Effects of Plant-Based Protein Interventions, with and without an Exercise Component, on Body Composition, Strength and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Information Source and Search Strategy
2.2. Eligibility, Inclusion, and Exclusion Criteria
2.3. Data Collection
2.4. Risk of Bias Assessment
2.5. Data Extraction
2.6. Meta-Analysis
(2 × SDpre × SDpost)
3. Results
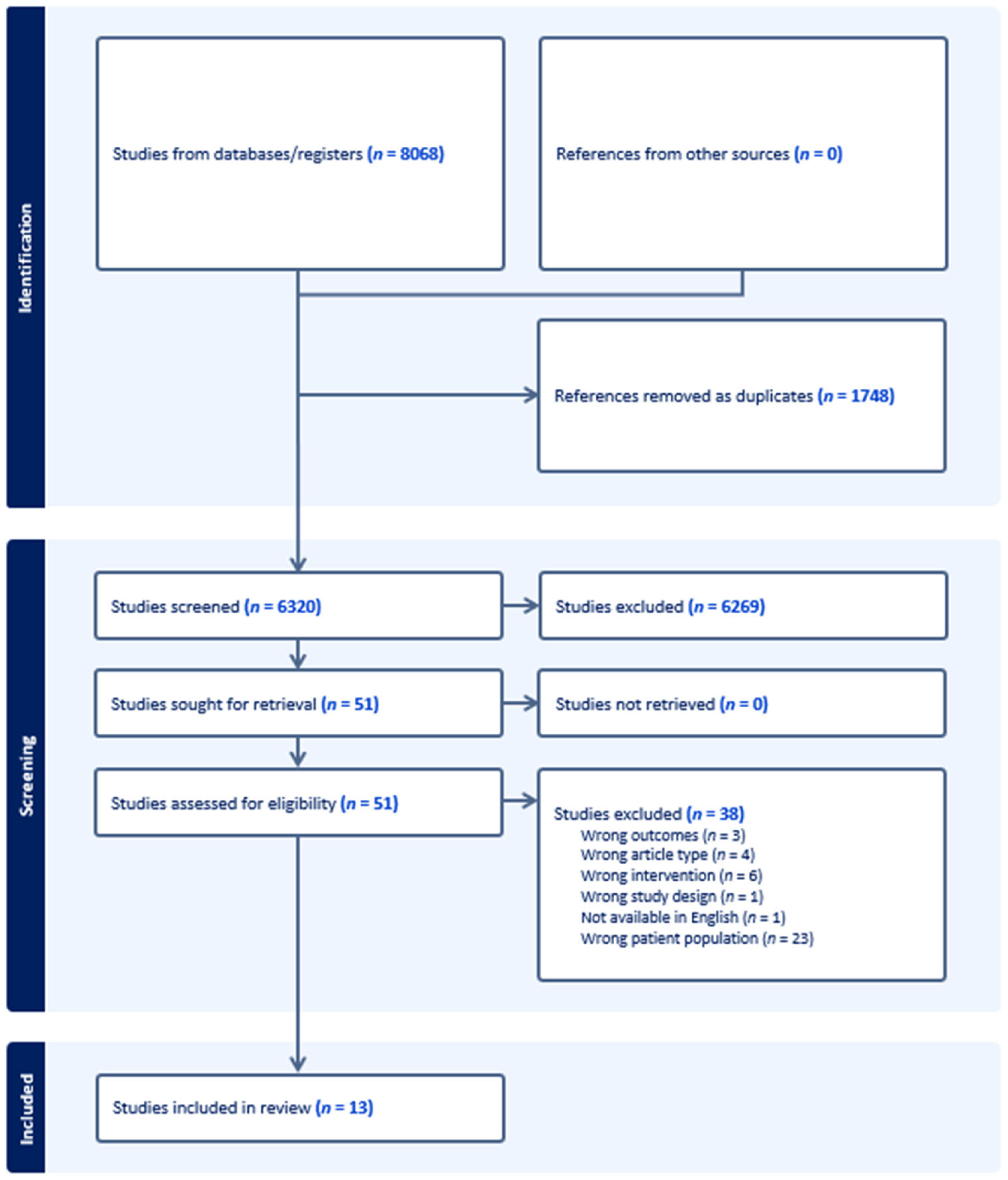
3.1. Study Selection
3.2. Study Characteristics
3.3. Intervention Characteristics
| Author, Year Published | Country | Study Design | Age | Inclusion Criteria | Exclusion Criteria | Sample Size and Sex | Quality |
|---|---|---|---|---|---|---|---|
| Bakhtiari [53] | Iran | Non-blinded RCT | 60–70 | Metabolic Syndrome defined as ≥3 of following: waist circumference >80 cm; serum HDL-C < 50 mg/dL; triglyceride ≥150 mg/dL; fasting blood glucose ≥100 mg/dL; and systolic blood pressure ≥130 mmHg and diastolic ≥85 mmHg) | Medication for diabetes, hypertension, hyperlipidaemia; estrogen therapy; soy consumption; history of CVD; thyroid condition; kidney or liver conditions; infectious disease; cancer; vegetarian; smokers or soy allergy | 75♀ | + |
| Beavers [49] | United States | Single-blind RCT | 60–79 | BMI ≥ 27 kg/m2, waist circumference ≥102 cm ♂ and 88 cm ♀, willing to consume prepared meals and meal replacement products; and no contraindications for participation in a weight loss program | Weight change (±5%) in the past 6 months; body mass >136 kg; regular smoker; alcohol or substance abuse ≤2 years; insulin-dependent or uncontrolled diabetes; abnormal kidney or liver function; past or current ischemic heart disease; uncontrolled blood pressure (>160/90 mmHg), pulmonary disease; thyroid disease; known significant haematological disease; cancer requiring treatment in past year, or life expectancy <2 years; and regular use of any medications that could influence study variables (growth/steroid hormones, including estrogen replacements, thiazolidinediones, statins, regular anti-inflammatory medications, blood thinners, or weight loss medications) | 21♀ 3♂ | + |
| Bijeh [54] | Iran | Double-blind RCT | 60–80 | Physically independent | CVD, neurological, respiratory, muscular, metabolic, inflammatory, bone problems, joints, and movement disorders; consuming nutritional supplements; consuming drugs affecting muscle metabolism; consuming alcohol or smoking ≥1 year; soy milk allergy/sensitivity, and history of regular physical activity ≥1 year. | 60♂ | + |
| Haub [45,47] | United States | Non-blinded RCT | 65 ± 5 | Medical conditions that might place them at risk if they participated in the study | 21♂ | + (2002), Ø (2005) | |
| Imaoka [51] | Japan | Non-blinded RCT | ≥60 | Community dwelling, physically independent | Collagen disease; depression; CVD; medical contraindications to exercise; or Parkinson’s disease | 61♀ 13♂ | + |
| Imaoka [50] | Japan | Non-blinded RCT | ≥60 | Community dwelling, physically independent | Doctors’ orders to stop exercise, medicalcontraindications to exercise; dementia | 59♀ 13♂ | + |
| Kenny [48] | United States | Double-blind RCT | ≥60 | Diseases that could affect bone metabolism (Paget’s disease, thyroid conditions, osteomalacia, multiple myeloma); cancer ≤5 years; calcitonin, calcitriol, heparin, phenytoin, or phenobarbital use ≤2 years; bisphosphonates or corticosteroid use ≥6 months; methotrexate or fluoride use; creatinine clearance <50 mL/min; liver disease; history of hip fracture; known vertebral fracture ≤1 year; and vegan | 131♀ | + | |
| Kok [44] Kreijkamp-Kaspers [46] | Netherlands | Double-blind RCT | 60–75 | Normal mammography ≤1 year | Liver disease; renal disease; thrombosis; malignant disease; hormone replacement therapy ≤6 months; soy or casein allergy; lactose intolerance; endometrium thickness over 4 mm | 202♀ | Ø (2005), + (2004) |
| Li [55] | China | Double-blind RCT | ≥65 | Low appendicular skeletal muscle mass index (♂ < 7.0 kg/m2, ♀ < 5.4 kg/m2) | Diseases with impaired movement (stroke, fracture, and arthritis); kidney disease; nervous system disease; joint replacement; musculoskeletal injuries; whey or soy allergy; supplement use ≤1 year; and unwillingness to adhere to the study protocol | 62♀ 61♂ | + |
| Matsuda [52] | Japan | Single-blind RCT | 65–80 | HbA1c 6.5 to <8.5%; HbA1c change of ≤1.0% ≤6 months | Diabetes other than T2DM; receiving insulin, growth hormone, glucocorticoids, or anabolic steroids; eGFR < 30 mL/min/1.73 m2; proliferative retinopathy; contraindication to exercise due to bone and joint disease; current treatment for malignancy | 13♀ 23♂ | + |
| Roschel [56] | Brazil | Double-blind RCT | >65 years old | Pre-frail or frail based on Fried’s criteria—unintentional weight loss, weakness, self-reported exhaustion, slow walking speed, and low physical activity | Insulin or steroid-based drugs; protein supplements; caloric or food restriction; resistance training; untreated chronic disease or any musculoskeletal condition contraindicated for exercising | 60♀ | + |
3.4. Outcomes of Plant Protein Interventions on Body Composition, Strength, and Physical Function
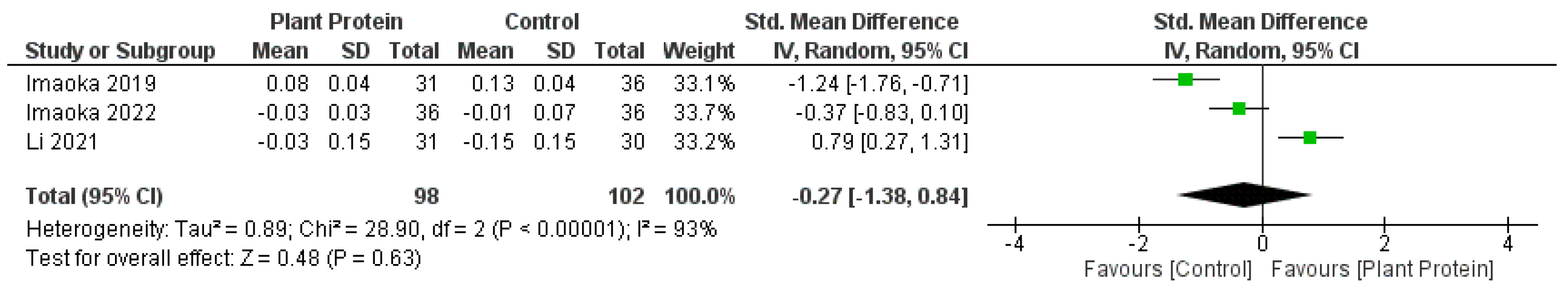
3.5. Meta-Analyses for the Effect of Plant Protein Interventions on Body Composition, Strength, and Physical Function
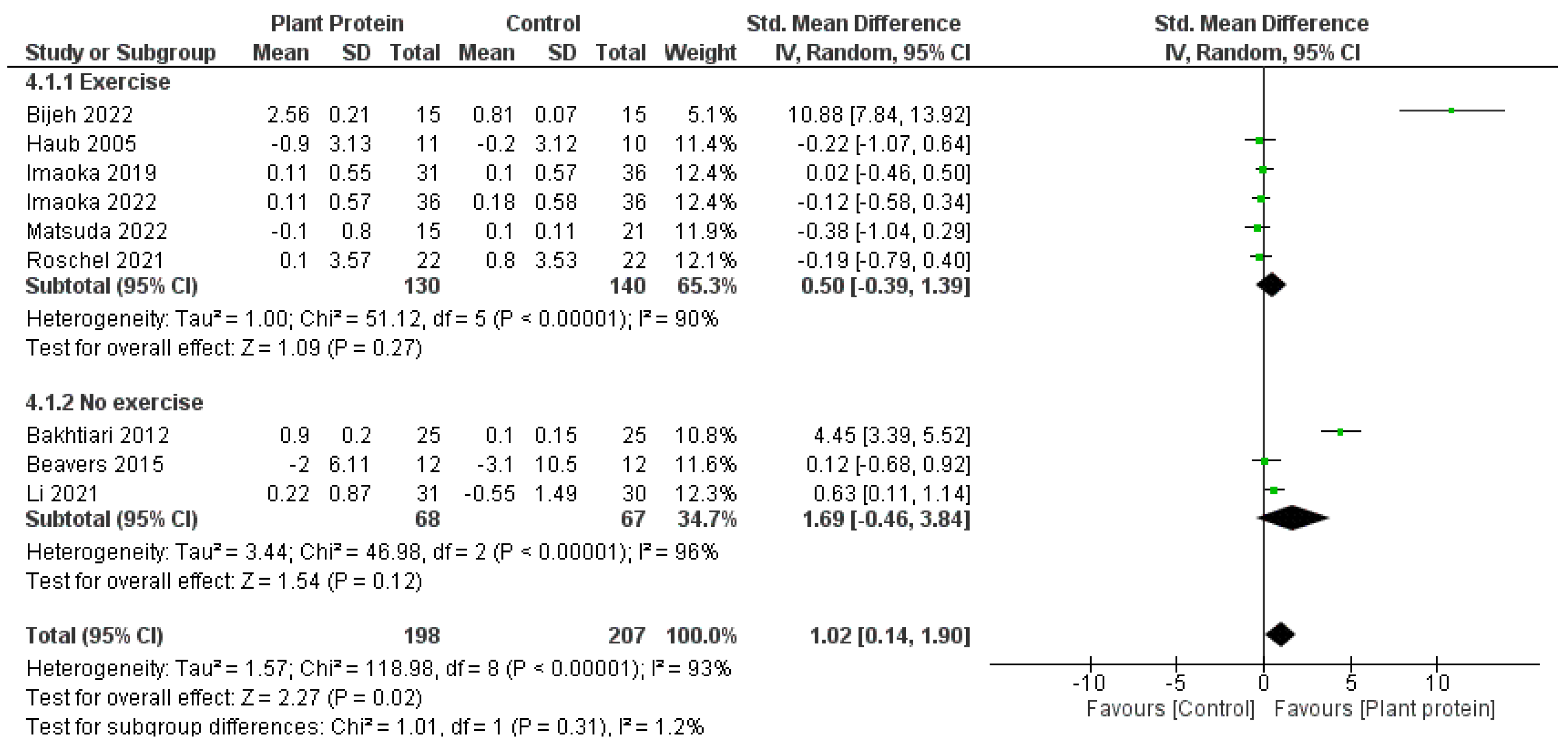
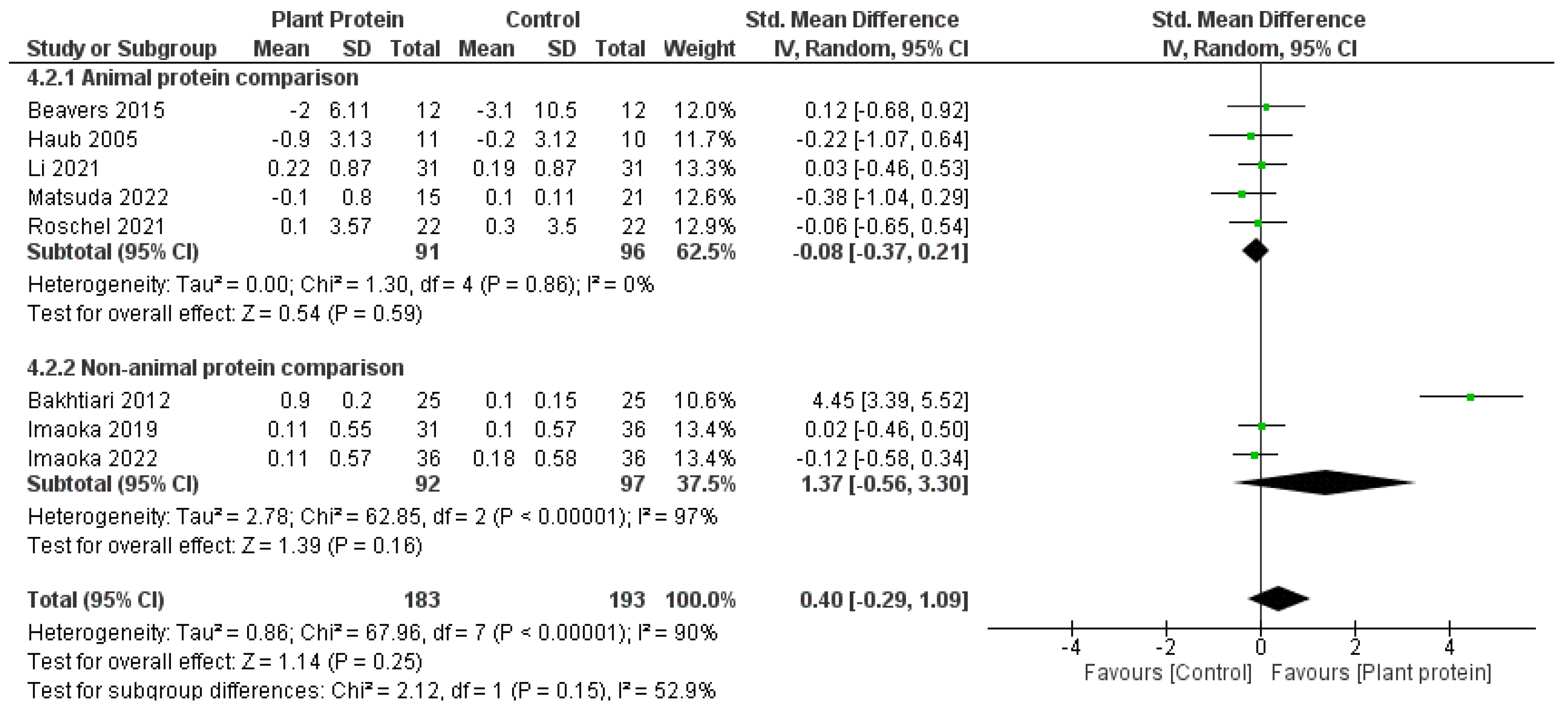
3.6. Subgroup Meta-Analyses
3.7. Sensitivity Analysis and Publication Bias
4. Discussion
4.1. Plant-Based Proteins and Body Composition Outcomes
4.2. Plant-Based Proteins and Strength and Physical Function Outcomes
4.3. Plant-Based Proteins in Combination with Exercise
4.4. Plant-Based Proteins and Dietary Patterns
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kyle, U.G.; Genton, L.; Hans, D.; Karsegard, L.; Slosman, D.O.; Pichard, C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur. J. Clin. Nutr. 2001, 55, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Mendes, A.F. Physiology and pathophysiology of musculoskeletal aging: Current research trends and future priorities. Front. Physiol. 2013, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznaric, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Zaaria, M.; Pasleau, F.; Reginster, J.Y.; Bruyere, O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0169548. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef]
- Beckwée, D.; Delaere, A.; Aelbrecht, S.; Baert, V.; Beaudart, C.; Bruyere, O.; de Saint-Hubert, M.; Bautmans, I. Exercise Interventions for the Prevention and Treatment of Sarcopenia. A Systematic Umbrella Review. J. Nutr. Health Aging 2019, 23, 494–502. [Google Scholar] [CrossRef]
- Beaudart, C.; Dawson, A.; Shaw, S.C.; Harvey, N.C.; Kanis, J.A.; Binkley, N.; Reginster, J.Y.; Chapurlat, R.; Chan, D.C.; Bruyere, O.; et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: Systematic review. Osteoporos. Int. 2017, 28, 1817–1833. [Google Scholar] [CrossRef]
- Cheng, H.; Kong, J.; Underwood, C.; Petocz, P.; Hirani, V.; Dawson, B.; O’Leary, F. Systematic review and meta-analysis of the effect of protein and amino acid supplements in older adults with acute or chronic conditions. Br. J. Nutr. 2018, 119, 527–542. [Google Scholar] [CrossRef]
- Colonetti, T.; Grande, A.J.; Milton, K.; Foster, C.; Alexandre, M.C.; Uggioni, M.L.; Rosa, M.I. Effects of whey protein supplement in the elderly submitted to resistance training: Systematic review and meta-analysis. Int. J. Food Sci. Nutr. 2017, 68, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Finger, D.; Goltz, F.R.; Umpierre, D.; Meyer, E.; Rosa, L.H.; Schneider, C.D. Effects of protein supplementation in older adults undergoing resistance training: A systematic review and meta-analysis. Sports Med. 2015, 45, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Lei, Y.; Li, X.; Huo, C.; Jia, X.; Yang, J.; Xu, R.; Wang, X. Effect of Protein Supplementation Combined with Resistance Training on Muscle Mass, Strength and Function in the Elderly: A Systematic Review and Meta-Analysis. J. Nutr. Health Aging 2019, 23, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; Bergia, R.E., 3rd; Campbell, W.W. Effects of protein supplements consumed with meals, versus between meals, on resistance training-induced body composition changes in adults: A systematic review. Nutr. Rev. 2018, 76, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Calicioglu, O.; Flammini, A.; Bracco, S.; Bellù, L.; Sims, R. The Future Challenges of Food and Agriculture: An Integrated Analysis of Trends and Solutions. Sustainability 2019, 11, 222. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Pereira, R.N.; Pinheiro, A.C.; Martins, J.T.; Andrade, C.C.P.; Ramos, O.L.; Vicente, A.A. Emergent food proteins—Towards sustainability, health and innovation. Food Res. Int. 2019, 125, 108586. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.Y.J.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant Proteins for Future Foods: A Roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef]
- Shepon, A.; Eshel, G.; Noor, E.; Milo, R. The opportunity cost of animal based diets exceeds all food losses. Proc. Natl. Acad. Sci. USA 2018, 115, 3804–3809. [Google Scholar] [CrossRef]
- Lappi, J.; Silventoinen-Veijalainen, P.; Vanhatalo, S.; Rosa-Sibakov, N.; Sozer, N. The nutritional quality of animal-alternative processed foods based on plant or microbial proteins and the role of the food matrix. Trends Food Sci. Technol. 2022, 129, 144–154. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Berrazaga, I.; Micard, V.; Gueugneau, M.; Walrand, S. The Role of the Anabolic Properties of Plant- versus Animal-Based Protein Sources in Supporting Muscle Mass Maintenance: A Critical Review. Nutrients 2019, 11, 1825. [Google Scholar] [CrossRef] [PubMed]
- Nichele, S.; Phillips, S.M.; Boaventura, B.C.B. Plant-based food patterns to stimulate muscle protein synthesis and support muscle mass in humans: A narrative review. Appl. Physiol. Nutr. Metab. 2022, 47, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Putra, C.; Konow, N.; Gage, M.; York, C.G.; Mangano, K.M. Protein Source and Muscle Health in Older Adults: A Literature Review. Nutrients 2021, 13, 743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Hughes, J.; Grafenauer, S. Got Mylk? The Emerging Role of Australian Plant-Based Milk Alternatives as a Cow’s Milk Substitute. Nutrients 2020, 12, 1254. [Google Scholar] [CrossRef] [PubMed]
- Marzani, B.; Balage, M.; Venien, A.; Astruc, T.; Papet, I.; Dardevet, D.; Mosoni, L. Antioxidant supplementation restores defective leucine stimulation of protein synthesis in skeletal muscle from old rats. J. Nutr. 2008, 138, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E. Anorexia of aging: A true geriatric syndrome. J. Nutr. Health Aging 2012, 16, 422–425. [Google Scholar] [CrossRef]
- Gueugneau, M. The value of dietary plant protein in older people. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 3–7. [Google Scholar] [CrossRef]
- Draganidis, D.; Karagounis, L.G.; Athanailidis, I.; Chatzinikolaou, A.; Jamurtas, A.Z.; Fatouros, I.G. Inflammaging and Skeletal Muscle: Can Protein Intake Make a Difference? J. Nutr. 2016, 146, 1940–1952. [Google Scholar] [CrossRef]
- Messina, M.; Lynch, H.; Dickinson, J.M.; Reed, K.E. No Difference Between the Effects of Supplementing with Soy Protein Versus Animal Protein on Gains in Muscle Mass and Strength in Response to Resistance Exercise. Int. J. Sport. Nutr. Exerc. Metab. 2018, 28, 674–685. [Google Scholar] [CrossRef]
- Lim, M.T.; Pan, B.J.; Toh, D.W.; Sutanto, C.N.; Kim, J.E. Animal Protein versus Plant Protein in Supporting Lean Mass and Muscle Strength: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 661. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Tang, J.E.; Moore, D.R. The Role of Milk- and Soy-Based Protein in Support of Muscle Protein Synthesis and Muscle Protein Accretion in Young and Elderly Persons. J. Am. Coll. Nutr. 2009, 28, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Nasimi, N.; Sohrabi, Z.; Nunes, E.A.; Sadeghi, E.; Jamshidi, S.; Gholami, Z.; Akbarzadeh, M.; Faghih, S.; Akhlaghi, M.; Phillips, S.M. Whey Protein Supplementation with or without Vitamin D on Sarcopenia-Related Measures: A Systematic Review and Meta-Analysis. Adv. Nutr. 2023, 14, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M.S.; Rachubińska, K.; Grochans, S.; Skonieczna-Żydecka, K.; Cybulska, A.M.; Grochans, E.; Karakiewicz, B. The Impact of Whey Protein Supplementation on Sarcopenia Progression among the Elderly: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2039. [Google Scholar] [CrossRef] [PubMed]
- Cuyul-Vásquez, I.; Pezo-Navarrete, J.; Vargas-Arriagada, C.; Ortega-Díaz, C.; Sepúlveda-Loyola, W.; Hirabara, S.M.; Marzuca-Nassr, G.N. Effectiveness of Whey Protein Supplementation during Resistance Exercise Training on Skeletal Muscle Mass and Strength in Older People with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3424. [Google Scholar] [CrossRef] [PubMed]
- Messina, M. A brief historical overview of the past two decades of soy and isoflavone research. J. Nutr. 2010, 140, 1350s–1354s. [Google Scholar] [CrossRef] [PubMed]
- Curtain, F.; Grafenauer, S. Plant-Based Meat Substitutes in the Flexitarian Age: An Audit of Products on Supermarket Shelves. Nutrients 2019, 11, 2603. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- American Dietetic Association. ADA Evidence Analysis Manual, 4th ed.; American Dietetic Association: Chicago, IL, USA, 2003. [Google Scholar]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Chapter 13: Fixed-Effect Versus Random-Effects Models. In Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 77–86. [Google Scholar]
- Higgins, J.P.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions, 6.2th ed.; The Cochrane Collaboration: London, UK, 2021. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Oxfordshire, UK, 2013. [Google Scholar]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, 6.2th ed.; Iggins, J.P.T.T.J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; The Cochrane Collaboration: London, UK, 2021. [Google Scholar]
- Kok, L.; Kreijkamp-Kaspers, S.; Grobbee, D.E.; Lampe, J.W.; van der Schouw, Y.T. Soy isoflavones, body composition, and physical performance. Maturitas 2005, 52, 102–110. [Google Scholar] [CrossRef]
- Haub, M.D.; Wells, A.M.; Tarnopolsky, M.A.; Campbell, W.W. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am. J. Clin. Nutr. 2002, 76, 511–517. [Google Scholar] [CrossRef]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; De Haan, E.H.F.; Aleman, A.; Lampe, J.W.; Van Der Schouw, Y.T. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipilds in postmenopausal women: A randomized controlled trial. JAMA 2004, 292, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Haub, M.D.; Wells, A.M.; Campbell, W.W. Beef and soy-based food supplements differentially affect serum lipoprotein-lipid profiles because of changes in carbohydrate intake and novel nutrient intake ratios in older men who resistive-train. Metab. Clin. Exp. 2005, 54, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; Anamani, D.E.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; Kerstetter, J.E. Soy proteins and isoflavones affect bone mineral density in older women: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Gordon, M.M.; Easter, L.; Beavers, D.P.; Hairston, K.G.; Nicklas, B.J.; Vitolins, M.Z. Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: A pilot feeding study. J. Nutr. Health Aging 2015, 19, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, M.; Nakao, H.; Nakamura, M.; Tazaki, F.; Hida, M.; Imai, R.; Maebuchi, M.; Ibuki, M.; Takeda, M. Improvement of memory function via a combination of exercise and soy peptide supplementation in community-dwelling older adults: A randomized controlled trial. Contemp. Clin. Trials Commun. 2022, 30, 100998. [Google Scholar] [CrossRef] [PubMed]
- Imaoka, M.; Nakao, H.; Nakamura, M.; Tazaki, F.; Maebuchi, M.; Ibuki, M.; Takeda, M. Effect of multicomponent exercise and nutrition support on the cognitive function of older adults: A randomized controlled trial. Clin. Interv. Aging 2019, 14, 2145–2153. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Suzuki, H.; Sugano, Y.; Suzuki, Y.; Yamanaka, D.; Araki, R.; Yahagi, N.; Sekiya, M.; Kawakami, Y.; Osaki, Y.; et al. Effects of Branched-Chain Amino Acids on Skeletal Muscle, Glycemic Control, and Neuropsychological Performance in Elderly Persons with Type 2 Diabetes Mellitus: An Exploratory Randomized Controlled Trial. Nutrients 2022, 14, 3917. [Google Scholar] [CrossRef]
- Bakhtiari, A.; Yassin, Z.; Hanachi, P.; Rahmat, A.; Ahmad, Z.; Sajadi, P.; Shojaei, S. Effects of Soy on Body Composition: A 12-Week Randomized Controlled Trial among Iranian Elderly Women with Metabolic Syndrome. Iran. J. Public Health 2012, 41, 9–18. [Google Scholar]
- Bijeh, N.; Mohammadnia-Ahmadi, M.; Hooshamnd-Moghadam, B.; Eskandari, M.; Golestani, F. Effects of Soy Milk in Conjunction With Resistance Training on Physical Performance and Skeletal Muscle Regulatory Markers in Older Men. Biol. Res. Nurs. 2022, 24, 294–307. [Google Scholar] [CrossRef]
- Li, C.; Meng, H.; Wu, S.; Fang, A.; Liao, G.; Tan, X.; Chen, P.; Wang, X.; Chen, S.; Zhu, H. Daily Supplementation with Whey, Soy, or Whey-Soy Blended Protein for 6 Months Maintained Lean Muscle Mass and Physical Performance in Older Adults With Low Lean Mass. J. Acad. Nutr. Diet. 2021, 121, 1035–1048. [Google Scholar] [CrossRef]
- Roschel, H.; Hayashi, A.P.; Fernandes, A.L.; Jambassi-Filho, J.C.; Hevia-Larraín, V.; de Capitani, M.; Santana, D.A.; Gonçalves, L.S.; de Sá-Pinto, A.L.; Lima, F.R.; et al. Supplement-based nutritional strategies to tackle frailty: A multifactorial, double-blind, randomized placebo-controlled trial. Clin. Nutr. 2021, 40, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R. Rise in Vegetarianism Not Halting the March of Obesity. Available online: https://www.roymorgan.com/findings/rise-in-vegetarianism-not-halting-the-march-of-obesity (accessed on 18 June 2023).
- Burd, N.A.; Gorissen, S.H.; van Loon, L.J. Anabolic resistance of muscle protein synthesis with aging. Exerc. Sport Sci. Rev. 2013, 41, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Genton, L.; Slosman, D.O.; Pichard, C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition 2001, 17, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Douchi, T.; Yamamoto, S.; Yoshimitsu, N.; Andoh, T.; Matsuo, T.; Nagata, Y. Relative contribution of aging and menopause to changes in lean and fat mass in segmental regions. Maturitas 2002, 42, 301–306. [Google Scholar] [CrossRef]
- Sedlmeier, A.M.; Baumeister, S.E.; Weber, A.; Fischer, B.; Thorand, B.; Ittermann, T.; Dörr, M.; Felix, S.B.; Völzke, H.; Peters, A.; et al. Relation of body fat mass and fat-free mass to total mortality: Results from 7 prospective cohort studies. Am. J. Clin. Nutr. 2021, 113, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Koster, A.; Ding, J.; Stenholm, S.; Caserotti, P.; Houston, D.K.; Nicklas, B.J.; You, T.; Lee, J.S.; Visser, M.; Newman, A.B.; et al. Does the Amount of Fat Mass Predict Age-Related Loss of Lean Mass, Muscle Strength, and Muscle Quality in Older Adults? J. Gerontol. Ser. A 2011, 66A, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Bendsen, N.T.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21, B16–B31. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The Effects of High Protein Diets on Thermogenesis, Satiety and Weight Loss: A Critical Review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Chung, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Shi, J.; Wallace, T.C.; et al. Animal versus plant protein and adult bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. PLoS ONE 2018, 13, e0192459. [Google Scholar] [CrossRef]
- Groenendijk, I.; Grootswagers, P.; Santoro, A.; Franceschi, C.; Bazzocchi, A.; Meunier, N.; Caille, A.; Malpuech-Brugere, C.; Bialecka-Debek, A.; Pietruszka, B.; et al. Protein intake and bone mineral density: Cross-sectional relationship and longitudinal effects in older adults. J. Cachexia Sarcopenia Muscle 2023, 14, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Miguel-Berges, M.L.; Gómez-Bruton, A.; Moreno, L.A.; Julián, C. Veganism, vegetarianism, bone mineral density, and fracture risk: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, S.T.; Päivärinta, E.; Pellinen, T.; Viitakangas, H.; Risteli, J.; Erkkola, M.; Lamberg-Allardt, C.; Pajari, A.-M. Partial Replacement of Animal Proteins with Plant Proteins for 12 Weeks Accelerates Bone Turnover Among Healthy Adults: A Randomized Clinical Trial. J. Nutr. 2021, 151, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lee, S.-K.; Chun, O.K. Soy Isoflavones and Osteoporotic Bone Loss: A Review with an Emphasis on Modulation of Bone Remodeling. J. Med. Food 2015, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bailón-Uriza, R.; Ayala-Méndez, J.A.; Celis-González, C.; Chávez-Brambila, J.; Hernández Marín, I.; Maldonado-Alvarado, J.d.D.; Montoya-Cossío, J.; Molina-Segui, F.; May-Hau, A.; Riobó Serván, P.; et al. Soy beverages and women’s health: Evidence review and experts opinion. Nutr. Hosp. 2023. [Google Scholar] [CrossRef]
- Hernández, A.; Cheng, A.; Westerblad, H. Antioxidants and Skeletal Muscle Performance: “Common Knowledge” vs. Experimental Evidence. Front. Physiol. 2012, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy Aging and Dietary Patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Tosato, M.; Landi, F.; Picca, A.; Marzetti, E. Protein intake and physical function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 81, 101731. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. Clinical effectiveness of protein and amino acid supplementation on building muscle mass in elderly people: A meta-analysis. PLoS ONE 2014, 9, e109141. [Google Scholar] [CrossRef]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Exercise Physiology: Nutrition, Energy, and Human Performance; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian Adult Soy Protein and Isoflavone Intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tsugane, S. Why has Japan become the world’s most long-lived country: Insights from a food and nutrition perspective. Eur. J. Clin. Nutr. 2021, 75, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Buoite Stella, A.; Gortan Cappellari, G.; Barazzoni, R.; Zanetti, M. Update on the Impact of Omega 3 Fatty Acids on Inflammation, Insulin Resistance and Sarcopenia: A Review. Int. J. Mol. Sci. 2018, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Luk, H.-Y.; Appell, C.; Chyu, M.-C.; Chen, C.-H.; Wang, C.-Y.; Yang, R.-S.; Shen, C.-L. Impacts of Green Tea on Joint and Skeletal Muscle Health: Prospects of Translational Nutrition. Antioxidants 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.Q.; Xiao, W.F.; Tang, K.; Wu, Y.X.; Hu, P.W.; Li, Y.S.; Duan, Y.; Lv, S. Caloric restriction: Implications for sarcopenia and potential mechanisms. Aging 2020, 12, 24441–24452. [Google Scholar] [CrossRef] [PubMed]
- Besora-Moreno, M.; Llauradó, E.; Valls, R.M.; Tarro, L.; Pedret, A.; Solà, R. Antioxidant-rich foods, antioxidant supplements, and sarcopenia in old-young adults ≥55 years old: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin. Nutr. 2022, 41, 2308–2324. [Google Scholar] [CrossRef]
- Nazri, N.S.M.; Vanoh, D.; Soo, K.L. Natural Food for Sarcopenia: A Narrative Review. Malays. J. Med. Sci. 2022, 29, 28–42. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Robinson, S.M. Dietary Patterns, Skeletal Muscle Health, and Sarcopenia in Older Adults. Nutrients 2019, 11, 745. [Google Scholar] [CrossRef]
- Boushey, C.; Ard, J.; Bazzano, L.; Heymsfield, S.; Mayer-Davis, E.; Sabaté, J.; Snetselaar, L.; Van Horn, L.; Schneeman, B.; English, L.K.; et al. Dietary Patterns and Sarcopenia: A Systematic Review. U.S. Department of Agriculture, Food and Nutrition Service, Center for Nutrition Policy and Promotion, Nutrition Evidence Systematic Review. 2020. Available online: https://nesr.usda.gov/sites/default/files/2022-07/DP-dietary-patterns-sarcopenia-full-SR.pdf (accessed on 18 June 2023).
- Doleman, B.; Mathiesen, O.; Jakobsen, J.C.; Sutton, A.J.; Freeman, S.; Lund, J.N.; Williams, J.P. Methodologies for systematic reviews with meta-analysis of randomised clinical trials in pain, anaesthesia, and perioperative medicine. Br. J. Anaesth. 2021, 126, 903–911. [Google Scholar] [CrossRef]
- Owens, J.K. Systematic reviews: Brief overview of methods, limitations, and resources. Nurse Author Ed. 2021, 31, 69–72. [Google Scholar] [CrossRef]



| Search Line | Search Terms |
|---|---|
| 1 | SARCOPENIA/ |
| 2 | AGED/ |
| 3 | AGING/ |
| 4 | “Aged, 80 and over” |
| 5 | FRAIL ELDERLY/ |
| 6 | FRAILTY/ |
| 7 | (dynapen* OR anabolic resistance).mp. |
| 8 | (older adult OR older OR senior OR elder OR elderly OR ?enarian OR geriatric).mp. |
| 9 | (sarcop?eni* OR aged OR aging OR frail elderly OR frailty).tw. |
| 10 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 |
| 11 | Plant Proteins/ |
| 12 | Vegetable Proteins/ |
| 13 | Soybean Proteins/ |
| 14 | (plant protein* OR vegetable protein* OR soybean protein* OR plant-base*).tw. |
| 15 | Soy milk/ |
| 16 | soy milk.tw. |
| 17 | 11 OR 12 OR 13 OR 14 OR 15 OR 16 |
| 18 | animals/NOT (humans/AND animals/) |
| 19 | 10 AND 17 |
| 20 | 19 AND 18 |
| PICO Component | Inclusion Criteria |
|---|---|
| Participants | Community dwelling older adults of either sex, ≥60 years old |
| Intervention | Plant-based protein interventions, with or without an exercise component, at least 6 weeks in duration. Supplements or whole foods that can clearly be attributed to plant sources can be included. Any protein sources of unclear origin (e.g., isolated amino acids) will be excluded. Any exercise intervention can be included (aerobic training, resistance training, combined, etc.) Setting: gym facility or home-based interventions, with any supervision type (face to face training, online supervision, no supervision at all, etc.) |
| Controls | Placebo interventions (with or without an exercise component). This may include non-protein dietary interventions or animal-based protein interventions. Exercise only controls. |
| Outcomes | Body composition: lean muscle mass, appendicular muscle mass, fat mass, bone density or bone content (e.g., dual energy X-ray absorptiometry—DEXA, bioelectrical impedance analysis—BIA, computer tomography—CT, or air displacement plethysmography—BodPod). Strength: Grip strength (e.g., dynamometer), knee extension strength (e.g., dynamometer), thirty second sit-to-stand, 5 chair stand test. Function: gait speed (e.g., 3–10 m walk tests, 400 m walk test), short physical performance battery, timed up and go. |
| Study | Duration | Plant Protein Intervention Protein Type Protein Dose/Serve Frequency Total Daily Dose * Exercise Component | Comparison Protein Type Protein Dose/Serve Frequency Total Daily Dose * Exercise Component | Narrative Summary Body Composition | Narrative Summary Strength | Narrative Summary Physical Function |
|---|---|---|---|---|---|---|
| Bakhtiari [53] | 12 weeks | Soy nut 13.8 g 1/day 13.8 g/day | (1) Control (nothing) (2) Textured soy protein 18.2 g/day | Mild positive effect of both soy groups but not significant between groups. Lean mass (BIA) increased in soy nut group compared to control, and both soy groups decreased fat mass (BIA) over time. | ||
| Beavers [49] | 12 weeks | Soy protein meal replacement products 11–15 g 4/day 44–60 g/day | (1) Non-soy (whey and egg) meal replacement products 11–15 g 4/day 44–60 g/day | Lean mass, fat mass (DEXA) reduced in both groups, no between group interactions. | Knee extensor strength (isokinetic dynamometer) significantly reduced in both groups. Grip strength (dynamometer) did not change over time in either group. | Gait speed (400 m walk time) and SPPB did not change over time in either group. |
| Bijeh [54] | 12 weeks | Soy milk 6.75 g 1/day 6.75 g/day RT 3/week | (1) RT 3/week (2) RT + Soy milk 6.75 g/day RT 3/week (3) Control | Muscle mass (BIA) increased in RT and RT+ soy group over time. Significant group and time effect interaction for fat mass (BIA). No change in control group over time. | Significant group and time effect interaction for grip strength (dynamometer), with RT + soy milk group performing best. | |
| Haub [45,47] | 12 weeks | Textured vegetable protein products (soy) 0.6 g/kg/day RT 3/week | (1) Beef foods + RT 0.6 g/kg/day RT 3/week | No overall response in either group for muscle mass or fat mass (BodPod). Mid-thigh muscle (CT) increased in both groups over time. No significant differences between groups. | Knee extension strength (pneumatically adjusted leg extension machine) increased in both groups, no significant differences between groups. | |
| Imaoka [51] | 3 months | Soy peptide drink 4.4 g 1/week 0.6 g/day AE 1/week | (1) AE 1/week | Skeletal muscle (BIA) improved in both groups over time, with no group interaction. | Grip strength (dynamometer) improved in both groups over time, with no group interaction. | Gait speed (2.4 m walk test) improved in both groups over time, with no group interaction. |
| Imaoka [50] | 3 months | Soy peptide drink 4.4 g 1/week 0.6 g/day AE 1/week | (1) AE 1/week | Skeletal muscle (BIA) improved in both groups over time, with no group interaction. | No group or time effects for grip strength (dynamometer). | No time or group effects for gait speed (2.4 m walk test). |
| Kenny [48] | 1 year | Soy protein isolate (placebo isoflavone tablets) 18 g 1/day 18 g/day | (1) Control protein: casein (50%), whey (25%) and egg white (25%) isolate + placebo isoflavone tablets 18 g/day (2) Soy protein + isoflavone tablets (3) Control protein + isoflavone tablets | No group or time effects for BMD (DEXA). | ||
| Kok [44] Kreijkamp-Kaspers [46] | 1 year | Soy protein 25.6 g 1/day 25.6 g/day | (1) Milk protein 25.6 g 1/day 25.6 g/day | Both groups decreased BMD (DEXA) after a year. Hip (intertrochanter region), had significant difference between groups with increase in soy group, reduction in control group. No other differences in other hip regions or spine. | No significant difference between groups, however hand grip (dynamometer) not measured at baseline. Results adjusted by baseline age, BMI, past use of HRT, postmenopausal years, fertile years, and height did not impact results. | Slight increases in SPPB score in both groups, no significant differences between groups. |
| Li [55] | 6 months | Soy protein 8.80 g 2/day 17.6 g/day | (1) Whey Protein 7.89 g 2/day 15.78 g/day (2) Combined whey-soy blend 8.39 g 2/day 16.78 g/day (3) control | ASMMI, lean mass in legs (DEXA) maintained in the supplement groups compared to control which decreased from baseline. No significant differences between protein groups. | No change in hand grip strength (dynamometer), no significant difference between all 4 groups. | SPPB and gait speed (4 m walk test) maintained in protein groups, decreased in control group over time. The 5 chair stand test component increased in time taken for control group but decreased for all protein groups. No differences between protein groups. |
| Matsuda [52] | 24 weeks | Soy protein drink 7.5 g 1/day 7.5 g/day RT + AE 3/week | (1) BCAA 8 g 1/day 8 g/day RT + AE 3/week | Skeletal muscle mass (BIA) did not change over time or between groups | Knee extension strength (dynamometer) significantly improved in the soy group but not in the BCAA group. No significant differences between groups. Grip strength (dynamometer) improved in the BCAA group not soy, but no significant differences between groups. | |
| Roschel [56] | 16 weeks | Soy protein 15 g 2/day 30 g/day RT 2/week | (1) Whey protein 15 g 2/day 30 g/day RT 2/week (2) Corn Starch 15 g 2/day 30 g/day RT 2/week | Total and ASMM (DEXA) improved over time but no significant differences between groups. Total fat mass (DEXA) did not change throughout trial in either group. | Hand grip strength (dynamometer) did not change over time in either group, with no significant group differences. | TUG did not change over time or between groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoodley, I.L.; Williams, L.M.; Wood, L.G. Effects of Plant-Based Protein Interventions, with and without an Exercise Component, on Body Composition, Strength and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 4060. https://doi.org/10.3390/nu15184060
Stoodley IL, Williams LM, Wood LG. Effects of Plant-Based Protein Interventions, with and without an Exercise Component, on Body Composition, Strength and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2023; 15(18):4060. https://doi.org/10.3390/nu15184060
Chicago/Turabian StyleStoodley, Isobel L., Lily M. Williams, and Lisa G. Wood. 2023. "Effects of Plant-Based Protein Interventions, with and without an Exercise Component, on Body Composition, Strength and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Nutrients 15, no. 18: 4060. https://doi.org/10.3390/nu15184060
APA StyleStoodley, I. L., Williams, L. M., & Wood, L. G. (2023). Effects of Plant-Based Protein Interventions, with and without an Exercise Component, on Body Composition, Strength and Physical Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients, 15(18), 4060. https://doi.org/10.3390/nu15184060






