Association between Dietary Inflammatory Index and Risk of Colorectal Adenomatous Polyps in Kashgar Prefecture of Xinjiang, China
Abstract
:1. Introduction
2. Materials and Methods
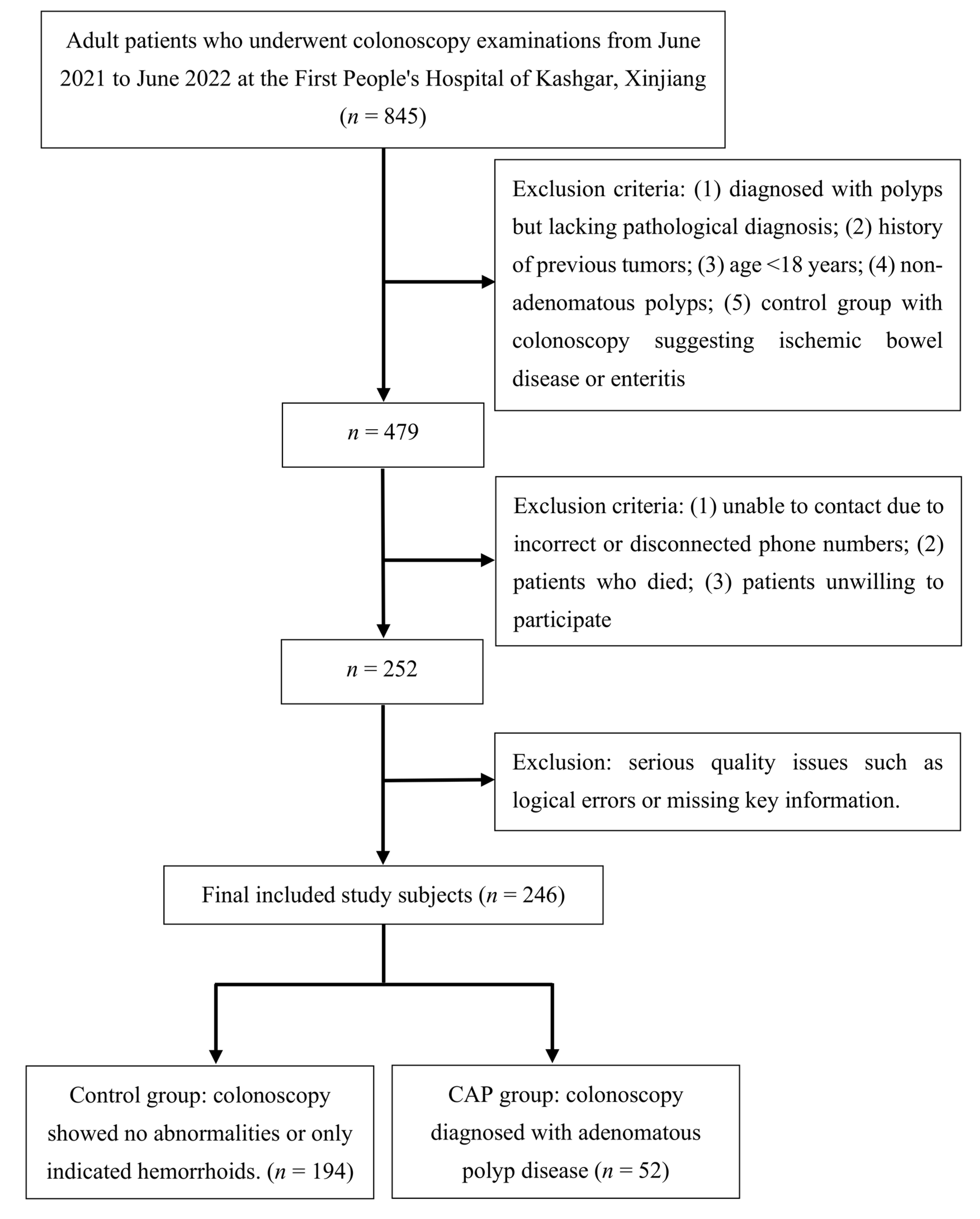
2.1. Patients
2.2. Inclusion and Exclusion Criteria
2.3. Assessment of Dietary Intake
2.3.1. Data Collection
2.3.2. Calculation of DII Score
2.4. Statistical Analysis
2.5. Ethical Approval
3. Results
3.1. Basic Characteristics of the Study Population
3.2. Logistic Analysis of the Relationship between E-DII and the Risk of CAP
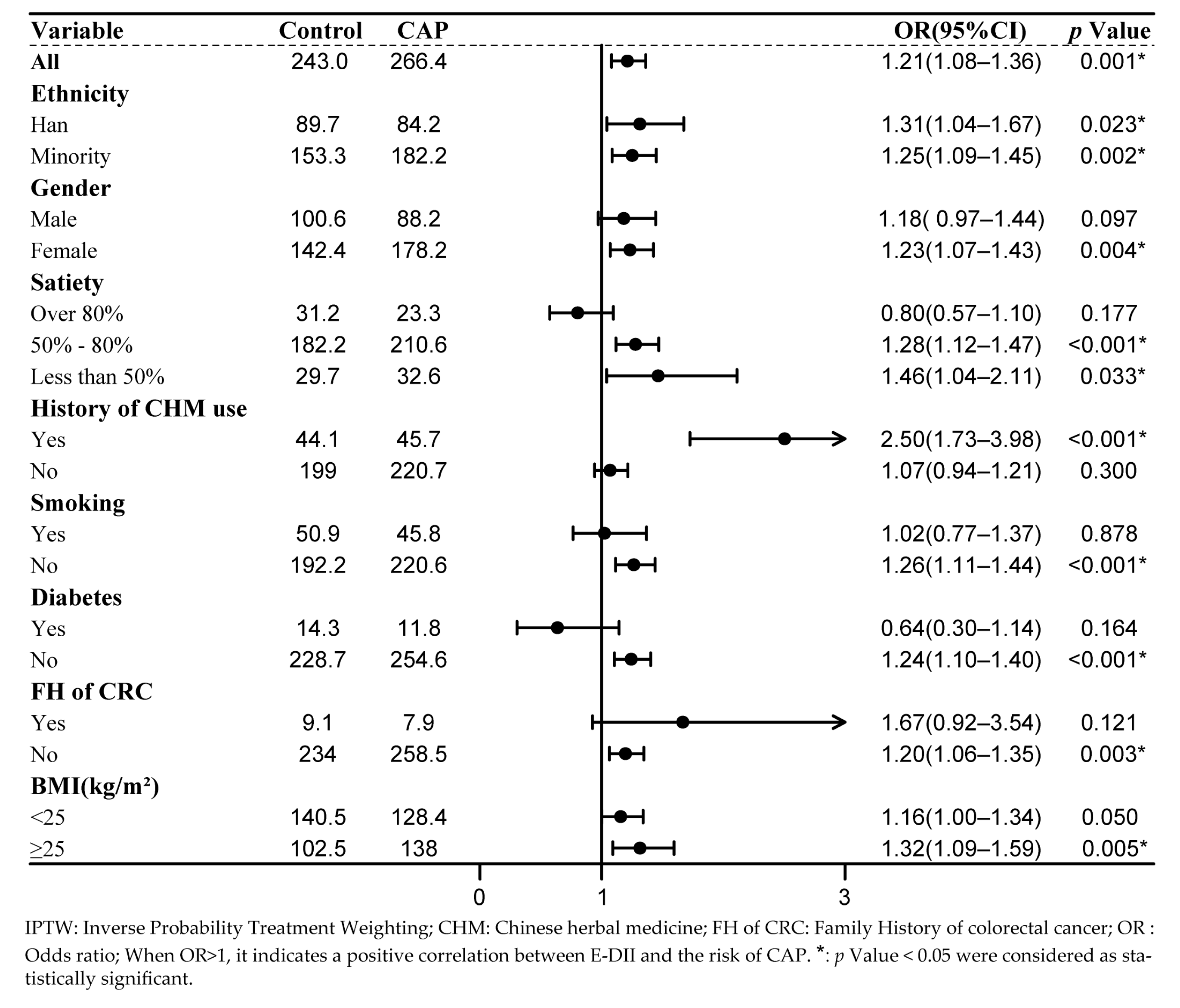
3.3. Subgroup Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Clinical Research Center for Digestive Diseases (Shanghai); Chinese Society of Digestive Endoscopology; Cancer Endoscopy Professional Committee of China Anti-Cancer Association; Digestive Endoscopy Professional Committee of Chinese Endoscopist Association; Endoscopic Health Management and Medical Examination Professional Committee of Chinese Endoscopist Association. Expert Consensus on Management Strategies for Precancerous Lesions and Conditions of Colorectal Cancer in China. Chin. J. Dig. Endosc. 2022, 39, 1–18. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal Cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Tanaka, S.; Sano, Y. Aim to Unify the Narrow Band Imaging (NBI) Magnifying Classification for Colorectal Tumors: Current Status in Japan from a Summary of the Consensus Symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig. Endosc. 2011, 23 (Suppl. S1), 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Zhou, K.; Su, W.; Yu, J.; Zhou, P. Endoscopic Management of Colorectal Polyps. Gastroenterol. Rep. 2023, 11, goad027. [Google Scholar] [CrossRef] [PubMed]
- Conteduca, V.; Sansonno, D.; Russi, S.; Dammacco, F. Precancerous Colorectal Lesions (Review). Int. J. Oncol. 2013, 43, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Sano, W.; Hirata, D.; Teramoto, A.; Iwatate, M.; Hattori, S.; Fujita, M.; Sano, Y. Serrated Polyps of the Colon and Rectum: Remove or Not? World J. Gastroenterol. 2020, 26, 2276–2285. [Google Scholar] [CrossRef]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, Inflammation and Cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Vernia, F.; Longo, S.; Stefanelli, G.; Viscido, A.; Latella, G. Dietary Factors Modulating Colorectal Carcinogenesis. Nutrients 2021, 13, 143. [Google Scholar] [CrossRef]
- Chen, J.; Pitmon, E.; Wang, K. Microbiome, Inflammation and Colorectal Cancer. Semin. Immunol. 2017, 32, 43–53. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Qiao, S. Research Progress on the Relationship Between Inflammation and Colorectal Cancer. Ann. Gastroenterol. Surg. 2022, 6, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J. The Association of Diet, Gut Microbiota and Colorectal Cancer: What We Eat May Imply What We Get. Protein Cell 2018, 9, 474–487. [Google Scholar] [CrossRef]
- Li, A.; Chen, Y.T.; Schuller, A.A.; van der Sluis, L.W.M.; Tjakkes, G.-H.E. Dietary Inflammatory Potential Is Associated with Poor Periodontal Health: A Population-Based Study. J. Clin. Periodontol. 2021, 48, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Zhuravleva, Y. Inflammation: A New Look at an Old Problem. Int. J. Mol. Sci. 2022, 23, 4596. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 2017, 9, 1043. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-L.; Shu, L.; Zheng, P.-F.; Zhang, X.-Y.; Si, C.-J.; Yu, X.-L.; Gao, W.; Zhang, L. Dietary Patterns and Colorectal Cancer Risk: A Meta-Analysis. Eur. J. Cancer Prev. 2017, 26, 201–211. [Google Scholar] [CrossRef]
- Shafiee, N.H.; Razalli, N.H.; Shahril, M.R.; Muhammad Nawawi, K.N.; Mohd Mokhtar, N.; Abd Rashid, A.A.; Ashari, L.S.; Jan Mohamed, H.J.; Raja Ali, R.A. Dietary Inflammatory Index, Obesity, and the Incidence of Colorectal Cancer: Findings from a Hospital-Based Case-Control Study in Malaysia. Nutrients 2023, 15, 982. [Google Scholar] [CrossRef]
- Li, Z.Y.; Wang, K.; Shivappa, N.; Hébert, J.R.; Chen, H.; Liu, H.; Jiang, X.L. Inflammatory Potential of Diet and Colorectal Carcinogenesis: A Prospective Longitudinal Cohort. Br. J. Cancer 2022, 126, 1735–1743. [Google Scholar] [CrossRef]
- Rafiee, P.; Shivappa, N.; Hébert, J.R.; Jaafari Nasab, S.; Bahrami, A.; Hekmatdoost, A.; Rashidkhani, B.; Sadeghi, A.; Houshyari, M.; Hejazi, E. Dietary Inflammatory Index and Odds of Colorectal Cancer and Colorectal Adenomatous Polyps in a Case-Control Study from Iran. Nutrients 2019, 11, 1213. [Google Scholar] [CrossRef]
- Sardo Molmenti, C.L.; Steck, S.E.; Thomson, C.A.; Hibler, E.A.; Yang, J.; Shivappa, N.; Greenlee, H.; Wirth, M.D.; Neugut, A.I.; Jacobs, E.T.; et al. Dietary Inflammatory Index and Risk of Colorectal Adenoma Recurrence: A Pooled Analysis. Nutr. Cancer 2017, 69, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.C.; Deng, L.; Sun, X.Q.; Chen, Z.Y.; Shivappa, N.; Sheth, A.K.; Cooper, G.S.; Hebert, J.R.; Li, L. Dietary Inflammatory Index and Risk of Colorectal Adenoma: Effect Measure Modification by Race, Nonsteroidal Anti-Inflammatory Drugs, Cigarette Smoking and Body Mass Index? Cancer Causes Control 2021, 32, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-C.; Zhao, W.-Q.; Fan, Z.-H.; Lan, X.-F.; Zhu, R.-X.; Zheng, F.-F. Satiety Score Comparison of Five Kinds of Cooked Rice-Vegetable Meals. Food Nutr. China 2020, 26, 57–62. [Google Scholar]
- Ness, K.M.; Strayer, S.M.; Nahmod, N.G.; Schade, M.M.; Chang, A.-M.; Shearer, G.C.; Buxton, O.M. Four Nights of Sleep Restriction Suppress the Postprandial Lipemic Response and Decrease Satiety. J. Lipid Res. 2019, 60, 1935–1945. [Google Scholar] [CrossRef]
- WHO Expert Consultation. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, L.; Zeng, M.R.; Liu, F.Y.; Sun, L.; Liu, Y.; Xiao, L. Energy-Adjusted Dietary Inflammatory Index Is Associated With 5-Year All Cause and Cardiovascular Mortality Among Chronic Kidney Disease Patients. Front. Nutr. 2022, 9, 899004. [Google Scholar] [CrossRef]
- Liang, Z.; Feng, Y.; Shivappa, N.; Hebert, J.R.; Xu, X. Dietary Inflammatory Index and Mortality from All Causes, Cardiovascular Disease, and Cancer: A Prospective Study. Cancers 2022, 14, 4609. [Google Scholar] [CrossRef]
- Shivappa, N.; Wirth, M.D.; Murphy, E.A.; Hurley, T.G.; Hébert, J.R. Association between the Dietary Inflammatory Index (DII) and Urinary Enterolignans and C-Reactive Protein from the National Health and Nutrition Examination Survey-2003-2008. Eur. J. Nutr. 2019, 58, 797–805. [Google Scholar] [CrossRef]
- Andersen, N.N.; Pasternak, B.; Basit, S.; Andersson, M.; Svanström, H.; Caspersen, S.; Munkholm, P.; Hviid, A.; Jess, T. Association Between Tumor Necrosis Factor-α Antagonists and Risk of Cancer in Patients With Inflammatory Bowel Disease. JAMA 2014, 311, 2406. [Google Scholar] [CrossRef]
- Dai, C.L.; Wang, H.Y.; Zhang, W.L.; Wang, P.; Gao, X.H.; Li, W.Y.; Wang, J.; Liu, F.Q.; Chen, F.; He, B.C. Association between Serum Nickel and Oral Cancer Incidence Using Propensity Score Matching and Inverse Probability of Treatment Weighting. J. Environ. Occup. Med. 2022, 39, 1329–1335. [Google Scholar]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Allan, V.; Ramagopalan, S.V.; Mardekian, J.; Jenkins, A.; Li, X.Y.; Pan, X.Y.; Luo, X.M. Propensity Score Matching and Inverse Probability of Treatment Weighting to Address Confounding by Indication in Comparative Effectiveness Research of Oral Anticoagulants. J. Comp. Effect Res. 2020, 9, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical Primer: Propensity Score Matching and Its Alternatives. Eur. J. Cardiothorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Zhang, L.; Chen, Y.M.; Chen, Y.L.; Wang, X.J.; Wu, D.R. Application of Propensity Score Methods in Observational Studies. Chin. J. Evid.-Based Med. 2021, 21, 469–474. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical Activity, Sedentary Behaviour, Diet, and Cancer: An Update and Emerging New Evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Baldi, S.; Menicatti, M.; Nannini, G.; Niccolai, E.; Russo, E.; Ricci, F.; Pallecchi, M.; Romano, F.; Pedone, M.; Poli, G.; et al. Free Fatty Acids Signature in Human Intestinal Disorders: Significant Association between Butyric Acid and Celiac Disease. Nutrients 2021, 13, 742. [Google Scholar] [CrossRef]
| Variables | Control (n = 194) | CAP (n = 52) | p | SMD | |
|---|---|---|---|---|---|
| Age a | - | 49.53(12.76) | 52.83(12.74) | 0.099 | 0.259 |
| Ethnicity b | Han | 66(34.0) | 27(51.9) | 0.06 | 0.369 |
| Uygur | 119(61.3) | 23(44.2) | |||
| Others | 9(4.6) | 2(3.8) | |||
| Gender b | Male | 75(38.7) | 30(57.7) | 0.021 * | 0.388 |
| Female | 119(61.3) | 22(42.3) | |||
| Smoking b | Yes | 38(19.6) | 14(26.9) | 0.337 | 0.174 |
| No | 156(80.4) | 38(73.1) | |||
| Diabetes b | Yes | 10(5.2) | 6(11.5) | 0.18 | 0.232 |
| No | 184(94.8) | 46(88.5) | |||
| FH of CRC b | Yes | 6(3.1) | 4 (7.7) | 0.273 | 0.205 |
| No | 188(96.9) | 48(92.3) | |||
| Mealtime b | Regular | 166(85.6) | 48(92.3) | 0.293 | 0.216 |
| Irregular | 28(14.4) | 4(7.7) | |||
| Meat and vegetable pairing b | Mainly vegetarian | 40(20.6) | 10(19.2) | 0.774 | 0.105 |
| Mainly carnivorous | 7(3.6) | 3(5.8) | |||
| Equally balanced | 147(75.8) | 39(75.0) | |||
| Number of breakfasts per week a | - | 6.48(1.40) | 6.69(1.17) | 0.316 | 0.165 |
| Satiety b | Over 80% | 24(12.4) | 10(19.2) | 0.287 | 0.249 |
| 50–80% | 144(74.2) | 38(73.1) | |||
| Below 50% | 26(13.4) | 4(7.7) | |||
| History of CHM use b | Yes | 34(17.5) | 11(21.2) | 0.69 | 0.092 |
| No | 160(82.5) | 41(78.8) | |||
| BMI a | - | 24.40(4.61) | 24.95(3.30) | 0.421 | 0.137 |
| Variables | PSM | IPTW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | CAP | p | SDM | Control | CAP | p | SDM | ||
| n = 104 | n = 42 | n = 243.03 a | n = 266.42 a | ||||||
| Age b | - | 50.57 (11.11) | 51.31 (12.20) | 0.723 | 0.064 | 50.17 (12.70) | 51.23 (11.67) | 0.615 | 0.087 |
| Ethnicity c | Han | 37 (35.6) | 20 (47.6) | 0.402 | 0.246 | 89.7 (36.9) | 84.2 (31.6) | 0.722 | 0.143 |
| Uygur | 61 (58.7) | 20 (47.6) | 141.9 (58.4) | 162.8 (61.1) | |||||
| Others | 6 (5.8) | 2 (4.8) | 11.5 (4.7) | 19.3 (7.3) | |||||
| Gender c | Male | 44 (42.3) | 22 (52.4) | 0.356 | 0.203 | 100.6 (41.4) | 88.2 (33.1) | 0.298 | 0.172 |
| Female | 60 (57.7) | 20 (47.6) | 142.4 (58.6) | 178.2 (66.9) | |||||
| Smoking c | Yes | 27 (26.0) | 11 (26.2) | 1 | 0.005 | 50.9 (20.9) | 45.8 (17.2) | 0.541 | 0.095 |
| No | 77 (74.0) | 31 (73.8) | 192.2 (79.1) | 220.6 (82.8) | |||||
| Diabetes c | Yes | 5 (4.8) | 2 (4.8) | 1 | 0.002 | 14.3 (5.9) | 11.8 (4.4) | 0.588 | 0.067 |
| No | 99 (95.2) | 40 (95.2) | 228.7 (94.1) | 254.6 (95.6) | |||||
| FH of CRC c | Yes | 4 (3.8) | 0 (0.0) | 0.466 | 0.283 | 9.1 (3.7) | 7.9 (3.0) | 0.733 | 0.042 |
| No | 100 (96.2) | 42 (100.0) | 234.0 (96.3) | 258.5 (97.0) | |||||
| Mealtime c | Regular | 92 (88.5) | 38 ( 90.5) | 0.952 | 0.066 | 211.1 (86.9) | 227.2 (85.3) | 0.841 | 0.045 |
| Irregular | 12 (11.5) | 4 (9.5) | 31.9 (13.1) | 39.2 (14.7) | |||||
| Meat and vegetable pairing c | Mainly vegetarian | 19 (18.3) | 9 (21.4) | 0.838 | 0.111 | 49.6 (20.4) | 46.3 (17.4) | 0.685 | 0.123 |
| Mainly carnivorous | 4 (3.8) | 1 (2.4) | 8.5 (3.5) | 5.4 (2.0) | |||||
| Equally balanced | 81 (77.9) | 32 (76.2) | 185.0 (76.1) | 214.7 (80.6) | |||||
| Number of breakfasts per week b | - | 6.59 (1.20) | 6.62 (1.30) | 0.902 | 0.022 | 6.52 (1.34) | 6.72 (1.15) | 0.304 | 0.156 |
| Satiety c | Over 80% | 16 (15.4) | 6 (14.3) | 0.935 | 0.068 | 31.2 (12.8) | 23.3 (8.7) | 0.708 | 0.134 |
| 50–80% | 79 (76.0) | 33 (78.6) | 182.2 (75.0) | 210.6 (79.0) | |||||
| Below 50% | 9 (8.7) | 3 (7.1) | 29.7 (12.2) | 32.6 (12.2) | |||||
| History of CHM use c | Yes | 21 (20.2) | 9 (21.4) | 1 | 0.03 | 44.1 (18.1) | 45.7 (17.2) | 0.879 | 0.026 |
| No | 83 (79.8) | 33 (78.6) | 199.0 (81.9) | 220.7 (82.8) | |||||
| BMI b | - | 24.70 (4.55) | 25.09 (3.54) | 0.619 | 0.096 | 24.53 (4.56) | 24.59 (3.77) | 0.942 | 0.014 |
| Variables | OR | 95%CI | p Value |
|---|---|---|---|
| Continuous of E-DII a | 1.22 | 1.00–1.51 | 0.055 |
| Tertiles of E-DII a | |||
| T1 | 1.00 | ||
| T2 | 1.53 | 0.68–3.51 | 0.308 |
| T3 | 2.27 | 1.06–5.09 | 0.039 * |
| Continuous of E-DII b | 1.30 | 1.02–1.67 | 0.035 * |
| Tertiles of E-DII b | |||
| T1 | 1.00 | ||
| T2 | 2.15 | 0.84–5.77 | 0.116 |
| T3 | 3.07 | 1.23–8.14 | 0.019 * |
| Continuous of E-DII c | 1.40 | 1.09–1.80 | 0.009 * |
| Tertiles of E-DII c | |||
| T1 | 1.00 | ||
| T2 | 2.06 | 0.74–5.73 | 0.166 |
| T3 | 4.05 | 1.53–10.69 | 0.005 * |
| Continuous of E-DII d | 1.22 | 1.08–1.37 | 0.001 * |
| Tertiles of E-DII d | |||
| T1 | 1.00 | ||
| T2 | 2.19 | 1.38–3.51 | 0.001 * |
| T3 | 2.91 | 1.84–4.67 | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.-J.; Yusufu, W.; Zhang, S.; Luo, M.-Y.; Chen, Y.-C.; Peng, H.; Wan, X.-Y. Association between Dietary Inflammatory Index and Risk of Colorectal Adenomatous Polyps in Kashgar Prefecture of Xinjiang, China. Nutrients 2023, 15, 4067. https://doi.org/10.3390/nu15184067
He Z-J, Yusufu W, Zhang S, Luo M-Y, Chen Y-C, Peng H, Wan X-Y. Association between Dietary Inflammatory Index and Risk of Colorectal Adenomatous Polyps in Kashgar Prefecture of Xinjiang, China. Nutrients. 2023; 15(18):4067. https://doi.org/10.3390/nu15184067
Chicago/Turabian StyleHe, Zhuo-Jie, Weili Yusufu, Shuang Zhang, Min-Yi Luo, Yong-Cheng Chen, Hui Peng, and Xing-Yang Wan. 2023. "Association between Dietary Inflammatory Index and Risk of Colorectal Adenomatous Polyps in Kashgar Prefecture of Xinjiang, China" Nutrients 15, no. 18: 4067. https://doi.org/10.3390/nu15184067
APA StyleHe, Z.-J., Yusufu, W., Zhang, S., Luo, M.-Y., Chen, Y.-C., Peng, H., & Wan, X.-Y. (2023). Association between Dietary Inflammatory Index and Risk of Colorectal Adenomatous Polyps in Kashgar Prefecture of Xinjiang, China. Nutrients, 15(18), 4067. https://doi.org/10.3390/nu15184067







