.R.; Farcas, A.C.; Pop, O.L.; Socaci, S.A. Blackthorn—A Valuable Source of Phenolic Antioxidants with Potential Health Benefits. Molecules 2023, 28, 3456. [Google Scholar] [CrossRef]
- Yuksel, A.K. The Effects of Blackthorn (Prunus spinosa L.) Addition on certain quality characteristics of ice cream. J. Food Qual. 2015, 38, 413–421. [Google Scholar] [CrossRef]
- Balta, V.; Đikić, D.; Crnić, I.; Odeh, D.; Oršolić, N.; Kmetič, I.; Murati, T.; Dragović-Uzelac, V.; Landeka Jurčević, I. The effects of four-week intake of blackthorn flower extract on mice tissue antioxidant status and phenolic content. Pol. J. Food Nutr. Sci. 2020, 70, 361–375. [Google Scholar] [CrossRef]
- Đikić, D.; Balta, V.; Pedisić, S.; Zorić, Z.; Padovan, J.; Butorac, D.; Milić, A.; Jurić, D.; Landeka Jurčević, I. Polyphenol bioavailability and modulatory potential on brain antioxidative markers in C57BL/6 mouse. Period. Biol. 2022, 124, 41–54. [Google Scholar] [CrossRef]
- Calvo, M.I.; Cavero, R.Y. Medicinal plants used for cardiovascular diseases in Navarra and their validation from Officinal sources. J. Ethnopharmacol. 2014, 157, 268–273. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Owczarek, A.; Rutkowska, M.; Michel, P.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. New insights into antioxidant activity of Prunus spinosa flowers: Extract, model polyphenols and their phenolic metabolites in plasma towards multiple in vivo-relavant oxidants. Phytochem. Lett. 2019, 30, 288–295. [Google Scholar] [CrossRef]
- Coppari, S.; Colomba, M.; Fraternale, D.; Brinkmann, V.; Romeo, M.; Rocchi, M.B.L.; Di Giacomo, B.; Mari, M.; Guidi, L.; Ramakrishna, S.; et al. Antioxidant and Anti-Inflammaging Ability of Prune (Prunus Spinosa L.) Extract Result in Improved Wound Healing Efficacy. Antioxidants 2021, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-inflammatory Enzymes Inhibition and Protective Effects Against Oxidative Stress In Vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Pierluigi Spugnini, E.; Baldi, A.; Amadio, B.; Vincenzi, B.; Occhionero, G.; Delfine, S.; Mastrodonato, F.; Meschini, S. Anticancer activity of “Trigno M”, extract of Prunus spinosa drupes, against in vitro 3D and in vivo colon cancer models. Biomed. Pharmacother. 2019, 118, 109281. [Google Scholar] [CrossRef]
- Murati, T.; Miletić, M.; Kolarić, J.; Lovrić, V.; Kovačević, D.B.; Putnik, P.; Jurčević Landeka, I.; Đikić, D.; Dragović-Uzelac, V.; Kmteič, I. Toxic activity of Prunus spinosa L. flower extract in hepatocarcinoma cells. Arh. Za Hig. Rada I Toksikol. 2019, 70, 303–309. [Google Scholar] [CrossRef]
- Karakas, N.; Mehmet, E.O.; Ozturk, I.; Ayla, S.; Karadag, A.E.; Derya, Ç.P. Antioxidant Activity of blackthorn (Prunus spinosa L.) fruit extract and cytotoxic effects on various cancer cell lines. Medeni. Med. J. 2019, 34, 297–304. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure-activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of plant phenols with food macronutrients: Characterisation and nutritional-physiological consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef]
- Kardum, N.; Glibetić, M. Polyphenols and their interactions with other dietary compounds: Implications for human health. Adv. Food Nutr. 2018, 84, 103–144. [Google Scholar] [CrossRef]
- Pinarli, B.; Simge Karliga, E.; Gulay Ozkan, G.; Capanoglu, E. Interaction of phenolics with food matrix: In vitro and in vivo approaches. Mediterr. J. Nutr. Metab. 2020, 12, 63–74. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Tomas, M.; Ozdal, T.; Capanoglu, E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci. 2020, 117, 15–33. [Google Scholar] [CrossRef]
- Lovrić, V.; Putnik, P.; Kovačević, D.B.; Jukić, M.; Dragović-Uzelac, V. Effect of microwave-assisted extraction on the phenolic compounds and antioxidant capacity of blackthorn flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Narodne, N. Zakon o Zaštiti Životinja (Law on Animal Welfare); Narodne Novine: Zagreb, Croatia, 2017; p. 102/17. [Google Scholar]
- Narodne, N. Zakon o Izmjenama i Dopunama Zakona o Zaštiti Životinja (Law on Amendments to the Law on Animal Welfare); Narodne Novine: Zagreb, Croatia, 2013; p. 37/13. [Google Scholar]
- Narodne, N. Pravilnik o Zaštiti Životnja Koje se Koriste u Znanstvene Svrhe (Regulation on the Protection of Animals Used for Scientific Purposes); Narodne Novine: Zagreb, Croatia, 2013; p. 55/13. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Ganguly, S.G.; Mantha, S.; Panda, K. Simultaneous Determination of Black Tea—Derived Catechins and Theaflavnis in Tissue of Tea Consuming Animals Using Ultra13 Performance Liquid-Chromatography Tandem Mass Spectrometry. PLoS ONE 2016, 11, e0163498. [Google Scholar] [CrossRef]
- Landeka Jurčević, I.; Dora, M.; Guberović, I.; Petras, M.; Rimac-Brnčić, S.; Đikić, D. Polyphenols from Wine Lees as a Novel Functional Bioactive Compound in the Protection Against Oxidative Stress and Hyperlipidaemia. Food Technol. Biotechnol. 2017, 55, 109–116. [Google Scholar] [CrossRef]
- Lowry, D.H.; Rosebrough, N.J.; Farr, A.L. Protein measurement with the Folin–phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Flohé, L.; Ötting, F. Superoxide dismutase assays. Meth. Enzymol. 1984, 105, 93–104. [Google Scholar]
- Tietze, F. Enzyme method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- IBM SPSS Statistics; Version 17.0; SPSS Inc.: Chicago, IL, USA, 2008; Available online: https://www.ibm.com/products/spss-statistics (accessed on 13 March 2023).
- GraphPad Prism, version 9.0.0; GraphPad Software: San Diego, CA, USA, 2020.
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of In Vitro Digestion on Composition, Bioaccessibility and Antioxidant Activity of Food Polyphenols—A Non-Systematic Review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Vitali Čepo, D.; Radić, K.; Turčić, P.; Anić, D.; Komar, B.; Šalov, M. Food (Matrix) Effects on Bioaccessibility and Intestinal Permeability of Major Olive Antioxidants. Foods 2020, 9, 1831. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.I.; Torcello-Gómez, A.; Saha, S.; Mackie, A.R.; Wilde, P.J.; Brodkorb, A. Impact of caseins and whey proteins ratio and lipid content on in vitro digestion and ex vivo absorption. Food Chem. 2020, 319, 126514. [Google Scholar] [CrossRef] [PubMed]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Simon, C.D.; Sagdic, O.; Capanoglu, E. Effect of dietary fiber (inulin) addition on phenolics and in vitro bioaccessibility of tomato sauce. Int. Food Res. J. 2018, 106, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Vardakou, M.; Faulks, R.; Bisignano, C.; Martorana, M.; Smeriglio, A.; Trombetta, D. Food Matrix Effects of Polyphenol Bioaccessibility from Almond Skin during Simulated Human Digestion. Nutrients 2016, 8, 568. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemicalphysiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Buitimea-Cantua, N.E.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Phenolic–protein interactions: Effects on food properties and health benefits. J. Med. Food 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Sae-Leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Baba, W.N.; McClements, D.J.; Maqsood, S. Whey protein–polyphenol conjugates and complexes: Production, characterization, and applications. Food Chem. 2021, 365, 130455. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Liy, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Chima, B.; Mathews, P.; Morgan, S.; Johnson, S.A.; Van Buiten, C.B. Physicochemical Characterization of Interactions between Blueberry Polyphenols and Food Proteins from Dairy and Plant Sources. Nutrients 2022, 11, 2846. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed]
- Xiue, H.; Zhiqiang, L.; Songfan, T.; Lu, L.; Song, W. Epigallocatechin gallate (EGCG) modification of structural and functional properties of whey protein isolate. Food Res. Int. 2022, 158, 11534. [Google Scholar] [CrossRef]
- Xuejiao, Q.; Wu, Y.; Chen, Y.; Liu, C. Competitive interactions among tea catechins, proteins, and digestive enzymes modulate in vitro protein digestibility, catechin bioaccessibility, and antioxidant activity of milk tea beverage model systems. Food Res. Int. 2021, 140, 110050. [Google Scholar] [CrossRef]
- Sabouri, S.; Wright, A.J.; Corredig, M. In vitro, digestion of sodium caseinate emulsions loaded with epigallocatechin gallate. Food Hydrocoll. 2017, 69, 350–358. [Google Scholar] [CrossRef]
- Hu, S.; Wang, T.; Fernandez, M.L.; Luo, Y. Development of tannic acid cross-linked hollow zein nanoparticles as potential oral delivery vehicles for curcumin. Food Hydrocoll. 2016, 61, 821–831. [Google Scholar] [CrossRef]
- Shi, M.; Huang, L.Y.; Nie, N.; Ye, J.H.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Binding of tea catechins to rice bran protein isolate: Interaction and protective effect during in vitro digestion. Food Res. Int. 2017, 93, 1–7. [Google Scholar] [CrossRef]
- De Morais, F.P.; Pessato, T.B.; Rodrigues, E.; Mallmann, L.P.; Mariutti, L.R.; Netto, F.M. Whey protein and phenolic compound complexation: Effects on antioxidant capacity before and after in vitro digestion. Food Res. Int. 2020, 133, 109104. [Google Scholar] [CrossRef]
- Świeca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyż, J. The influence of protein–flavonoid interactions on protein digestibility in vitro and the antioxidant quality of breads enriched with onion skin. Food Chem. 2013, 141, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Oksuz, T.; Tacer-Caba, Z.; Nilufer-Erdil, D.; Boyacioglu, D. Changes in bioavailability of sour cherry (Prunus cerasus L.) phenolics and anthocyanins when 1031 consumed with dairy food matrices. J. Food Sci. Technol. 2019, 56, 4177–4188. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Edwards, C.A.; Serafini, M.; Crozier, A. Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream. J. Agric. Food Chem. 2008, 56, 713–719. [Google Scholar] [CrossRef]
- Serafini, M.; Testa, M.F.; Villaño, D.; Pecorari, M.; van Wieren, K.; Azzini, E.; Brambilla, A.; Maian, G. Antioxidant activity of blueberry fruit is impaired by association with milk. Free Radic. Biol. Med. 2009, 46, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Sengul, H.; Surek, E.; Nilufer-Erdil, D. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; de Ancos, B.; Sánchez-Moreno, C.; Pilar Cano, M.; Elez-Martínez, P.; Martín-Belloso, O. Impact of food matrix and processing on the in vitro bioaccessibility of vitamin C, phenolic compounds, and hydrophilic antioxidant activity from fruit juice-based beverages. J. Funct. Foods 2015, 14, 33–43. [Google Scholar] [CrossRef]
- Budryn, G.; Bartłomiej Pałecz, B.; Rachwał-Rosiak, D.; Oracz, J.; Zaczyńska, D.; Belica, S.; Navarro-González, I.; Vegara Meseguer, J.M.; Pérez-Sánchez, H. Effect of inclusion of hydroxycinnamic and chlorogenic acids from green coffee bean in β-cyclodextrin on their interactions with whey, egg white and soy protein isolates. Food Chem. 2015, 168, 276–287. [Google Scholar] [CrossRef]
- Ibrahim, A.H. Physicochemical characterization of interactions between phenolics and camel’s milk proteins and their bioavailability. Anim. Sci. Rep. 2019, 12, 24. [Google Scholar]
- Pozzo, L.; Russo, R.; Frassinetti, S.; Vizzarri, F.; Arvay, J.; Vornoli, A.; Casamassima, D.; Palazzo, M.; Della Croce, C.M.; Longo, V. Wild Italian Prunus spinosa L. Fruit Exerts In Vitro Antimicrobial Activity and Protects Against In Vitro and In vivo Oxidative Stress. Foods 2020, 9, 5. [Google Scholar] [CrossRef]
- El-Desouky, W.I.; Mahmoud, A.H.; Abbas, M.M. Antioxidant potential and hypolipidemic effect of whey protein against gamma irradiation induced damages in rats. Appl. Radiat. Isot. 2017, 129, 103–107. [Google Scholar] [CrossRef]
- Veskoukis, A.; Kerasioti, E.; Skaperda, Z.; Apostolos Papapostolou, P.; Nepka, C.; Spandidos, D.A.; Asporodini, E.; Taitzoglou, I.; Kouretas, D. Whey protein boosts the antioxidant profile of rats by enhancing the activities of crucial antioxidant enzymes in a tissue-specific manner. Food Chem. Toxicol. 2020, 142, 111508. [Google Scholar] [CrossRef] [PubMed]



| a Small Intestine Bioavailability | 1–28 Day, Subchronic Dose * | |||
|---|---|---|---|---|
| Compound | Group | cmax (µg/g Tissue) | tmax (h) | AUClast (h·µg/g Tissue) |
| Flavonols | ||||
| Isorhamnetin-3-rutinoside | Cont. | 0.39 ± 0.01 | 24 ± 2 | 252.9 ± 1.5 |
| WP | 0.41 ± 0.00 | 504 ± 0 | 263.9 ± 0.0 | |
| EPS | 0.43 ± 0.25 | 24 ± 0 | 273.2 ± 4.5 | |
| WP + EPS | 0.41 ± 0.00 | 24 ± 0 | 263.1 ± 0.0 | |
| Quercetin-3-O-glucoside | Cont. | 0.17 ± 0.00 | 168 ± 0 | 63.9 ± 10.2 |
| WP | 0.55 ± 0.00 | 168 ± 0 | 115.1 ± 1.1 | |
| EPS | 0.56 ± 0.05 * | 504 ± 19 | 151.0 ± 35.5 * | |
| WP + EPS | 0.38 ± 0.00 | 336 ± 0 | 88.9 ± 0.5 | |
| Quercetin-3-O-rutinoside | Cont. | 0.78 ± 0.00 | 168 ± 0 | 270.3 ± 4.3 |
| WP | 1.36 ± 0.00 | 24 ± 8 | 546.2 ± 1.0 | |
| EPS | 1.73 ± 0.21 * | 24 ± 3 | 952.0 ± 120.8 * | |
| WP + EPS | 1.40 ± 0.03 | 24 ± 2 | 909.1 ± 114.6 | |
| Quercetin-acetyl-hexoside | Cont. | 0.52 ± 0.01 | 672 ± 291 | 219.2 ± 23.8 |
| WP | 0.54 ± 0.00 | 504 ± 0 | 305 ± 0.0 | |
| EPS | 0.67 ± 0.39 | 672 ± 0 | 429.9 ± 0.9 * | |
| WP + EPS | 0.56 ± 0.00 | 168 ± 0 | 352.4 ± 0.0 | |
| Quercetin-pentoside | Cont. | 1.37 ± 0.07 | 168 ± 83 | 654.5 ± 19.2 |
| WP | 0.14 ± 0.00 | 504 ± 0 | 93.5 ± 1.1 | |
| EPS | 1.94 ± 1.03 | 24 ± 0 | 814.6 ± 8.0 | |
| WP + EPS | 0.14 ± 0.00 ▲ | 336 ± 0 | 93.6 ± 5.2 ▲ | |
| Quercetin-pentosyl-hexoside | Cont. | 0.34 ± 0.01 | 24 ± 8 | 152.3 ± 13.9 |
| WP | 0.30 ± 0.00 | 672 ± 0 | 126.7 ± 0.5 | |
| EPS | 0.45 ± 0.04 | 24 ± 0 | 179.9 ± 10.2 | |
| WP + EPS | 0.35 ± 0.04 | 336 ± 0 | 153.5 ± 25.4 | |
| Qurercetin-rhamnoside | Cont. | 0.96 ± 0.10 | 336 ± 83 | 387.7 ± 11.8 |
| WP | 1.68 ± 0.00 | 672 ± 0 | 235.2 ± 1.2 | |
| EPS | 1.68 ± 0.95 * | 24 ± 0 | 723.7 ± 17.3 * | |
| WP + EPS | 1.52 ± 0.70 | 672 ± 97 | 213.2 ± 131.2 ▲ | |
| Kaempferol-3-glucoside | Cont. | 0.16 ± 0.00 | 168 ± 83 | 93.5 ± 2.1 |
| WP | 0.22 ± 0.00 | 672 ± 0 | 89.0 ± 0.0 | |
| EPS | 0.30 ± 0.17 * | 168 ± 0 | 146.1 ± 3.8 * | |
| WP + EPS | 0.16 ± 0.00 | 336 ± 0 | 87.6 ± 0.0 | |
| Kaempferol-3-rutinoside | Cont. | 0.24 ± 0.00 | 168 ± 0 | 116.3 ± 3.2 |
| WP | <LOD | - | - | |
| EPS | 0.28 ± 0.16 | 168 ± 83 | 132.1 ± 1.2 | |
| WP + EPS | <LOD ▲ | - | - | |
| Kaempferol-pentoside | Cont. | 2.75 ± 0.00 | 168 ± 0 | 1344.3 ± 6.0 |
| WP | 2.55 ± 0.00 | 672 ± 0 | 1282.6 ± 0.0 | |
| EPS | 5.29 ± 2.91 * | 24 ± 0 | 1559.4 ± 74.7 | |
| WP + EPS | 2.10 ± 0.00 ▲ | 168 ± 0 | 1071.4 ± 0.0 | |
| Kaempferol-3-O-rhamnoside | Cont. | 0.00 ± 0.00 | 168 ± 0 | 0.0 ± 0.0 |
| WP | <LOD | - | - | |
| EPS | 0.06 ± 0.00 | 672 ± 0 | 9.9 ± 0.0 * | |
| WP + EPS | 0.00 ± 0.00 ▲ | 24 ± 0 | 0.0 ± 0.0 ▲ | |
| Kaempferol-rhamnosyl-hexoside | Cont. | 0.69 ± 0.02 | 24 ± 8 | 334.8 ± 4.9 |
| WP | 0.68 ± 0.00 | 672 ± 0 | 300.1 ± 0.0 | |
| EPS | 0.84 ± 0.49 | 24 ± 0 | 384.6 ± 3.5 | |
| WP + EPS | 0.54 ± 0.00 | 336 ± 0 | 286.7 ± 0.0 | |
| Kaempferol-acetyl- hexoside | Cont. | 0.04 ± 0.01 | 504 ± 0 | 13.3 ± 4.7 |
| WP | 0.04 ± 0.00 | 24 ± 0 | 16.3 ± 0.0 | |
| EPS | 0.05 ± 0.05 | 672 ± 37 | 7.6 ± 2.7 | |
| WP + EPS | 0.06 ± 0.00 | 672 ± 0 | 24.7 ± 0.0 | |
| Kaempferol-pentosyl-hexoside | Cont. | 0.51 ± 0.01 | 168 ± 83 | 260.6 ± 13.9 |
| WP | 0.51 ± 0.00 | 672 ± 0 | 242.4 ± 0.0 | |
| EPS | 0.85 ± 0.45 * | 24 ± 0 | 329.4 ± 10.2 | |
| WP + EPS | 0.55 ± 0.00 | 336 ± 0 | 266.2 ± 0.0 | |
| Flavones | ||||
| Apigenin | Cont. | 0.89 ± 0.14 | 24 ± 0 | 188.3 ± 17.8 |
| WP | 0.08 ± 0.01 | 24 ± 14 | 1.1 ± 0.74 | |
| EPS | 2.50 ± 0.25 * | 24 ± 3 | 523.9 ± 72.5 * | |
| WP + EPS | <LOD ▲ | - | - | |
| Luteolin | Cont. | 0.20 ± 0.10 | 168 ± 83 | 89.5 ± 19.2 |
| WP | 0.20 ± 0.00 | 504 ± 0 | 17.5 ± 0.2 | |
| EPS | 0.64 ± 0.28 * | 24 ± 3 | 171.8 ± 30.6 * | |
| WP + EPS | 0.22 ± 0.02 ▲ | 504 ± 388 | 18.8 ± 2.0 ▲ | |
| Flavan-3-ols | ||||
| (+)-Catechin | Cont. | 0.13 ± 0.00 | 672 ± 374 | 83.5 ± 0.5 |
| WP | 0.23 ± 0.00 | 168 ± 0 | 102.3 ± 0.2 | |
| EPS | 0.16 ± 0.09 | 672 ± 0 | 86.8 ± 1.5 | |
| WP + EPS | 0.13 ± 0.00 | 168 ± 83 | 87.2 ± 11.2 | |
| (−)-Epicatechin | Cont. | 0.05 ± 0.04 | 168 ± 97 | 9.4 ± 1.1 |
| WP | 0.08 ± 0.00 | 504 ± 0 | 7.5 ± 0.2 | |
| EPS | 0.06 ± 0.03 | 168 ± 0 | 12.5 ± 0.0 | |
| WP + EPS | 0.09 ± 0.03 | 504 ± 83 | 8.3 ± 1.5 | |
| (−)-Epigallocatechin-3-gallate (EGCG) | Cont. | 0.02 ± 0.00 | 504 ± 291 | 6.3 ± 0.8 |
| WP | 0.05 ± 0.00 | 672 ± 0 | 9.6 ± 0.1 | |
| EPS | 0.17 ± 0.02 * | 672 ± 0 | 14.5 ± 13.9 * | |
| WP + EPS | 0.05 ± 0.01 ▲ | 168 ± 0 | 3.9 ± 1.3 ▲ | |
| Phenolic acids | ||||
| 3-O-Feruloylquinic acid | Cont. | 0.62 ± 0.13 | 168 ± 83 | 211.9 ± 37.3 |
| WP | 0.44 ± 0.00 | 672 ± 0 | 155.6 ± 0.0 | |
| EPS | 4.61 ± 0.42 * | 168 ± 37 | 768.4 ± 6.3 * | |
| WP + EPS | 1.40 ± 0.00 ▲ | 336 ± 0 | 471.7 ± 0 ▲ | |
| 3-O-p-Coumaroylquinic acid | Cont. | 0.00 ± 0.05 | 24 ± 8 | 0.0 ± 6.2 |
| WP | 0.05 ± 0.00 | 24 ± 0 | 22.4 ± 0.0 | |
| EPS | 0.20 ± 0.02 | 168 ± 14 | 71.8 ± 14.4 * | |
| WP + EPS | 0.28 ± 0.00 | 336 ± 0 | 81.5 ± 0.0 | |
| 4-O-Caffeoylquinic acid | Cont. | 1.64 ± 0.10 | 672 ± 374 | 896.5 ± 83.2 |
| WP | 1.54 ± 0.00 | 504 ± 0 | 953.5 ± 0.0 | |
| EPS | 1.81 ± 1.17 | 168 ± 34 | 1110.1 ± 25.4 | |
| WP + EPS | 1.50 ± 0.00 | 24 ± 0 | 910.6 ± 0.0 | |
| 4-O-p-Coumaroylquinic acid | Cont. | 1.22 ± 0.05 | 24 ± 0 | 627.7 ± 4.2 |
| WP | 0.95 ± 0.00 | 168 ± 0 | 623.6 ± 4.0 | |
| EPS | 2.16 ± 1.27 * | 504 ± 97. | 1095.0 ± 27.8 * | |
| WP + EPS | 0.93 ± 0.00 ▲ | 168 ± 0 | 570 ± 160.7 ▲ | |
| Caffeic acid | Cont. | 0.20 ± 0.05 | 168 ± 0 | 41.6 ± 17.4 |
| WP | 0.20 ± 0.00 | 672 ± 0 | 62.7 ± 0.0 | |
| EPS | 0.37 ± 0.21 * | 168 ± 0 | 111.8 ± 13.9 * | |
| WP + EPS | 0.22 ± 0.00 | 504 ± 0 | 108.2 ± 0.0 | |
| Ferullic acid | Cont. | 1.04 ± 0.30 | 504 ± 0 | 368.3 ± 61.5 |
| WP | 1.57 ± 0.00 | 504 ± 0 | 823.5 ± 1.2 | |
| EPS | 1.68 ± 0.95 * | 24 ± 2 | 740.9 ± 9.7 * | |
| WP + EPS | 1.54 ± 0.22 | 672 ± 374 | 847.4 ± 113.8 | |
| Gallic acid | Cont. | 1.28 ± 0.67 | 24 ± 0 | 215.1 ± 96.7 |
| WP | 0.63 ± 0.00 | 672 ± 0 | 183.6 ± 0.0 | |
| EPS | 2.21 ± 1.27 * | 168 ± 0 | 575.2 ± 12.6 * | |
| WP + EPS | 0.42 ± 0.00 ▲ | 336 ± 0 | 191.6 ± 0.0 ▲ | |
| p-Coumaric acid | Cont. | 0.48 ± 0.14 | 504 ± 277 | 186.7 ± 37.4 |
| WP | 0.57 ± 0.00 | 24 ± 0 | 302 ± 0.0 | |
| EPS | 1.67 ± 0.94 * | 24 ± 0 | 444.9 ± 6.3 * | |
| WP + EPS | 1.22 ± 0.00 | 504 ± 0 | 567.6 ± 0.0 | |
| a Liver Bioavailability | 1–28 Day, Subchronic Dose * | |||
|---|---|---|---|---|
| Compound | Group | cmax (µg/g Tissue) | tmax (h) | AUClast (h·µg/g Tissue) |
| Flavonols | ||||
| Isorhamnetin-3-rutinoside | Cont. | 0.39 ± 0.00 | 168 ± 29 | 248.0 ± 1.2 |
| WP | 0.43 ± 0.00 | 672 ± 356 | 268.7 ± 0.0 | |
| EPS | 0.41 ± 0.00 | 168 ± 83 | 265.4 ± 0.2 | |
| WP + EPS | 0.40 ± 0.02 | 504 ± 0 | 266.9 ± 0.0 | |
| Quercetin-3-O-glucoside | Cont. | 0.14 ± 0.09 | 24 ± 0 | 70.6 ± 0.8 |
| WP | 0.32 ± 0.35 | 336 ± 0 | 107.2 ± 0.0 | |
| EPS | 0.14 ± 0.09 | 24 ± 0 | 70.6 ± 0.8 | |
| WP + EPS | 0.65 ± 0.02 | 168 ± 0 | 171.4 ± 0.0 | |
| Quercetin-3-O-rutinoside | Cont. | 1.55 ± 0.32 | 336 ± 83 | 715.1 ± 78.9 |
| WP | 1.51 ± 0.04 | 168 ± 0 | 777.9 ± 6.6 | |
| EPS | 2.57 ± 0.03 * | 336 ± 83 | 1113.1 ± 0.6 * | |
| WP + EPS | 1.79 ± 0.04 | 168 ± 0 | 845.1 ± 24.8 | |
| Quercetin-acetyl-hexoside | Cont. | 0.55 ± 0.00 | 24 ± 3 | 302.8 ± 2.5 |
| WP | 0.55 ± 0.00 | 168 ± 0 | 355.7 ± 0.0 | |
| EPS | 0.64 ± 0.00 | 24 ± 3 | 419.2 ± 0.2 | |
| WP + EPS | 1.17 ± 0.04 | 24 ± 0 | 500.5 ± 0.0 | |
| Quercetin-pentoside | Cont. | 0.13 ± 0.00 | 168 ± 0 | 84.2 ± 1.1 |
| WP | 0.14 ± 0.00 | 168 ± 0 | 94.5 ± 0.0 | |
| EPS | 1.44 ± 0.02 * | 168 ± 83 | 670.0 ± 1.6 * | |
| WP + EPS | 0.14 ± 0.11 ▲ | 336 ± 37.2 | 92.7 ± 0.7 ▲ | |
| Quercetin pentosyl-hexoside | Cont. | 0.36 ± 0.02 | 168 ± 0 | 157.7 ± 0.8 |
| WP | 0.12 ± 0.00 | 168 ± 0 | 43.6 ± 0.0 | |
| EPS | 0.47 ± 0.06 | 168 ± 0 | 207.5 ± 0.2 | |
| WP + EPS | 0.31 ± 0.02 | 504 ± 0 | 141.5 ± 0.0 | |
| Qurercetin-rhamnoside | Cont. | 1.95 ± 0.03 | 168 ± 0 | 606.7 ± 1.6 |
| WP | 1.68 ± 0.1 | 168 ± 0 | 259.3 ± 0.0 | |
| EPS | 1.67 ± 0.01 | 168 ± 0 | 635.4 ± 1.0 | |
| WP + EPS | 1.52 ± 0.17 | 336 ± 0 | 659.3 ± 0.2 | |
| Kaempferol-3-glucoside | Cont. | 0.16 ± 0.00 | 24 ± 0 | 71.6 ± 0.4 |
| WP | 0.06 ± 0.00 | 24 ± 0 | 17.6 ± 0.0 | |
| EPS | 0.26 ± 0.01 | 168 ± 0 | 108.3 ± 0.3 | |
| WP + EPS | 0.15 ± 0.1 | 336 ± 0 | 71.6 ± 0.2 | |
| Kaempferol-3-rutinoside | Cont. | 0.23 ± 0.01 | 24 ± 8 | 114.9 ± 0.8 |
| WP | 0.04 ± 0.00 | 672 ± 0 | 3.7 ± 2.9 | |
| EPS | 0.29 ± 0.00 * | 168 ± 0 | 140.8 ± 0.1 * | |
| WP + EPS | 0.06 ± 0.01 ▲ | 336 ± 29 | 25.4 ± 6.9 ▲ | |
| Kampferol-pentoside | Cont. | 2.60 ± 0.00 | 168 ± 0 | 1243. 4 ± 1.1 |
| WP | 2.58 ± 0.00 | 168 ± 0 | 1230.1 ± 0.0 | |
| EPS | 2.69 ± 0.00 | 168 ± 0 | 1382.3 ± 1.0 | |
| WP + EPS | 2.67 ± 0.14 | 336 ± 0 | 1416.4 ± 0.0 | |
| Kaempferol-3-O-rhamnoside | Cont. | 0.03 ± 0.00 | 168 ± 34 | 8.0 ± 1.1 |
| WP | <LOD | - | - | |
| EPS | 0.12 ± 0.02 * | 168 ± 0 | 26.2 ± 0.5 * | |
| WP + EPS | 0.03 ± 0.00 ▲ | 336 ± 0 | 11.8 ± 2.4 ▲ | |
| Kaempferol-rhamnosyl- hexoside | Cont. | 0.70 ± 0.02 | 168 ± 83 | 330.5 ± 1.5 |
| WP | 0.26 ± 0.01 | 168 ± 0 | 134.9 ± 0.5 | |
| EPS | 0.80 ± 0.00 | 168 ± 0 | 374.2 ± 0.59 | |
| WP + EPS | 0.62 ± 0.04 | 336 ± 290 | 392.8 ± 0.8 | |
| Kaempferol-acetyl-hexoside | Cont. | 0.02 ± 0.00 | 168 ± 96 | 4.1 ± 0.8 |
| WP | 0.13 ± 0.00 | 504 ± 0 | 44.8 ± 0.0 | |
| EPS | 0.07 ± 0.00 * | 168 ± 0 | 13.4 ± 0.0 * | |
| WP + EPS | 0.05 ± 0.00 | 24 ± 0 | 22 ± 0.2 | |
| Flavones | ||||
| Apigenin | Cont. | 0.28 ± 0.01 | 24 ± 8 | 107.2 ± 0.9 |
| WP | 0.50 ± 0.00 | 168 ± 0 | 40.6 | |
| EPS | 0.20 ± 0.03 | 168 ± 27 | 121.1 ± 0.5 | |
| WP + EPS | 0.10 ± 0.03 ▲ | 24 ± 0 | 9.9 ± 0.1 ▲ | |
| Luteolin | Cont. | 0.19 ± 0.01 | 168 ± 83 | 51.3 ± 3.3 |
| WP | <LOD | - | - | |
| EPS | 0.19 ± 0.01 | 168 ± 83 | 51.3 ± 3.3 | |
| WP + EPS | 0.05 ± 0.01 ▲ | 24 ± 0 | 25.8 ± 0.1 ▲ | |
| Flavan-3-ols | ||||
| (+)-Catechin | Cont. | 0.13 ± 0.00 | 168 ± 37 | 84.1 ± 0.1 |
| WP | 0.13 ± 0.00 | 168 ± 118 | 86.3 ± 0.9 | |
| EPS | 0.14 ± 0.00 | 672 ± 290 | 90.5 ± 0.0 | |
| WP + EPS | 0.13 ± 0.00 | 504 ± 256 | 88.4 ± 16.5 | |
| (−)-Epicatechin-3-gallate | Cont. | 0.70 ± 0.00 | 336 ± 193 | 169.2 ± 2.4 |
| WP | 0.99 ± 0.00 | 168 ± 0 | 328.3 ± 0.0 | |
| EPS | 1.40 ± 0.00 * | 336 ± 0 | 265.9 ± 0.0 * | |
| WP + EPS | 4.56 ± 0.26 ▲ | 168 ± 0 | 783.4 ± 328.0 ▲ | |
| (−)-Epigallocatechin-3-gallate (EGCG) | Cont. | 1.07 ± 0.25 | 168 ± 193 | 400.0 ± 61.5 |
| WP | 1.07 ± 0.00 | 168 ± 33 | 406.8 ± 0.1 | |
| EPS | 0.88 ± 0.00 * | 336 ± 0 | 256.6 ± 0.0 | |
| WP + EPS | 2.18 ± 0.12 ▲ | 168 ± 0 | 596.3 ± 0.1 ▲ | |
| Phenolic acids | ||||
| 3-O-Feruloylquinic acid | Cont. | 0.21 ± 0.03 | 504 ± 290 | 34.9 ± 9.0 |
| WP | 0.45 ± 0.00 | 24 ± 0 | 134.8 ± 5.1 | |
| EPS | 0.45 ± 0.09 * | 24 ± 2 | 81.0 ± 4.6 * | |
| WP + EPS | 0.42 ± 0.04 | 24 ± 0 | 116.1 ± 21.9 | |
| 3-O-p-Coumaroylquinic acid | Cont. | 0.04 ± 0.00 | 24 ± 8 | 8.3 ± 0.7 |
| WP | 0.03 ± 0.03 | 168 ± 0 | 6.5 ± 0.1 | |
| EPS | 0.26 ± 0.00 * | 168 ± 0 | 68.5 ± 0.1 * | |
| WP + EPS | 0.16 ± 0.01 | 336 ± 0 | 65.4 ± 8.3 | |
| 4-O-Caffeoylquinic acid | Cont. | 1.42 ± 0.01 | 504 ± 96 | 710.6 ± 0.5 |
| WP | 1.61 ± 0.00 | 504 ± 0 | 1005.8 ± 0.0 | |
| EPS | 1.59 ± 0.00 | 672 ± 0 | 963.9 ± 1.7 | |
| WP + EPS | 1.43 ± 0.08 | 672 ± 0 | 890.8 ± 0.1 | |
| 4-O-p-Coumaroylquinic acid | Cont. | 0.54 ± 0.14 | 24 ± 1 | 317.5 ± 2.8 |
| WP | 0.96 ± 0.00 | 168 ± 0 | 606.2 ± 5.5 | |
| EPS | 0.54 ± 0.01 | 24 ± 1 | 317.5 ± 2.8 | |
| WP + EPS | 0.96 ± 0.06 | 168 ± 0 | 610.1 ± 0.8 | |
| Caffeic acid | Cont. | 0.24 ± 0.01 | 504 ± 0 | 54.5 ± 0.2 |
| WP | 0.05 ± 0.00 | 168 ± 0 | 10.6 ± 0 | |
| EPS | 1.79 ± 0.15 * | 504 ± 227 | 479.4 ± 39.1 * | |
| WP + EPS | 0.17 ± 0.01 ▲ | 504 ± 0 | 78.8 ± 0.0 ▲ | |
| Ferullic acid | Cont. | 0.77 ± 0.02 | 336 ± 83 | 494.6 ± 0.4 |
| WP | 0.17 ± 0.00 | 336 ± 48 | 98.4 ± 0.1 | |
| EPS | 1.64 ± 0.02 | 672 ± 290 | 835.8 ± 3.6 | |
| WP + EPS | 1.38 ± 0.09 | 24 ± 8 | 760.9 ± 0.1 | |
| Gallic acid | Cont. | 0.58 ± 0.14 | 672 ± 0 | 76.9 ± 8.8 |
| WP | 0.20 ± 0.00 | 24 ± 0 | 2.5 ± 0.0 | |
| EPS | 1.31 ± 0.19 * | 672 ± 387 | 110.1 ± 16.2 | |
| WP + EPS | 1.11 ± 0.00 | 336 ± 0 | 506.0 ± 0.0 ▲ | |
| p-Coumaric acid | Cont. | <LOD | - | - |
| WP | <LOD | - | - | |
| EPS | 0.17 ± 0.03 | 168 ± 83 | 38.4 ± 0.0 * | |
| WP + EPS | 0.07 ± 0.00 ▲ | 336 ± 0 | 27.3 ± 0.3 | |
| a Kidney’s Bioavailability | 1–28 Day, Subchronic Dose * | |||
|---|---|---|---|---|
| Compound | Group | cmax (µg/g Tissue) | tmax (h) | AUClast (h·µg/g Tissue) |
| Flavonols | ||||
| Kaempferol-pentoside | Cont. | 2.77 ± 0.02 | 24 ± 0 | 1317.0 ± 14.7 |
| WP | 1.33 ± 0.00 | 24 ± 0 | 536.7 ± 3.2 | |
| EPS | 2.94 ± 0.03 | 24 ± 0 | 1381.1 ± 12.5 | |
| WP + EPS | 2.76 ± 0.00 | 24 ± 0 | 1305.6 ± 8.9 | |
| Quercetin-3-O-glucoside | Cont. | 0.15 ± 0.00 | 168 ± 83 | 85.1 ± 1.0 |
| WP | <LOD | 24 ± 0 | 0.0 ± 0.0 | |
| EPS | 0.20 ± 0.00 | 24 ± 0 | 121.1 ± 0.6 | |
| WP + EPS | 0.14 ± 0.03 | 672 ± 194 | 33.4 ± 3.7 ▲ | |
| Quercetin-3-O-rutinoside | Cont. | 0.65 ± 0.35 | 168 ± 19 | 240.1 ± 100.8 |
| WP | 0.28 ± 0.01 | 336 ± 0 | 65.8 ± 15.2 | |
| EPS | 2.96 ± 0.02 * | 336 ± 0 | 1764.0 ± 6.7 * | |
| WP + EPS | 0.38 ± 0.00 ▲ | 504 ± 0 | 89.9 ± 34.3 ▲ | |
| Quercetin-pentosyl-hexoside | Cont. | 0.13 ± 0.00 | 24 ± 0 | 86.8 ± 1.2 |
| WP | 0.14 ± 0.00 | 24 ± 0 | 91.6 ± 0.3 | |
| EPS | 0.41 ± 0.00* | 24 ± 8 | 193.2 ± 1.1* | |
| WP + EPS | 0.36 ± 0.00 | 24 ± 9 | 163.9 ± 1.4 | |
| Quercetin-rhamnoside | Cont. | 1.35 ± 0.02 | 24 ± 0 | 609.0 ± 12.7 |
| WP | 0.66 ± 0.00 | 24 ± 0 | 395.7 ± 1.5 | |
| EPS | 1.46 ± 0.00 | 24 ± 0 | 699.5 ± 1.0 | |
| WP + EPS | 0.24 ± 0.14 ▲ | 672 ± 388 | 52.3 ± 11.7 ▲ | |
| Flavones | ||||
| Apigenin | Cont. | 0.45 ± 0.01 | 24 ± 0 | 128.2 ± 1.0 |
| WP | 0.46 ± 0.00 | 24 ± 0 | 134.9 ± 1.2 | |
| EPS | 0.96 ± 0.02 * | 504 ± 0 | 349.2 ± 0.6 * | |
| WP + EPS | 0.34 ± 0.00 ▲ | 168 ± 0 | 119.5 ± 0.0 ▲ | |
| Luteolin | Cont. | 0.13 ± 0.15 | 672 ± 0 | 11.3 ± 3.5 |
| WP | <LOD | - | - | |
| EPS | 0.52 ± 0.19 * | 24 ± 0 | 131.2 ± 13.7 * | |
| WP + EPS | 0.11 ± 0.06 ▲ | 672 ± 388 | 9.5 ± 5.5 ▲ | |
| Flavan-3-ols | ||||
| (+)-Catechin | Cont. | 0.21 ± 0.00 | 24 ± 0 | 92.9 ± 1.2 |
| WP | 1.41 ± 0.00 | 24 ± 0 | 654.0 ± 3.2 | |
| EPS | 1.41 ± 0.01 * | 168 ± 0 | 776.2 ± 1.4 * | |
| WP + EPS | 1.42 ± 0.03 | 24 ± 3 | 634.8 ± 95.4 | |
| (−)-Epicatechin | Cont. | 0.03 ± 0.00 | 24 ± 0 | 0.3 ± 0.0 |
| WP | 1.32 ± 0.02 | 336 ± 38 | 668.7 ± 2.1 | |
| EPS | 1.55 ± 0.00 * | 168 ± 0 | 848.4 ± 0.4 * | |
| WP + EPS | 1.65 ± 0.00 | 672 ± 0 | 686.0 ± 1.2 | |
| (−)-Epigallocatechin-3-gallate (EGCG) | Cont. | 0.83 ± 0.00 | 24 ± 0 | 514.9 ± 2.3 |
| WP | 0.91 ± 0.55 | 168 ± 0 | 500.7 ± 138.4 | |
| EPS | 0.91 ± 0.00 | 24 ± 0 | 589.1 ± 2.6 | |
| WP + EPS | 0.94 ± 0.14 | 168 ± 29 | 592.8 ± 72.6 | |
| Phenolic acids | ||||
| 4-O-p-Coumaroylquinic acid | Cont. | 0.65 ± 0.003 | 672 ± 291 | 412.3 ± 11.1 |
| WP | 1.30 ± 0.01 | 336 ± 0 | 341.6 ± 25.9 | |
| EPS | 2.07 ± 0.04 * | 672 ± 0 | 1143.1 ± 2.6 * | |
| WP + EPS | 0.79 ± 0.01 ▲ | 336 ± 37 | 272.5 ± 38.0 ▲ | |
| Ferullic acid | Cont. | 0.84 ± 0.56 | 504 ± 277 | 261.6 ± 30.79 |
| WP | 0.49 ± 0.20 | 504 ± 246 | 116.7 ± 27.8 | |
| EPS | 5.45 ± 0.00 * | 504 ± 0 | 1354.5 ± 1.5 * | |
| WP + EPS | 1.05 ± 0.00 ▲ | 504 ± 0 | 172.0 ± 0.5 ▲ | |
| Groups a | MDA (nmol/mg Proteins) | CAT (U/mg Proteins) | SOD (U/mg Proteins) | GSH (mU/mg Proteins) |
|---|---|---|---|---|
| Small Intestine | ||||
| Cont. | 1.52 ± 0.24 | 10.47 ± 2.05 | 10.17 ± 4.31 | 76.79 ± 12.69 |
| WP | 1.02 ± 0.28 | 10.96 ± 1.42 | 25.37 ± 3.31 # | 70.01 ± 8.53 |
| EPS | 0.52 ± 0.09 * | 8.78 ± 1.50 | 12.53 ± 2.17 * | 119.95 ± 12.90 * |
| WP + EPS | 0.98 ± 0.20 | 7.87 ± 1.13 | 17.11 ± 3.35 ▲ | 63.43 ± 20.71 ▲ |
| Liver | ||||
| Cont. | 1.69 ± 0.41 | 93.91 ± 35.84 | 15.44 ± 1,49 | 109.81 ± 25.45 |
| WP | 1.58 ± 0.50 | 145.35 ± 42.15 | 35.04 ± 5.04 | 157.48 ± 22.47 |
| EPS | 0.91 ± 0.22 * | 107.94 ± 25.75 * | 24.58 ± 6.16 * | 138.15 ± 26.51 * |
| WP + EPS | 1.26 ± 0.39 | 175.20 ± 52.56 ▲ | 27.42 ± 5.54 ▲ | 167.71 ± 29.55 ▲ |
| Kidney | ||||
| Cont. | 1.75 ± 0.38 | 60.92 ± 14.41 | 12.78 ± 1.81 | 62.64 ± 9.23 |
| WP | 1.26 ± 0.42 | 42.12 ± 9.48 | 26.28 ± 2.67 | 79.34 ± 12.75 |
| EPS | 1.06 ± 0.23 * | 80.9 ± 14.78 * | 22.16 ± 3.13 * | 106.10 ± 16.92 * |
| WP + EPS | 1.17 ± 0.35 | 61.66 ± 9.25 ▲ | 18.29 ± 2.55 ▲ | 73.96 ± 17.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balta, V.; Đikić, D.; Landeka Jurčević, I.; Odeh, D.; Oršolić, N.; Ferara, N.; Dilber, D.; Dragičević, P.; Dragović-Uzelac, V. The Effect of a High-Protein Diet Supplemented with Blackthorn Flower Extract on Polyphenol Bioavailability and Antioxidant Status in the Organs of C57BL/6 Mice. Nutrients 2023, 15, 4066. https://doi.org/10.3390/nu15184066
Balta V, Đikić D, Landeka Jurčević I, Odeh D, Oršolić N, Ferara N, Dilber D, Dragičević P, Dragović-Uzelac V. The Effect of a High-Protein Diet Supplemented with Blackthorn Flower Extract on Polyphenol Bioavailability and Antioxidant Status in the Organs of C57BL/6 Mice. Nutrients. 2023; 15(18):4066. https://doi.org/10.3390/nu15184066
Chicago/Turabian StyleBalta, Vedran, Domagoj Đikić, Irena Landeka Jurčević, Dyana Odeh, Nada Oršolić, Nikola Ferara, Dario Dilber, Petar Dragičević, and Verica Dragović-Uzelac. 2023. "The Effect of a High-Protein Diet Supplemented with Blackthorn Flower Extract on Polyphenol Bioavailability and Antioxidant Status in the Organs of C57BL/6 Mice" Nutrients 15, no. 18: 4066. https://doi.org/10.3390/nu15184066
APA StyleBalta, V., Đikić, D., Landeka Jurčević, I., Odeh, D., Oršolić, N., Ferara, N., Dilber, D., Dragičević, P., & Dragović-Uzelac, V. (2023). The Effect of a High-Protein Diet Supplemented with Blackthorn Flower Extract on Polyphenol Bioavailability and Antioxidant Status in the Organs of C57BL/6 Mice. Nutrients, 15(18), 4066. https://doi.org/10.3390/nu15184066








