Association of Vegetarian and Vegan Diets with Cardiovascular Health: An Umbrella Review of Meta-Analysis of Observational Studies and Randomized Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Information Source
2.2. Eligibility Criteria
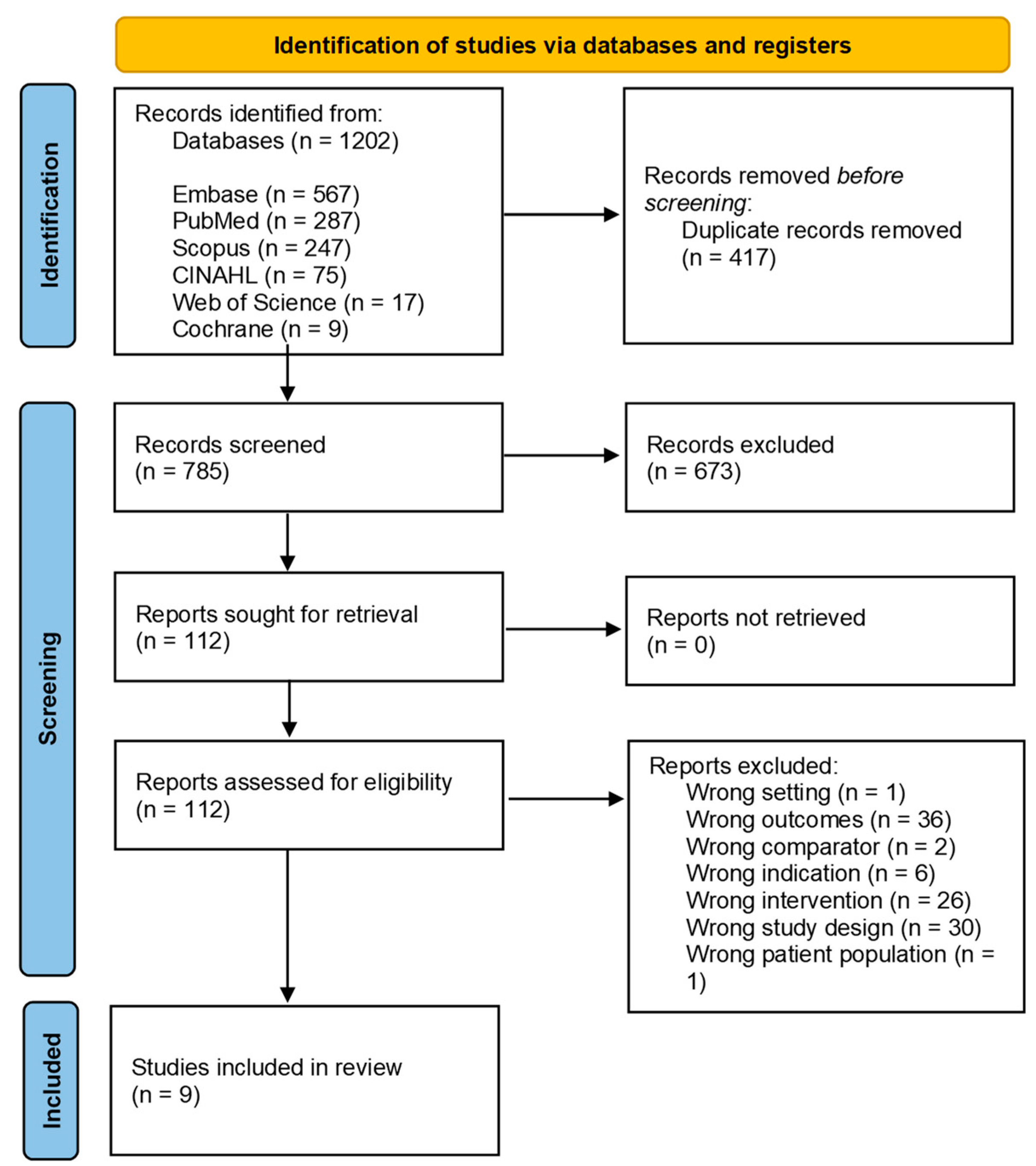
2.3. Selection Process
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
Non-Independence of Effect Sizes
2.7. Credibility Assessment
3. Results
3.1. Study Characteristics
3.1.1. Cardiovascular Events
3.1.2. CVD Mortality
3.1.3. Coronary Heart Disease and CHD Mortality
3.1.4. Ischemic Stroke
3.2. Definition of Vegetarian
3.3. Meta-Analysis Results Grouping by Author and Outcome
3.4. Meta-Analysis Results Grouping by Outcome
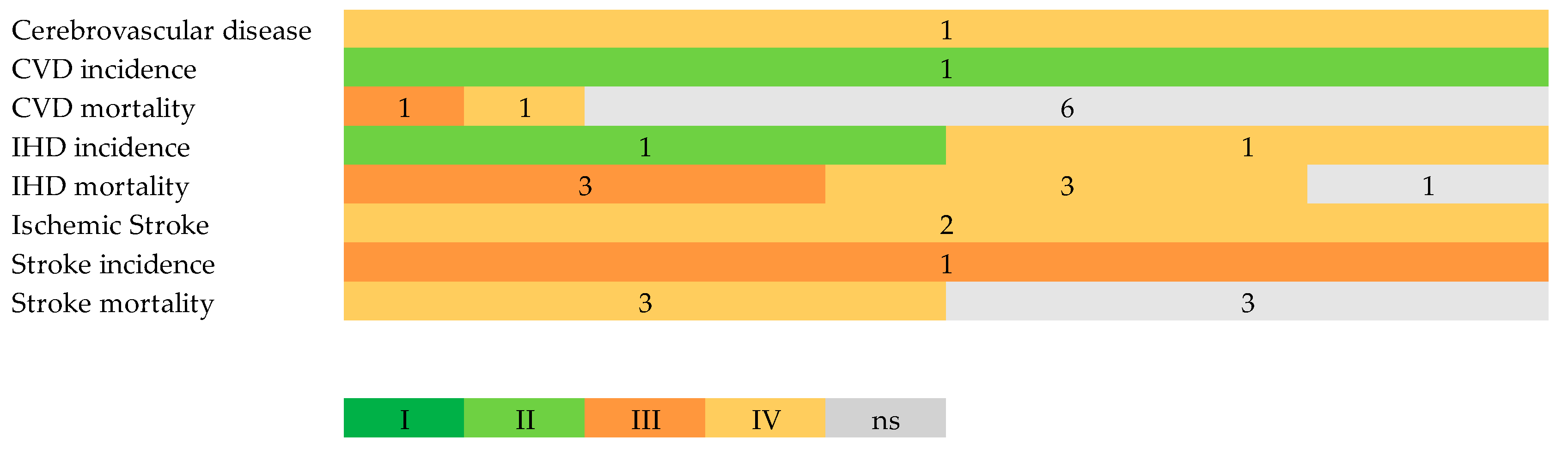
3.5. Evaluation of Bias, Heterogeneity, and Quality
3.6. Strength of Evidence
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the Global Burden of Cardiovascular Disease, Part 1. Circ. Res. 2017, 121, 677–694. [Google Scholar] [CrossRef]
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 31 July 2023).
- CDC Coronary Artery Disease|Cdc.Gov. Available online: https://www.cdc.gov/heartdisease/coronary_ad.htm (accessed on 20 August 2023).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Cerebrovascular Disease—Classifications, Symptoms, Diagnosis and Treatments. Available online: https://www.aans.org/ (accessed on 20 August 2023).
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Danaei, G.; Lu, Y.; Singh, G.M.; Carnahan, E.; Stevens, G.A.; Cowan, M.J.; Farzadfar, F.; Lin, J.K.; Finucane, M.M.; Rao, M. Cardiovascular Disease, Chronic Kidney Disease, and Diabetes Mortality Burden of Cardiometabolic Risk Factors from 1980 to 2010: A Comparative Risk Assessment. Lancet Diabetes Endocrinol. 2014, 2, 634–647. [Google Scholar]
- CDC Know Your Risk for Heart Disease|Cdc.Gov. Available online: https://www.cdc.gov/heartdisease/risk_factors.htm (accessed on 20 August 2023).
- Anand, S.S.; Hawkes, C.; de Souza, R.J.; Mente, A.; Dehghan, M.; Nugent, R.; Zulyniak, M.A.; Weis, T.; Bernstein, A.M.; Krauss, R.; et al. Food Consumption and Its Impact on Cardiovascular Disease: Importance of Solutions Focused on the Globalized Food System. J. Am. Coll. Cardiol. 2015, 66, 1590–1614. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Vadiveloo, M.; Hu, F.B.; Kris-Etherton, P.M.; Rebholz, C.M.; Sacks, F.M.; Thorndike, A.N.; Van Horn, L.; Wylie-Rosett, J. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e472–e487. [Google Scholar] [CrossRef]
- Rees, K.; Al-Khudairy, L.; Takeda, A.; Stranges, S. Vegan Dietary Pattern for the Primary and Secondary Prevention of Cardiovascular Diseases. Cochrane Database Syst. Rev. 2021, 2, CD013501. [Google Scholar] [CrossRef]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Pollock, M.; Fernandes, R.M.; Becker, L.A.; Pieper, D.; Hartling, L. Chapter V: Overviews of Reviews. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; version 6.4 (updated August 2023). Cochrane, 2023; Available online: www.training.cochrane.org/handbook (accessed on 20 August 2023).
- Dinu, M.; Pagliai, G.; Angelino, D.; Rosi, A.; Dall’Asta, M.; Bresciani, L.; Ferraris, C.; Guglielmetti, M.; Godos, J.; Del Bo’, C.; et al. Effects of Popular Diets on Anthropometric and Cardiometabolic Parameters: An Umbrella Review of Meta-Analyses of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 815–833. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, A.; Levy, J.; Berthezène, C.; Alpers, D.H.; Guéant, J.-L. Health Outcomes Associated with Vegetarian Diets: An Umbrella Review of Systematic Reviews and Meta-Analyses. Clin. Nutr. 2020, 39, 3283–3307. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.S.J.; Heng, F.K.X.; Tien, S.A.; Thian, J.Y.; Chou, H.S.; Loong, S.S.E.; Ang, W.H.D.; Chew, N.W.S.; Lo, K.-H.K. Effects of Plant-Based Diets on Anthropometric and Cardiometabolic Markers in Adults: An Umbrella Review. Nutrients 2023, 15, 2331. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Clark, J.; Glasziou, P.; Del Mar, C.; Bannach-Brown, A.; Stehlik, P.; Scott, A.M. A Full Systematic Review Was Completed in 2 Weeks Using Automation Tools: A Case Study. J. Clin. Epidemiol. 2020, 121, 81–90. [Google Scholar] [CrossRef]
- SR/MA/HTA/ITC—CINAHL. Available online: https://searchfilters.cadth.ca/link/98 (accessed on 14 July 2023).
- SR/MA/HTA/ITC—MEDLINE, Embase, PsycInfo. Available online: https://searchfilters.cadth.ca/link/33 (accessed on 14 July 2023).
- SR/MA/HTA/ITC—PubMed. Available online: https://searchfilters.cadth.ca/link/99 (accessed on 14 July 2023).
- SR/MA/HTA/ITC—Scopus. Available online: https://searchfilters.cadth.ca/link/105 (accessed on 14 July 2023).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-470-05724-7. [Google Scholar]
- Nakagawa, S.; Yang, Y.; Macartney, E.L.; Spake, R.; Lagisz, M. Quantitative Evidence Synthesis: A Practical Guide on Meta-Analysis, Meta-Regression, and Publication Bias Tests for Environmental Sciences. Environ. Evid. 2023, 12, 8. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 1st ed.; Wiley: Hoboken, NJ, USA, 2019; ISBN 978-1-119-53662-8. [Google Scholar]
- Gosling, C.J.; Solanes, A.; Fusar-Poli, P.; Radua, J. Metaumbrella: The First Comprehensive Suite to Perform Data Analysis in Umbrella Reviews with Stratification of the Evidence. BMJ Ment. Health 2023, 26, e300534. [Google Scholar] [CrossRef]
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of Random Effects Meta-Analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Radua, J. Ten Simple Rules for Conducting Umbrella Reviews. Evid. Based Ment. Health 2018, 21, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Vegetarian, Vegan Diets and Multiple Health Outcomes: A Systematic Review with Meta-Analysis of Observational Studies. Eur. Heart J. 2016, 37, 549. [Google Scholar] [CrossRef] [PubMed]
- Dybvik, J.S.; Svendsen, M.; Aune, D. Vegetarian and Vegan Diets and the Risk of Cardiovascular Disease, Ischemic Heart Disease and Stroke: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Eur. J. Nutr. 2023, 62, 51–69. [Google Scholar] [CrossRef]
- Quek, J.; Lim, G.; Lim, W.H.; Ng, C.H.; So, W.Z.; Toh, J.; Pan, X.H.; Chin, Y.H.; Muthiah, M.D.; Chan, S.P.; et al. The Association of Plant-Based Diet With Cardiovascular Disease and Mortality: A Meta-Analysis and Systematic Review of Prospect Cohort Studies. Front. Cardiovasc. Med. 2021, 8, 756810. [Google Scholar] [CrossRef]
- Glenn, A.J.; Viguiliouk, E.; Seider, M.; Boucher, B.A.; Khan, T.A.; Blanco Mejia, S.; Jenkins, D.J.; Kahleová, H.; Rahelić, D.; Salas-Salvadó, J.; et al. Relation of Vegetarian Dietary Patterns With Major Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Front. Nutr. 2019, 6, 80. [Google Scholar] [CrossRef]
- Jafari, S.; Hezaveh, E.; Jalilpiran, Y.; Jayedi, A.; Wong, A.; Safaiyan, A.; Barzegar, A. Plant-Based Diets and Risk of Disease Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 7760–7772. [Google Scholar] [CrossRef]
- Huang, T.; Yang, B.; Zheng, J.; Li, G.; Wahlqvist, M.L.; Li, D. Cardiovascular Disease Mortality and Cancer Incidence in Vegetarians: A Meta-Analysis and Systematic Review. Ann. Nutr. Metab. 2012, 60, 233–240. [Google Scholar] [CrossRef]
- Kwok, C.S.; Umar, S.; Myint, P.K.; Mamas, M.A.; Loke, Y.K. Vegetarian Diet, Seventh Day Adventists and Risk of Cardiovascular Mortality: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2014, 176, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Jabri, A.; Kumar, A.; Verghese, E.; Alameh, A.; Kumar, A.; Khan, M.S.; Khan, S.U.; Michos, E.D.; Kapadia, S.R.; Reed, G.W.; et al. Meta-Analysis of Effect of Vegetarian Diet on Ischemic Heart Disease and All-Cause Mortality. Am. J. Prev. Cardiol. 2021, 7, 100182. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Yu, L.H.; Tu, Y.K.; Cheng, H.Y.; Chen, L.Y.; Loh, C.H.; Chen, T.L. Risk of Incident Stroke among Vegetarians Compared to Nonvegetarians: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Nutrients 2021, 13, 3019. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schünemann, H.J.; Meerpohl, J.J. Improving the Trustworthiness of Findings from Nutrition Evidence Syntheses: Assessing Risk of Bias and Rating the Certainty of Evidence. Eur. J. Nutr. 2021, 60, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Ioannidis, J.P.A. Perspective: Limiting Dependence on Nonrandomized Studies and Improving Randomized Trials in Human Nutrition Research: Why and How. Adv. Nutr. 2018, 9, 367–377. [Google Scholar] [CrossRef]
- Laville, M.; Segrestin, B.; Alligier, M.; Ruano-Rodríguez, C.; Serra-Majem, L.; Hiesmayr, M.; Schols, A.; La Vecchia, C.; Boirie, Y.; Rath, A.; et al. Evidence-Based Practice within Nutrition: What Are the Barriers for Improving the Evidence and How Can They Be Dealt With? Trials 2017, 18, 425. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for Europe Plant-Based Diets and Their Impact on Health, Sustainability and the Environment: A Review of the Evidence; WHO European Office for the Prevention and Control of Noncommunicable Diseases; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2021.
- Chareonrungrueangchai, K.; Wongkawinwoot, K.; Anothaisintawee, T.; Reutrakul, S. Dietary Factors and Risks of Cardiovascular Diseases: An Umbrella Review. Nutrients 2020, 12, 1088. [Google Scholar] [CrossRef]
| Parameter | Inclusion | Exclusion |
|---|---|---|
| Population | Adults with age ≥ 16 years | Specific population (e.g., diabetic) |
| Intervention | Vegetarian diet, vegan diet, plant-based diet | An omnivorous diet with additional vegetable/fruit |
| Comparison | Non-vegetarian diet | |
| Outcome | Cardiovascular (CVD) events, CVD mortality, coronary heart disease (CHD), CHD mortality, ischemic stroke | Hemorrhagic stroke |
| Study design | Systematic reviews, including meta-analyses (Quantitative analysis) of prospective observational studies (cross-sectional, case-control, cohort) or randomized clinical trials (RCTs) | Systematic review without meta-analysis, narrative review |
| Author Year | Country | Outcome | N Studies | N Participants | Cases | Controls | Effect Size | eOR | eOR 95% CI | I2 | Class * | RoB % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dinu 2016 [36] | USA, Germany, UK | CVD mortality | 4 | 107,285 | 3778 | 103,507 | OR | 0.85 | 0.61, 1.17 | 93.205 | ns | 44.05 |
| IHD mortality | 6 | 225,618 | 5169 | 220,449 | OR | 0.84 | 0.62, 1.14 | 95.475 | ns | 40.69 | ||
| Dybvik 2023 [37] | USA, Germany, UK, Taiwan | Cerebrovascular disease | 3 | 121,850 | 1096 | 120,754 | RR | 0.588 | 0.36, 0.97 | 86.36 | IV | 0.00 |
| CVD incidence | 1 | 398,448 | 106,690 | 291,758 | RR | 0.71 | 0.68, 0.75 | 1 study | II | 0.00 | ||
| CVD mortality | 1 | 36,346 | 987 | 35,359 | HR | 0.87 | 0.75, 1.01 | 1 study | ns | 0.00 | ||
| IHD incidence | 3 | 919,768 | 32,800 | 886,968 | RR | 0.605 | 0.44, 0.83 | 88.903 | IV | 0.00 | ||
| IHD mortality | 7 | 266,473 | 8523 | 257,950 | OR | 0.733 | 0.59, 0.92 | 96.286 | IV | 0.00 | ||
| Stroke incidence | 1 | 422,102 | 5946 | 416,156 | RR | 0.609 | 0.48, 0.78 | 1 study | III | 0.00 | ||
| Stroke mortality | 3 | 98,072 | 1417 | 96,655 | RR | 1.136 | 1.02, 1.26 | 0 | IV | 0.00 | ||
| Glenn 2019 [39] | USA, Germany, UK | CVD mortality | 4 | 107,285 | 3778 | 103,507 | OR | 0.846 | 0.61, 1.17 | 93.123 | ns | 55.95 |
| IHD incidence | 1 | 44,561 | 1235 | 43,326 | RR | 1.474 | 1.32, 1.65 | 1 study | II | 0.00 | ||
| IHD mortality | 5 | 181,057 | 3934 | 177,123 | OR | 0.738 | 0.59, 0.92 | 80.08 | IV | 73.90 | ||
| Stroke mortality | 4 | 145,326 | 1682 | 143,644 | RR | 0.995 | 0.75, 1.32 | 79.157 | ns | 67.48 | ||
| Huang 2012 [41] | USA, Netherlands, UK, Germany, Japan | CVD mortality | 2 | 49,158 | 1205 | 47,953 | RR | 0.792 | 0.37, 1.68 | 96.297 | ns | 100.00 |
| IHD mortality | 6 | 92,314 | 2290 | 90,024 | RR | 0.63 | 0.49, 0.80 | 82.323 | III | 100.00 | ||
| Stroke mortality | 5 | 80,993 | 1508 | 79,485 | RR | 0.734 | 0.56, 0.97 | 82.995 | IV | 100.00 | ||
| Jabri 2021 [43] | USA, Netherlands, UK, Germany, Japan | CVD mortality | 1 | 47,254 | 950 | 46,304 | RR | 0.543 | 0.47, 0.63 | 1 study | IV | 0.00 |
| IHD mortality | 4 | 70,942 | 1342 | 69,600 | RR | 0.54 | 0.41, 0.70 | 60.845 | III | 0.00 | ||
| Stroke mortality | 3 | 60,103 | 1042 | 59,061 | RR | 0.722 | 0.46, 1.15 | 84.096 | ns | 0.00 | ||
| Jafari 2022 [40] | USA, UK, Germany, Europe, Spain, Australia | CVD mortality | 5 | 101,665 | 14,091 | 87,574 | OR | 0.827 | 0.63, 1.09 | 95.208 | ns | 35.75 |
| IHD mortality | 8 | 313,305 | 2912 | 310,393 | OR | 0.629 | 0.48, 0.82 | 89.477 | III | 31.08 | ||
| Stroke mortality | 3 | 68,144 | 731 | 67,413 | RR | 0.727 | 0.57, 0.93 | 50.186 | IV | 0.00 | ||
| Kwok 2014 [42] | USA, Netherlands, UK, Germany, Japan | CVD mortality | 6 | 156,443 | 4983 | 151,460 | OR | 0.827 | 0.62, 1.10 | 93.59 | ns | 62.84 |
| IHD mortality | 11 | 270,678 | 6985 | 263,693 | OR | 0.753 | 0.61, 0.94 | 93.969 | IV | 35.33 | ||
| Stroke mortality | 6 | 207,912 | 2721 | 205,191 | RR | 0.804 | 0.62, 1.05 | 96.291 | ns | 45.46 | ||
| Lu 2021 [44] | USA, UK, Taiwan | Ischemic Stroke | 6 | 679,034 | 12,791 | 666,243 | RR | 0.688 | 0.50, 0.95 | 73.083 | IV | 7.1 866 |
| Quek 2021 [38] | Europe, North America, Asia, Europe | CVD mortality | 1 | 4282 | 1565 | 2717 | HR | 0.681 | 0.58, 0.80 | 1 study | III | 0.00 |
| Ischemic Stroke | 2 | 61,540 | 604 | 60,936 | RR | 0.473 | 0.28, 0.79 | 61.765 | IV | 0.00 |
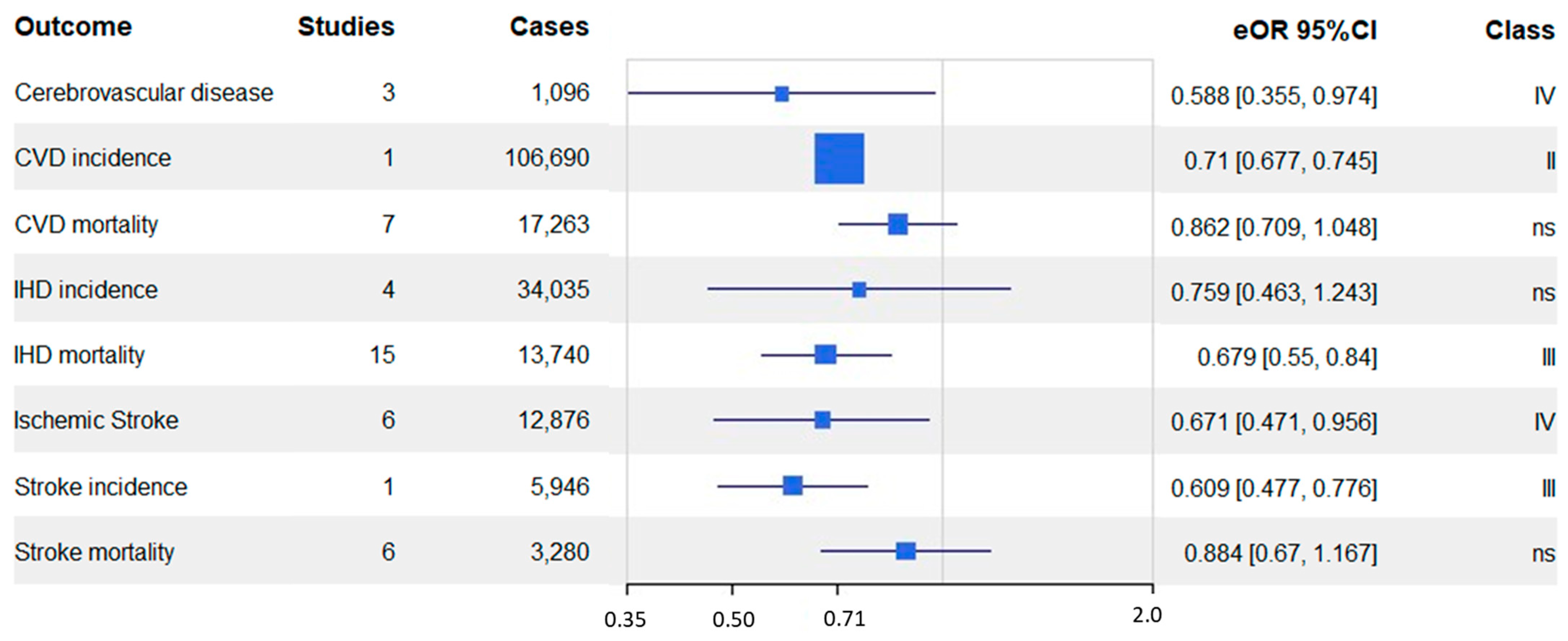
| Outcome | N Studies | Total N | Cases | Controls | Measure | eOR | eOR 95% CI | I2 | Class | RoB % |
|---|---|---|---|---|---|---|---|---|---|---|
| Cerebrovascular disease | 3 | 121,850 | 1096 | 120,754 | RR | 0.588 | 0.36, 0.97 | 86.36 | IV | 0 |
| CVD incidence | 1 | 398,448 | 106,690 | 291,758 | RR | 0.71 | 0.68, 0.75 | 1 study | II | 0 |
| CVD mortality | 7 | 145,227 | 17,263 | 127,964 | OR | 0.862 | 0.71, 1.05 | 92.83 | ns | 16.31 |
| IHD incidence | 4 | 964,329 | 34,035 | 930,294 | RR | 0.759 | 0.46, 1.24 | 98.24 | ns | 0 |
| IHD mortality | 15 | 619,430 | 13,740 | 605,690 | OR | 0.679 | 0.55, 0.84 | 96.68 | III | 18.49 |
| Ischemic stroke | 6 | 692,386 | 12,876 | 679,510 | RR | 0.671 | 0.47, 0.96 | 78.19 | IV | 0 |
| Stroke incidence | 1 | 422,102 | 5946 | 416,156 | RR | 0.609 | 0.48, 0.78 | 1 study | III | 0 |
| Stroke mortality | 6 | 196,925 | 3280 | 193,645 | RR | 0.884 | 0.67, 1.17 | 97.13 | ns | 40.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocagli, H.; Berti, G.; Rango, D.; Norbiato, F.; Chiaruttini, M.V.; Lorenzoni, G.; Gregori, D. Association of Vegetarian and Vegan Diets with Cardiovascular Health: An Umbrella Review of Meta-Analysis of Observational Studies and Randomized Trials. Nutrients 2023, 15, 4103. https://doi.org/10.3390/nu15194103
Ocagli H, Berti G, Rango D, Norbiato F, Chiaruttini MV, Lorenzoni G, Gregori D. Association of Vegetarian and Vegan Diets with Cardiovascular Health: An Umbrella Review of Meta-Analysis of Observational Studies and Randomized Trials. Nutrients. 2023; 15(19):4103. https://doi.org/10.3390/nu15194103
Chicago/Turabian StyleOcagli, Honoria, Giacomo Berti, Davide Rango, Federica Norbiato, Maria Vittoria Chiaruttini, Giulia Lorenzoni, and Dario Gregori. 2023. "Association of Vegetarian and Vegan Diets with Cardiovascular Health: An Umbrella Review of Meta-Analysis of Observational Studies and Randomized Trials" Nutrients 15, no. 19: 4103. https://doi.org/10.3390/nu15194103
APA StyleOcagli, H., Berti, G., Rango, D., Norbiato, F., Chiaruttini, M. V., Lorenzoni, G., & Gregori, D. (2023). Association of Vegetarian and Vegan Diets with Cardiovascular Health: An Umbrella Review of Meta-Analysis of Observational Studies and Randomized Trials. Nutrients, 15(19), 4103. https://doi.org/10.3390/nu15194103






