Erythritol Can Inhibit the Expression of Senescence Molecules in Mouse Gingival Tissues and Human Gingival Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Body Weight and General Health
2.3. Blood Tests
2.3.1. Preparation of Blood Sera
2.3.2. Serum Biochemistry
2.4. Hematoxylin and Eosin Histology
2.5. Immunofluorescence Analysis
2.6. Cell Culture
2.7. HGF Senescence Induced by High-Concentration Glucose
2.8. Measurement of SA-β-gal
2.9. Hydrogen Peroxide-Induced HGF Senescence
2.10. Lipopolysaccharide-Induced HGF Senescence
2.11. Real-Time PCR
2.12. Glycolytic Molecule Inhibition Experiment
2.13. Experiments to Verify the Effect of Erythritol on the Glycolytic System
2.14. Statistical Analysis
3. Results
3.1. Changes in Body Weight and Serum Components after Drinking Erythritol Water
3.2. Effect of Erythritol Administration on Aging of Gingiva in Aged Mice
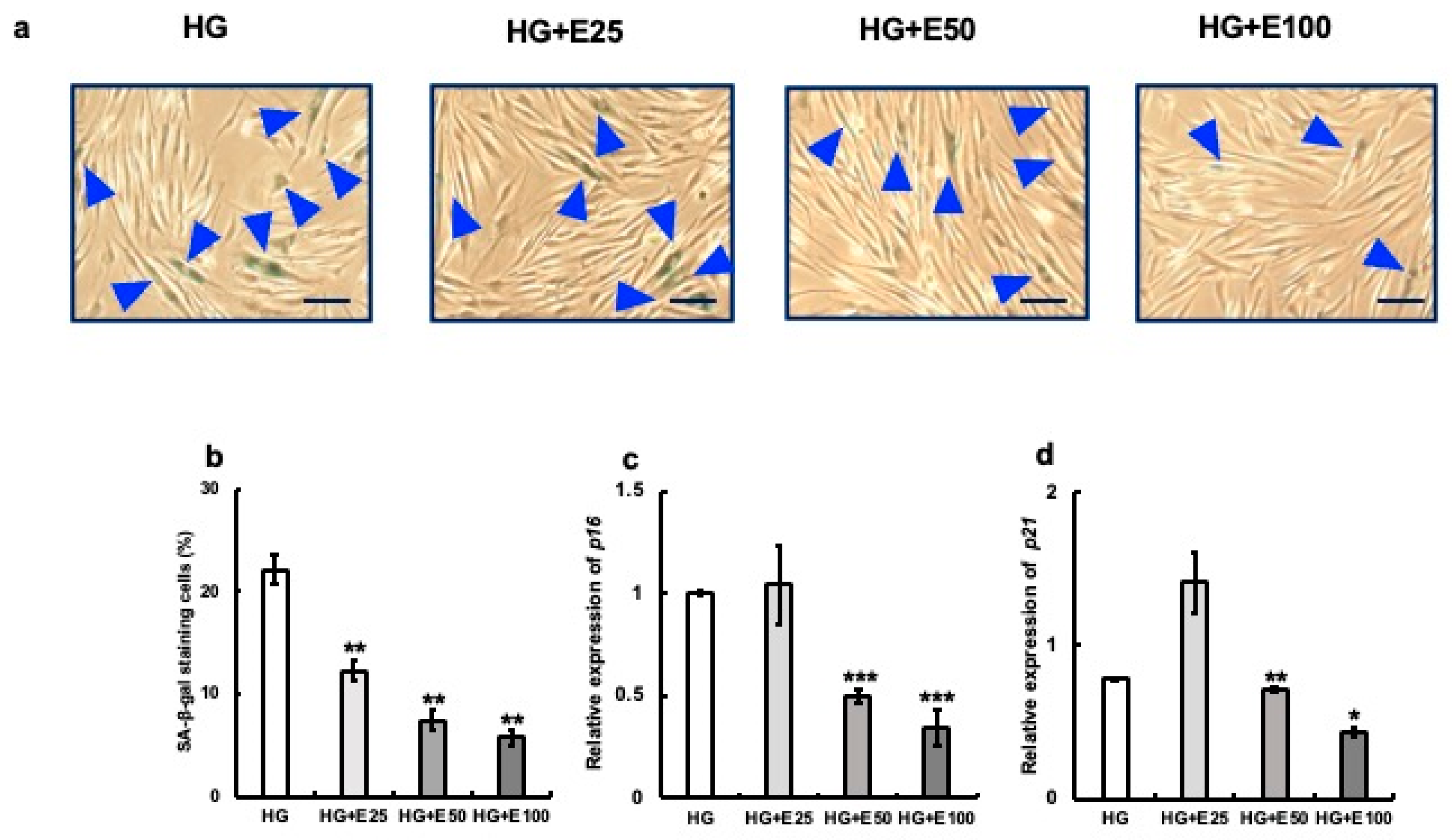
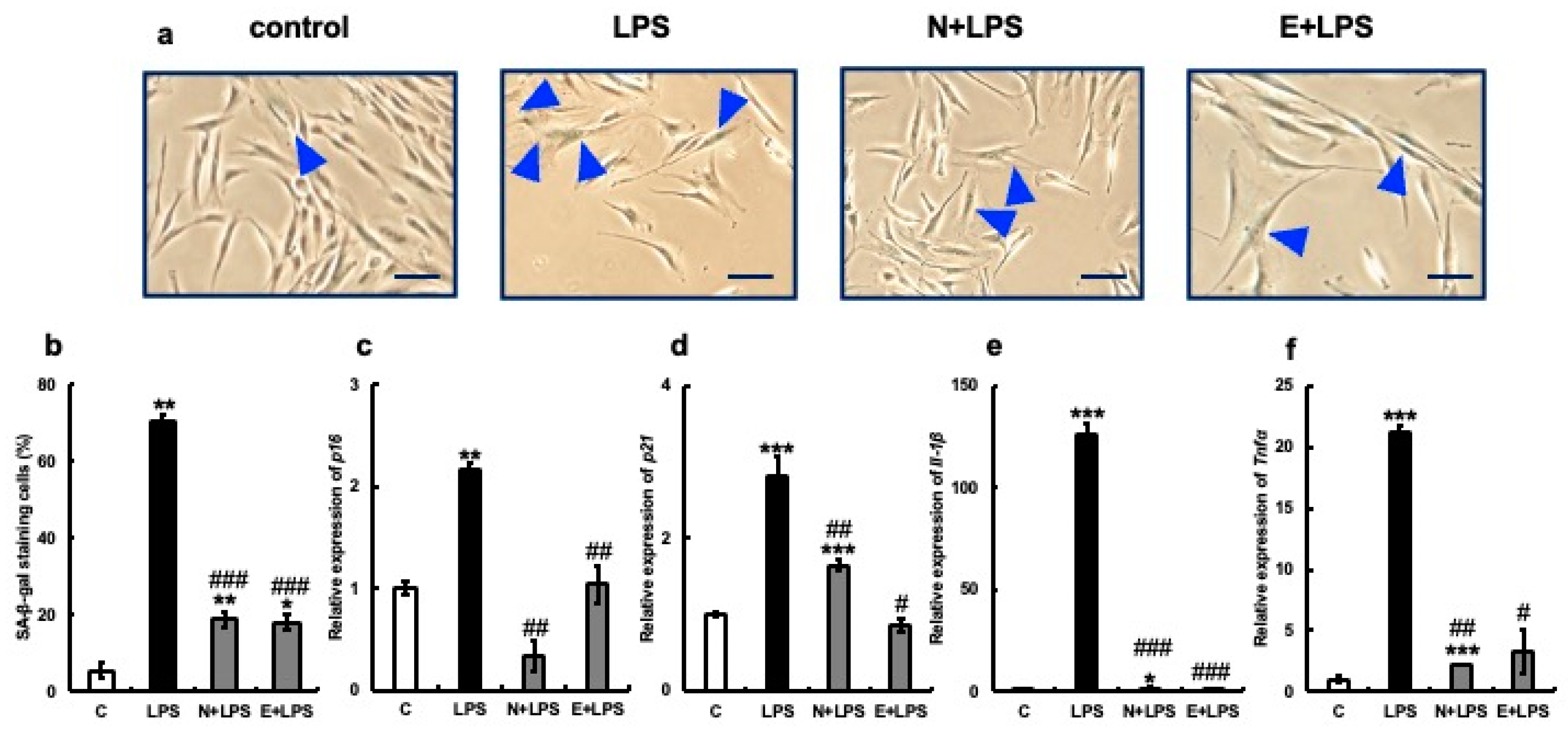
3.3. Anti-Aging Effect of Erythritol on Cultured Gingival Fibroblasts
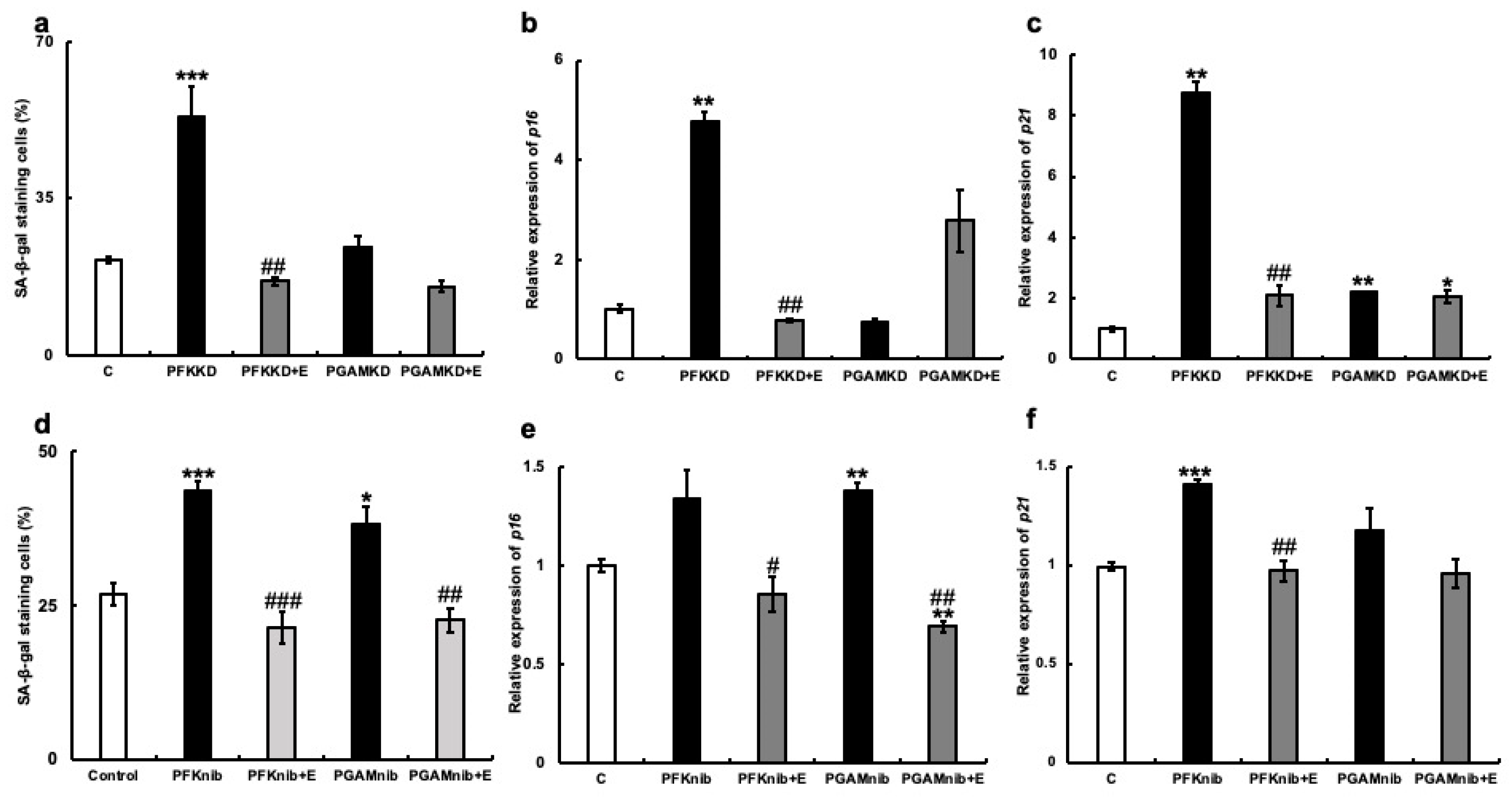
3.4. Relationship between the Glycolytic System, Cellular Senescence, and the Effects of Erythritol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.; O’Toole, S. Tooth wear and aging. Aust. Dent. J. 2019, 64 (Suppl. S1), S59–S62. [Google Scholar] [CrossRef]
- Ástvaldsdóttir, Á.; Boström, A.M.; Davidson, T.; Gabre, P.; Gahnberg, L.; Sandborgh Englund, G.; Skott, P.; Ståhlnacke, K.; Tranaeus, S.; Wilhelmsson, H.; et al. Oral health and dental care of older persons-A systematic map of systematic reviews. Gerodontology 2018, 35, 290–304. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Della Vella, F.; Di Stasio, D.; Carinci, F.; Lucchese, A.; Petruzzi, M. Oral Health Status and Need for Oral Care in an Aging Population: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4558. [Google Scholar] [CrossRef]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal tract disorders in older age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef]
- Hodjat, M.; Khan, F.; Saadat, K.A.S.M. Epigenetic alterations in aging tooth and the reprogramming potential. Ageing Res. Rev. 2020, 63, 101140. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Graves, C.L.; Gonzalez, O.A.; Dawson, D.; Morford, L.A.; Huja, P.E.; Hartsfield, J.K.; Huja, S.S.; Pandruvada, S.; Wallet, S.M. Aging, inflammation, immunity and periodontal disease. Periodontol. 2000 2016, 72, 54–75. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E.; Hayflick, H. Hayflick, his Limit, and cellular ageing. Nat. Rev. Mol. Cell Biol. 2000, 1, 72–76. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed]
- Saldías, M.P.; Fernández, C.; Morgan, A.; Díaz, C.; Morales, D.; Jaña, F.; Gómez, A.; Silva, A.; Briceño, F.; Oyarzún, A.; et al. Aged blood factors decrease cellular responses associated with delayed gingival wound repair. PLoS ONE 2017, 12, e0184189. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Matsuda, K.; Aoki, Y.; Yamada, M.; Wang, J.; Watanabe, M.; Kurosawa, M.; Shikama, Y.; Matsushita, K. Analysis of senescence in gingival tissues and gingival fibroblast cultures. Clin. Exp. Dent. Res. 2022, 8, 939–949. [Google Scholar] [CrossRef]
- Giri, S.; Takada, A.; Paudel, D.; Yoshida, K.; Furukawa, M.; Kuramitsu, Y.; Matsushita, K.; Abiko, Y.; Furuichi, Y. An in vitro senescence model of gingival epithelial cell induced by hydrogen peroxide treatment. Odontology 2022, 110, 44–53. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBiomedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Q.; Nie, L.; Zhu, R.; Zhou, X.; Zhao, P.; Ji, N.; Liang, X.; Ding, Y.; Yuan, Q.; et al. Hyperglycemia-induced Inflamm-aging accelerates gingival senescence via NLRC4 phosphorylation. J. Biol. Chem. 2019, 294, 18807–18819. [Google Scholar] [CrossRef]
- Shen, S.; Ji, C.; Wei, K. Cellular Senescence and Regulated Cell Death of Tubular Epithelial Cells in Diabetic Kidney Disease. Front. Endocrinol. 2022, 13, 924299. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Z.; Wu, A.; Khan, A.H.; Zhu, Y.; Ding, S.; Li, X.; Zhao, Y.; Dai, X.; Zhou, J.; et al. Hyperglycemia promotes endothelial cell senescence through AQR/PLAU signaling axis. Int. J. Mol. Sci. 2022, 23, 2879. [Google Scholar] [CrossRef]
- Liang, P.; Cao, M.; Li, J.; Wang, Q.; Dai, Z. Expanding sugar alcohol industry: Microbial production of sugar alcohols and associated chemocatalytic derivatives. Biotechnol. Adv. 2023, 64, 108105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Salamanca, E.; Chen, I.W.; Su, J.N.; Chen, Y.C.; Wang, S.Y.; Sun, Y.S.; Teng, N.C.; Chang, W.J. Xylitol-Containing Chewing Gum Reduces Cariogenic and Periodontopathic Bacteria in Dental Plaque-Microbiome Investigation. Front. Nutr. 2022, 9, 882636. [Google Scholar] [CrossRef] [PubMed]
- de Cock, P. Erythritol Functional Roles in Oral-Systemic Health. Adv. Dent. Res. 2018, 29, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.Y.; Lam, W.Y.; Wong, A.W.; Duangthip, D.; Chu, C.H. Nonrestorative Management of Dental Caries. Dent. J. 2021, 9, 121. [Google Scholar] [CrossRef]
- Kawano, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Sasano, R.; et al. Erythritol ameliorates small intestinal inflammation induced by high-fat diets and improves glucose tolerance. Int. J. Mol. Sci. 2021, 22, 5558. [Google Scholar] [CrossRef]
- Msomi, N.Z.; Erukainure, O.L.; Islam, M.S. Suitability of sugar alcohols as antidiabetic supplements: A review. J. Food Drug Anal. 2021, 29, 1–14. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Saxena, S.; Vekaria, H.; Sullivan, P.G.; Seifert, A.W. Connective tissue fibroblasts from highly regenerative mammals are refractory to ROS-induced cellular senescence. Nat. Commun. 2019, 10, 4400. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, J.; Li, Z.; Song, Q.; Yang, Z.; Wang, L.; Meng, Q. Identification of aging-related biomarkers and immune infiltration characteristics in osteoarthritis based on bioinformatics analysis and machine learning. Front. Immunol. 2023, 14, 1168780. [Google Scholar] [CrossRef]
- Bussian, T.J.; Aziz, A.; Meyer, C.F.; Swenson, B.L.; van Deursen, J.M.; Baker, D.J. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018, 562, 578–582. [Google Scholar] [CrossRef]
- Kovacovicova, K.; Skolnaja, M.; Heinmaa, M.; Mistrik, M.; Pata, P.; Pata, I.; Bartek, J.; Vinciguerra, M. Senolytic cocktail dasatinib+quercetin (D+Q) does not enhance the efficacy of senescence-inducing chemotherapy in liver cancer. Front. Oncol. 2018, 8, 459. [Google Scholar] [CrossRef]
- Elbini Dhouib, I.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing Research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular senescence: Molecular targets, biomarkers, and senolytic drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef]
- Raffaele, M.; Vinciguerra, M. The costs and benefits of senotherapeutics for human health. Lancet Healthy Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Martău, G.A.; Coman, V.; Vodnar, D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020, 40, 608–622. [Google Scholar] [CrossRef]
- Mazi, T.A.; Stanhope, K.L. Erythritol: An in-depth discussion of its potential to be a beneficial dietary component. Nutrients 2023, 15, 204. [Google Scholar] [CrossRef]
- Rice, T.; Zannini, E.; Arendt, K.; Coffey, A. A review of polyols—Biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef]
- Kondoh, H.; Lleonart, M.E.; Gil, J.; Wang, J.; Degan, P.; Peters, G.; Martinez, D.; Carnero, A.; Beach, D. Glycolytic enzymes can modulate cellular life span. Cancer Res. 2005, 65, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Shibata, E.; Shimada, M.; Ito, K.; Ito, T.; Kanda, H.; Takubo, K.; Shimada, A.; Lleonart, M.E.; Inagaki, N.; et al. Characterization of genetically modified mice for phosphoglycerate mutase, a vitally-essential enzyme in glycolysis. PLoS ONE 2021, 16, e0250856. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.; Qin, Z.H.; Sheng, R. Structure, regulation, and biological functions of TIGAR and its role in diseases. Acta Pharmacol. Sin. 2021, 42, 1547–1555. [Google Scholar] [CrossRef]
- Sano, H.; Nakamura, A.; Yamane, M.; Niwa, H.; Nishimura, T.; Araki, K.; Takemoto, K.; Ishiguro, K.-I.; Aoki, H.; Kato, Y.; et al. The polyol pathway is an evolutionarily conserved system for sensing glucose uptake. PLoS Biol. 2022, 20, e3001678. [Google Scholar] [CrossRef]
- Söderling, E.; Pienihäkkinen, K.; Gursoy, U.K. Effects of sugar-free polyol chewing gums on gingival inflammation: A systematic review. Clin. Oral. Investig. 2022, 26, 6881–6891. [Google Scholar] [CrossRef] [PubMed]
- Loimaranta, V.; Mazurel, D.; Deng, D.; Söderling, E. Xylitol and erythritol inhibit real-time biofilm formation of Streptococcus mutans. BMC Microbiol. 2020, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Onisor, F.; Mester, A.; Mancini, L.; Voina-Tonea, A. Effectiveness and clinical performance of erythritol air-polishing in non-surgical periodontal therapy: A systematic review of randomized clinical trials. Medicina 2022, 58, 866. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Miyawaki, S.; Kuroda, T.; Umeta, M.; Kawabe, M.; Watanabe, K. Erythritol inhibits the growth of periodontal-disease-associated bacteria isolated from canine oral cavity. Heliyon 2022, 8, e10224. [Google Scholar] [CrossRef]
- Baima, G.; Romandini, M.; Citterio, F.; Romano, F.; Aimetti, M. Periodontitis and Accelerated Biological Aging: A Geroscience Approach. J. Dent. Res. 2022, 101, 125–132. [Google Scholar] [CrossRef]




| Name | Forward | Reverse |
|---|---|---|
| Mouse p16 | CGTACCCCGATTCAGGTGAT | TTGAGCAGAAGAGCTGCTACG |
| Mouse p21 | GTGGGTCTGACTCCAGCCC | CCTTCTCGTGAGACGCTTAC |
| Mouse Il-1β | GCACTACAGGCTCCGAGATGAAC | TTGTCGTTGCTTGGTTCTCCTTGT |
| Mouse Tnfα | ATGAGCACAGAAAGCATGA | AGTAGACAGAAGAGCGTGGT |
| Mouse Gapdh | AACCTGCCAAGTATGATGA | GGAGTTGCTGTTGAAGTC |
| Human p16 | CTCGTGCTGATGCTACTGAGGA | GGTCGGCGCAGTTGGGCTCC |
| Human p21 | CCGAAGTCAGTTCCTTGTGG | CATGGGTTCTGACGGACAT |
| Human IL-1β | GCAGCCATGGCAGAAGTACCTGA | CCAGAGGGCAGAGGTCCAGGTC |
| Human TNFα | CCCAGGGACCTCTCTCTAATC | ATGGCTACAGGCTTGTCACT |
| Human GAPDH | TGTCAGTGGTGGACCTGACCT | AGGGGAGATTCAGTGTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoi, H.; Furukawa, M.; Wang, J.; Aoki, Y.; Raju, R.; Ikuyo, Y.; Yamada, M.; Shikama, Y.; Matsushita, K. Erythritol Can Inhibit the Expression of Senescence Molecules in Mouse Gingival Tissues and Human Gingival Fibroblasts. Nutrients 2023, 15, 4050. https://doi.org/10.3390/nu15184050
Yokoi H, Furukawa M, Wang J, Aoki Y, Raju R, Ikuyo Y, Yamada M, Shikama Y, Matsushita K. Erythritol Can Inhibit the Expression of Senescence Molecules in Mouse Gingival Tissues and Human Gingival Fibroblasts. Nutrients. 2023; 15(18):4050. https://doi.org/10.3390/nu15184050
Chicago/Turabian StyleYokoi, Haruna, Masae Furukawa, Jingshu Wang, Yu Aoki, Resmi Raju, Yoriko Ikuyo, Mitsuyoshi Yamada, Yosuke Shikama, and Kenji Matsushita. 2023. "Erythritol Can Inhibit the Expression of Senescence Molecules in Mouse Gingival Tissues and Human Gingival Fibroblasts" Nutrients 15, no. 18: 4050. https://doi.org/10.3390/nu15184050
APA StyleYokoi, H., Furukawa, M., Wang, J., Aoki, Y., Raju, R., Ikuyo, Y., Yamada, M., Shikama, Y., & Matsushita, K. (2023). Erythritol Can Inhibit the Expression of Senescence Molecules in Mouse Gingival Tissues and Human Gingival Fibroblasts. Nutrients, 15(18), 4050. https://doi.org/10.3390/nu15184050





