Aspartic Acid in Health and Disease
Abstract
1. Introduction
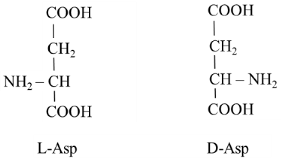
2. Aspartate Transporters
2.1. Plasma Membrane Transporters
2.2. Mitochondrial Transporters
2.2.1. Aspartate–Glutamate Carrier (AGC)
2.2.2. Uncoupling Protein 2 (UCP2, Aspartate/Pi + H+ Exchanger)
3. Aspartate Origin
3.1. Origin of L-Asp
3.2. Origin of D-Asp
4. Metabolism and Physiologic Importance of Aspartic Acid
4.1. Aspartate and Protein Synthesis
4.2. L-Asp and Malate–Aspartate Shuttle (MAS)
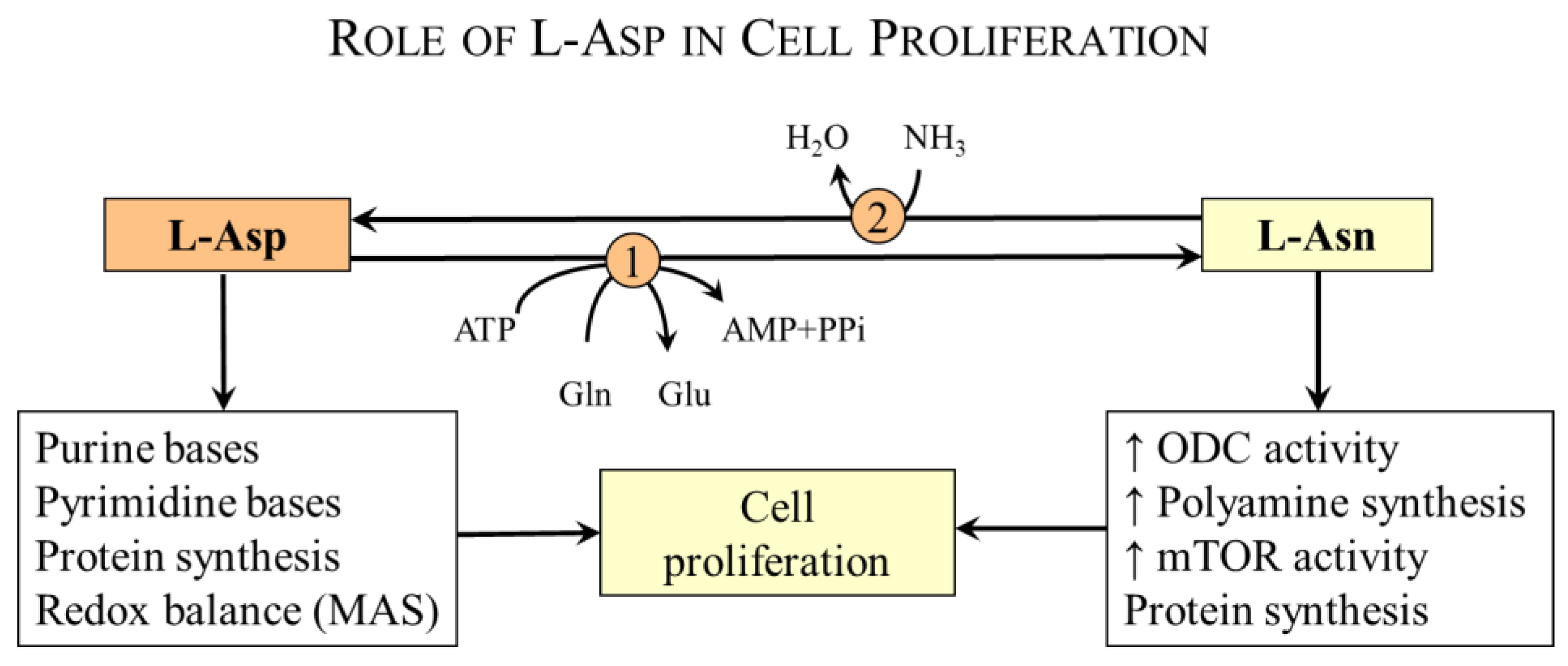
4.3. Aspartate and Cell Proliferation
4.3.1. L-Asp and Nucleotide Synthesis
4.3.2. L-Asp and Asparagine Synthesis
- Protein synthesis—Asparagine is a proteinogenic amino acid with a polar side chain that easily forms hydrogen bonds and increases the solubility of proteins in water. Together with L-Asp, it participates in the formation of asx turns and asx motifs that enable the folding of proteins. Its content in proteins is about 4% [56]. Like L-Asp, asparagine side chains can be post-translationally modified by a variety of chemical reactions, notably deamidation, isomerization, and racemization, resulting in various alterations in the structure and function of proteins [59].
- Activation of ornithine decarboxylase [69]—Ornithine decarboxylase is the rate-controlling enzyme in the biosynthesis of polyamines, e.g., spermine, spermidine, and putrescine, which are important in the regulation of cell proliferation and differentiation.
- Intracellular asparagine exchanges with extracellular amino acids, especially serine, arginine, and histidine, to promote mTOR activation, protein and nucleotide synthesis, and cell proliferation [70].
4.4. L-Asp and Arginine Synthesis
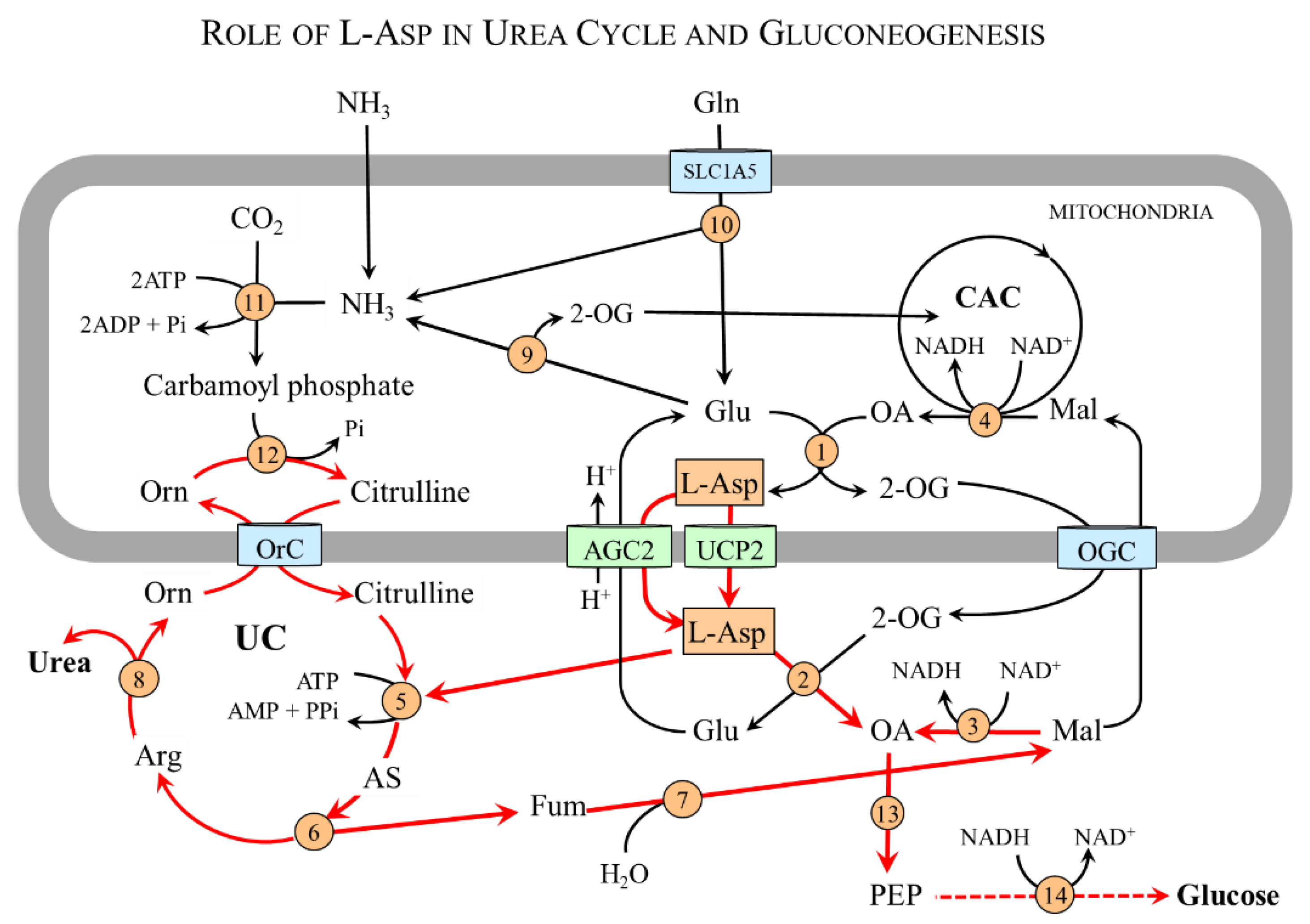
4.5. L-Asp and the Liver
4.5.1. L-Asp and Urea Cycle
4.5.2. L-Asp and Gluconeogenesis
4.6. L-Asp and Skeletal Muscle
4.6.1. L-Asp and Purine-Nucleotide Cycle (PNC)
| Mitochondria: | BCAA + 2-OG → BCKA + Glu |
| Glu + OA → 2-OG + L-Asp | |
| AGC1: | L-Asp transport to the cytosol |
| Cytosol: | L-Asp + IMP + GTP → adenylosuccinate + GDP + Pi |
| adenylosuccinate → fumarate + AMP | |
| AMP + H2O → IMP + NH3 | |
| or | |
| AMP + ATP → ADP + ADP | |
| ADP + creatine phosphate → ATP + creatine |
4.6.2. L-Asp and Synthesis of Alanine and Glutamine in Muscles
| Mitochondria: | BCAA + 2-OG → BCKA + Glu |
| Glu + OA → 2-OG + L-Asp | |
| AGC1: | L-Asp transport to the cytosol |
| Cytosol: | L-Asp + 2-OG → OA + Glu |
| Glu + pyruvate → 2-OG + Ala or | |
| Glu + NH3 + ATP → Gln + ADP + Pi |
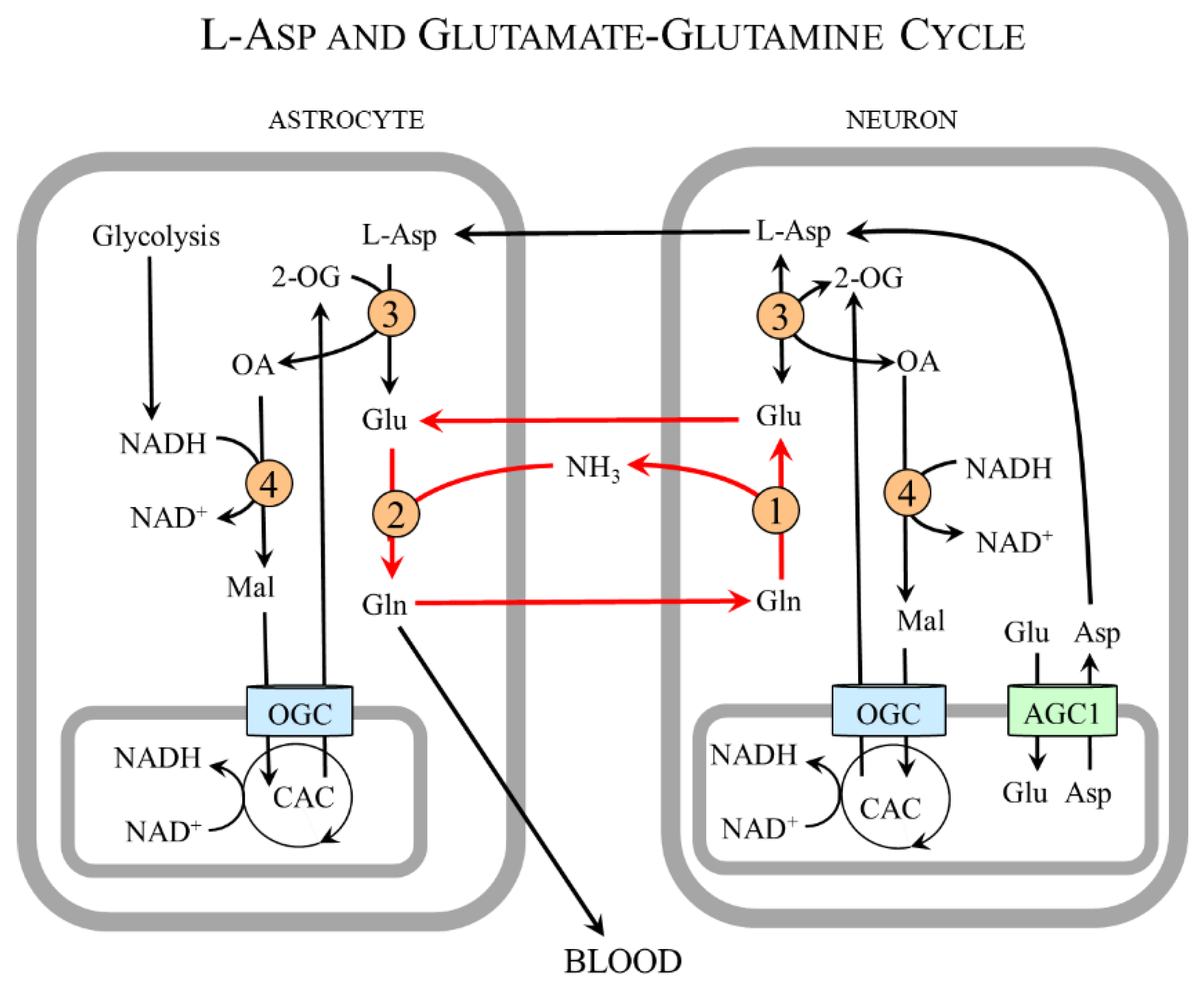
4.7. Aspartate and Nervous System
4.7.1. Aspartate and Neurotransmission
4.7.2. D-Asp and Brain Development
4.7.3. Aspartate-Derived Substances
- N-acetyl-L-aspartate (NAA) is an abundant brain metabolite synthesized by aspartate N-acetyltransferase from L-Asp and acetyl-CoA in neurons. It has been shown that NAA is released from neurons and transported to oligodendrocytes and astrocytes by sodium-coupled high-affinity carboxylate transporter NaC3/NaDC3, where it is cleaved by aspartoacylase into L-Asp and acetate anion that can be subsequently used for synthesis of acetyl-CoA by acetyl-CoA synthase [9,93,94]. In oligodendrocytes, acetate moiety is used for the synthesis of fatty acids and steroids, which are used for the synthesis of myelin, the basic component of the white matter of the brain and spinal cord [9].
- N-acetyl-L-aspartyl-L-glutamate (NAAG) is a neurotransmitter synthesized by peptide bond formation between NAA and glutamate that modulates glutamatergic neurotransmission [9]. NAAG is degraded by a specific dipeptidase known as NAAG peptidase, glutamate carboxypeptidase II, or prostate-specific membrane antigen to glutamate and N-acetyl-L-aspartate. The enzyme is highly expressed in the prostate and used as a biomarker of prostate cancer [95]. Inhibitors of NAAG peptidase are investigated in ischemic damage and diseases of the nervous system, including schizophrenia, diabetic neuropathy, Alzheimer’s disease, and amyotrophic lateral sclerosis [10].
- N-Methyl-D-aspartate (NMDA) is synthesized by an S-adenosyl-L-methionine-dependent enzyme from D-Asp [96]. The NMDA is a specific agonist of NMDA receptors that is used widely in neurological research [51]. It has been shown that NMDA administration can induce the release of some hypothalamic and pituitary hormones [89].
4.7.4. L-Asp and Cell-to-Cell Interactions
5. Aspartic Acid and Disease
5.1. Primary Disorders of L-Asp Metabolism
5.1.1. AGC1 (Aralar 1) Deficiency (OMIM ID #612949)
5.1.2. AGC2 (Citrin) Deficiency (OMIM ID #603471 and #605814)
5.1.3. Asparagine Synthetase Deficiency (OMIM ID #615574)
5.1.4. Canavan Disease (OMIM ID #271900)
5.1.5. Citrullinemia Type 1 (OMIM ID #215700)
5.1.6. Dicarboxylic Aminoaciduria (OMIM ID #222730)
5.2. Aspartate and Cancer
- cAST—The upregulation of cAST has been observed in pancreatic adenocarcinoma, colorectal cancer, breast cancer, acute myeloid leukemia, and hepatocellular carcinoma [121]. Although several cAST inhibitors have been developed and investigated as potential anti-cancer drugs, none has entered clinical trial [122].
- AGC1—Increased AGC1 (aralar 1, SLC25A12) expression and its mRNA levels are often elevated in tumors of the breast, pancreas, esophagus, colon, and ovaries [17,120,123,124,125]. It has been suggested that the expression of AGC1 plays a role in an increased incidence of cancer in patients with AGC2 deficiency [111,112]. SLC25A12 silencing by small interfering RNA significantly impaired HepG2 cell proliferation [111].
- Asparagine synthetase—It has been demonstrated that asparagine synthetase expression correlates with tumor grade and poor prognosis, and tumors that exhibit low asparagine synthetase activity and acquire asparagine from the extracellular environment are sensitive to therapy by asparaginase [37,67,70,126,127]. Currently, the therapy using asparaginase that depletes circulating asparagine is an integral component of chemotherapy for children and young adults with acute lymphoblastic leukemia [128]. Low expression of asparagine synthetase has also been demonstrated in human pancreatic carcinomas, suggesting the possible therapeutic potential of asparaginase therapy [129].
5.3. Aspartate and Psychiatric and Neurologic Disorders
5.4. L-Asp and BCAA in Diabetes
5.5. L-Asp and Hyperammonemia
| Mitochondria: | BCAA + 2-OG → BCKA + Glu |
| Glu + OA → 2-OG + L-Asp | |
| AGC1: | L-Asp transport to the cytosol |
| Cytosol: | L-Asp + 2-OG → OA + Glu |
| Glu + NH3 + ATP → Gln + ADP + Pi |
6. Aspartate as a Dietary Supplement
6.1. L-Aspartate
6.1.1. L-Asp as an Ergogenic Supplement
6.1.2. L-Asp as Ammonia Decreasing Supplement
6.1.3. Other Possible Indications
6.2. Aspartate-Containing Peptides as Artificial Sweeteners
6.2.1. Aspartame
6.2.2. Neotame
6.3. D-Aspartate
6.4. Asparagine
6.5. Side Effects of Aspartate and Aspartate-Containing Supplements
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Plisson, A.A.; Henry, É.O. Recherches sur les substances organiques azotées [Research on nitrogenous organic substances]. J. Pharm. Sci. Acc. 1830, 16, 729. [Google Scholar]
- Thangaratnarajah, C.; Ruprecht, J.J.; Kunji, E.R. Calcium-induced conformational changes of the regulatory domain of human mitochondrial aspartate/glutamate carriers. Nat. Commun. 2014, 5, 5491. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Wang, T.; Chen, W.W.; Freinkman, E.; Abu-Remaileh, M.; Sabatini, D.M. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 2015, 162, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Monné, M.; Vozza, A.; Lasorsa, F.M.; Porcelli, V.; Palmieri, F. Mitochondrial carriers for aspartate, glutamate and other amino acids: A review. Int. J. Mol. Sci. 2019, 20, 4456. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Aspartate-glutamate carrier 2 (citrin): A role in glucose and amino acid metabolism in the liver. BMB Rep. 2023, 56, 385–391. [Google Scholar] [CrossRef]
- Cavallero, A.; Marte, A.; Fedele, E. L-aspartate as an amino acid neurotransmitter: Mechanisms of the depolarization-induced release from cerebrocortical synaptosomes. J. Neurochem. 2009, 110, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Alleva, C.; Machtens, J.P.; Kortzak, D.; Weyand, I.; Fahlke, C. Molecular basis of coupled transport and anion conduction in excitatory amino acid transporters. Neurochem. Res. 2022, 47, 9–22. [Google Scholar] [CrossRef]
- Errico, F.; Mothet, J.P.; Usiello, A. D-Aspartate: An endogenous NMDA receptor agonist enriched in the developing brain with potential involvement in schizophrenia. J. Pharm. Biomed. Anal. 2015, 116, 7–17. [Google Scholar] [CrossRef]
- Moffett, J.R.; Ross, B.; Arun, P.; Madhavarao, C.N.; Namboodiri, A.M. N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Prog. Neurobiol. 2007, 81, 89–131. [Google Scholar] [CrossRef]
- Zhong, C.; Luo, Q.; Jiang, J. Blockade of N-acetylaspartylglutamate peptidases: A novel protective strategy for brain injuries and neurological disorders. Int. J. Neurosci. 2014, 124, 867–873. [Google Scholar] [CrossRef]
- Ramos, M.; del Arco, A.; Pardo, B.; Martínez-Serrano, A.; Martínez-Morales, J.R.; Kobayashi, K.; Yasuda, T.; Bogónez, E.; Bovolenta, P.; Saheki, T.; et al. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain. Res. Dev. Brain Res. 2003, 143, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Rodrigues, T.B.; Contreras, L.; Garzón, M.; Llorente-Folch, I.; Kobayashi, K.; Saheki, T.; Cerdan, S.; Satrústegui, J. Brain glutamine synthesis requires neuronal-born aspartate as amino donor for glial glutamate formation. J. Cereb. Blood Flow Metab. 2011, 31, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Contreras, L.; Satrústegui, J. De novo synthesis of glial glutamate and glutamine in young mice requires aspartate provided by the neuronal mitochondrial aspartate-glutamate carrier aralar/AGC1. Front. Endocrinol. 2013, 4, 149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ola, M.S.; Berkich, D.A.; Gardner, T.W.; Barber, A.J.; Palmieri, F.; Hutson, S.M.; LaNoue, K.F. Energy sources for glutamate neurotransmission in the retina: Absence of the aspartate/glutamate carrier produces reliance on glycolysis in glia. J. Neurochem. 2007, 101, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bermudez, J.; Baudrier, L.; La, K.; Zhu, X.G.; Fidelin, J.; Sviderskiy, V.O.; Papagiannakopoulos, T.; Molina, H.; Snuderl, M.; Lewis, C.A.; et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol. 2018, 20, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Luengo, A.; Danai, L.V.; Bush, L.N.; Diehl, F.F.; Hosios, A.M.; Lau, A.N.; Elmiligy, S.; Malstrom, S.; Lewis, C.A.; et al. Aspartate is an endogenous metabolic limitation for tumour growth. Nat. Cell Biol. 2018, 20, 782–788. [Google Scholar] [CrossRef]
- Alkan, H.F.; Walter, K.E.; Luengo, A.; Madreiter-Sokolowski, C.T.; Stryeck, S.; Lau, A.N.; Al-Zoughbi, W.; Lewis, C.A.; Thomas, C.J.; Hoefler, G.; et al. Cytosolic aspartate availability determines cell survival when glutamine is limiting. Cell Metab. 2018, 28, 706–720. [Google Scholar] [CrossRef]
- Holeček, M. Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 2023, 145, 155614. [Google Scholar] [CrossRef]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef]
- Holecek, M.; Sispera, L. Effects of arginine supplementation on amino acid profiles in blood and tissues in fed and overnight-fasted rats. Nutrients 2016, 8, 206. [Google Scholar] [CrossRef]
- Le Boucher, J.; Charret, C.; Coudray-Lucas, C.; Giboudeau, J.; Cynober, L. Amino acid determination in biological fluids by automated ion-exchange chromatography: Performance of Hitachi L-8500A. Clin. Chem. 1997, 43, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Windmueller, H.G.; Spaeth, A.E. Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch. Biochem. Biophys. 1976, 175, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. Renal arginine metabolism. J. Nutr. 2004, 134, 2791S–2795S. [Google Scholar] [CrossRef] [PubMed]
- Levillain, O. Expression and function of arginine-producing and consuming-enzymes in the kidney. Amino Acids 2012, 42, 1237–1252. [Google Scholar] [CrossRef]
- del Arco, A.; Satrústegui, J. Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J. Biol. Chem. 1998, 273, 23327–23334. [Google Scholar] [CrossRef]
- Borst, P. The malate-aspartate shuttle (Borst cycle): How it started and developed into a major metabolic pathway. IUBMB Life 2020, 72, 2241–2259. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Pardo, B.; Lasorsa, F.M.; del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.J.; Walker, J.E.; Saheki, T.; Satrústegui, J.; et al. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef]
- Bond, M.; Vadasz, G.; Somlyo, A.V.; Somlyo, A.P. Subcellular calcium and magnesium mobilization in rat liver stimulated in vivo with vasopressin and glucagon. J. Biol. Chem. 1987, 262, 15630–15636. [Google Scholar] [CrossRef]
- Keppens, S.; Vandenheede, J.R.; De Wulf, H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim. Biophys. Acta 1977, 496, 448–457. [Google Scholar] [CrossRef]
- Blackmore, P.F.; Waynick, L.E.; Blackman, G.E.; Graham, C.W.; Sherry, R.S. Alpha- and beta-adrenergic stimulation of parenchymal cell Ca2+ influx. Influence of extracellular pH. J. Biol. Chem. 1984, 259, 12322–12325. [Google Scholar] [CrossRef]
- Contreras, L.; Rial, E.; Cerdan, S.; Satrustegui, J. Uncoupling protein 2 (UCP2) Function in the Brain as Revealed by the Cerebral Metabolism of (1-13C)-Glucose. Neurochem. Res. 2017, 42, 108–114. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Liang, X.; Luo, Y.; Zen, K.; Zhang, C.Y. Uncoupling protein 2 negatively regulates glucose-induced glucagon-like peptide 1 secretion. J. Mol. Endocrinol. 2012, 48, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Lytovchenko, O.; Kunji, E.R.S. Expression and putative role of mitochondrial transport proteins in cancer. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 641–654. [Google Scholar] [CrossRef]
- Gorgoglione, R.; Porcelli, V.; Santoro, A.; Daddabbo, L.; Vozza, A.; Monné, M.; Di Noia, M.A.; Palmieri, L.; Fiermonte, G.; Palmieri, F. The human uncoupling proteins 5 and 6 (UCP5/SLC25A14 and UCP6/SLC25A30) transport sulfur oxyanions, phosphate and dicarboxylates. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar] [CrossRef]
- Sheid, B.; Morrris, H.P.; Roth, J.S. Distribution and activity of aspartate aminotransferase in some rapidly proliferating tissues. J. Biol. Chem. 1965, 240, 3016–3022. [Google Scholar] [CrossRef]
- Panteghini, M. Aspartate aminotransferase isoenzymes. Clin. Biochem. 1990, 23, 311–319. [Google Scholar] [CrossRef]
- Watford, M. Hepatic glutaminase expression: Relationship to kidney-type glutaminase and to the urea cycle. FASEB J. 1993, 7, 1468–1474. [Google Scholar] [CrossRef]
- Curthoys, N.P.; Watford, M. Regulation of glutaminase activity and glutamine metabolism. Annu. Rev. Nutr. 1995, 15, 133–159. [Google Scholar] [CrossRef]
- Márquez, J.; Matés, J.M.; Campos-Sandoval, J.A. Glutaminases. Adv. Neurobiol. 2016, 13, 133–171. [Google Scholar] [PubMed]
- Yoo, H.C.; Park, S.J.; Nam, M.; Kang, J.; Kim, K.; Yeo, J.H.; Kim, J.K.; Heo, Y.; Lee, H.S.; Lee, M.Y. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 2020, 31, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Suryawan, A.; Hawes, J.W.; Harris, R.A.; Shimomura, Y.; Jenkins, A.E.; Hutson, S.M. A molecular model of human branched-chain amino acid metabolism. Am. J. Clin. Nutr. 1998, 68, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef]
- Hutson, S.M.; Fenstermacher, D.; Mahar, C. Role of mitochondrial transamination in branched chain amino acid metabolism. J. Biol. Chem. 1988, 263, 3618–3625. [Google Scholar] [CrossRef] [PubMed]
- Morris, A. Newly characterized mitochondrial BCAA transporter. Nat. Rev. Endocrinol. 2019, 15, 626. [Google Scholar] [CrossRef] [PubMed]
- Haymond, M.W.; Miles, J.M. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 1982, 31, 86–89. [Google Scholar] [CrossRef]
- Galim, E.B.; Hruska, K.; Bier, D.M.; Matthews, D.E.; Haymond, M.W. Branched-chain amino acid nitrogen transfer to alamine in vivo in dogs. Direct isotopic determination with [15N]leucine. J. Clin. Investig. 1980, 66, 1295–1304. [Google Scholar] [CrossRef]
- Skeie, B.; Kvetan, V.; Gil, K.M.; Rothkopf, M.M.; Newsholme, E.A.; Askanazi, J. Branch-chain amino acids: Their metabolism and clinical utility. Crit. Care Med. 1990, 18, 549–571. [Google Scholar] [CrossRef]
- Genchi, G. An overview on D-amino acids. Amino Acids 2017, 49, 1521–1533. [Google Scholar] [CrossRef]
- D’Aniello, A. D-Aspartic acid: An endogenous amino acid with an important neuroendocrine role. Brain Res. Rev. 2007, 53, 215–234. [Google Scholar] [CrossRef]
- Topo, E.; Soricelli, A.; D’Aniello, A.; Ronsini, S.; D’Aniello, G. The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod. Biol. Endocrinol. 2009, 7, 120. [Google Scholar] [CrossRef]
- Di Fiore, M.M.; Boni, R.; Santillo, A.; Falvo, S.; Gallo, A.; Esposito, S.; Baccari, G.C. D-Aspartic acid in vertebrate reproduction: Animal models and experimental designs. Biomolecules 2019, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Usiello, A.; Di Fiore, M.M.; De Rosa, A.; Falvo, S.; Errico, F.; Santillo, A.; Nuzzo, T.; Chieffi Baccari, G. New evidence on the role of D-aspartate metabolism in regulating brain and endocrine system physiology: From preclinical observations to clinical applications. Int. J. Mol. Sci. 2020, 21, 8718. [Google Scholar] [CrossRef]
- Arkhipova, V.; Trinco, G.; Ettema, T.W.; Jensen, S.; Slotboom, D.J.; Guskov, A. Binding and transport of D-aspartate by the glutamate transporter homolog GltTk. eLife 2019, 8, e45286. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J.; Garlick, P.J. Protein and amino acid requirements and the composition of complementary foods. J. Nutr. 2003, 133, 2953S–2961S. [Google Scholar] [CrossRef]
- Wan, W.Y.; Milner-White, E.J. A natural grouping of motifs with an aspartate or asparagine residue forming two hydrogen bonds to residues ahead in sequence: Their occurrence at alpha-helical N termini and in other situations. J. Mol. Biol. 1999, 286, 1633–1649. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N. D-amino acid in elderly tissues. Biol. Pharm. Bull. 2005, 28, 1585–1589. [Google Scholar] [CrossRef]
- Geiger, T.; Clarke, S. Deamidation, isomerization, and racemization at asparaginyl and aspartyl residues in peptides. Succinimide-linked reactions that contribute to protein degradation. J. Biol. Chem. 1987, 262, 785–794. [Google Scholar] [CrossRef]
- Hooi, M.Y.; Raftery, M.J.; Truscott, R.J. Interconversion of the peptide isoforms of aspartate: Stability of isoaspartates. Mech. Ageing Dev. 2013, 134, 103–109. [Google Scholar] [CrossRef]
- Mamula, M.J.; Gee, R.J.; Elliott, J.I.; Sette, A.; Southwood, S.; Jones, P.J.; Blier, P.R. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J. Biol. Chem. 1999, 274, 22321–22327. [Google Scholar] [CrossRef]
- Rodwell, V.W.; Bender, D.; Botham, K. Harper’s Illustrated Biochemistry, 31st ed.; Lange medical book; McGraw-Hill’s Access Medicine: New York, NY, USA, 2018; 789p, ISSN 1045-5523. ISBN 1259837939/978-1259837937. [Google Scholar]
- Baynes, J.W.; Dominiczak, M.H. Medical Biochemistry, 6th ed.; Elsevier: Amsterdam, Netherlands, 2023; 744p, ISBN 9780323834506/9780323834513. [Google Scholar]
- Lane, A.N.; Fan, T.W. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Ali, E.S.; Sahu, U.; Ben-Sahra, I. Cancer cells tune the signaling pathways to empower de novo synthesis of nucleotides. Cancers 2019, 11, 688. [Google Scholar] [CrossRef]
- Milman, H.A.; Cooney, D.A. The distribution of L-asparagine synthetase in the principal organs of several mammalian and avian species. Biochem. J. 1974, 142, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, M.N.; Butterworth, E.A.; Kilberg, M.S. Asparagine synthetase: Regulation by cell stress and involvement in tumor biology. Am. J. Physiol. 2013, 304, E789–E799. [Google Scholar] [CrossRef] [PubMed]
- Boos, J.; Werber, G.; Ahlke, E.; Schulze-Westhoff, P.; Nowak-Göttl, U.; Würthwein, G.; Verspohl, E.J.; Ritter, J.; Jürgens, H. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur. J. Cancer 1996, 32A, 1544–1550. [Google Scholar] [CrossRef]
- Ray, R.M.; Viar, M.J.; Patel, T.B.; Johnson, L.R. Interaction of asparagine and EGF in the regulation of ornithine decarboxylase in IEC-6 cells. Am. J. Physiol. 1999, 276, G773–G780. [Google Scholar] [CrossRef]
- Krall, A.S.; Xu, S.; Graeber, T.G.; Braas, D.; Christofk, H.R. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat. Commun. 2016, 7, 11457. [Google Scholar] [CrossRef]
- Windmueller, H.G.; Spaeth, A.E. Source and fate of circulating citrulline. Am. J. Physiol. 1981, 241, E473–E480. [Google Scholar] [CrossRef]
- Morris, S.M., Jr. Arginine metabolism revisited. J. Nutr. 2016, 146, 2579S–2586S. [Google Scholar] [CrossRef]
- Yabaluri, N.; Bashyam, M.D. Hormonal regulation of gluconeogenic gene transcription in the liver. J. Biosci. 2010, 35, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, J.M. Ammonia production in muscle and other tissues: The purine nucleotide cycle. Physiol. Rev. 1972, 52, 382–414. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.; Hood, D.A.; Brown, O.M.; Terjung, R.L. Incorporation of 15N-leucine amine into ATP of fast-twitch muscle following stimulation. Biochem. Biophys. Res. Commun. 1985, 128, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Karl, I.E.; Garber, A.J.; Kipnis, D.M. Alanine and glutamine synthesis and release from skeletal muscle. III. Dietary and hormonal regulation. J. Biol. Chem. 1976, 251, 844–850. [Google Scholar] [CrossRef]
- Holecek, M.; Siman, P.; Vodenicarovova, M.; Kandar, R. Alterations in protein and amino acid metabolism in rats fed a branched-chain amino acid- or leucine-enriched diet during postprandial and postabsorptive states. Nutr. Metab. 2016, 13, 12. [Google Scholar] [CrossRef]
- She, P.; Reid, T.M.; Bronson, S.K.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef]
- Felig, P. Amino acid metabolism in man. Annu. Rev. Biochem. 1975, 44, 933–955. [Google Scholar] [CrossRef]
- Holeček, M. Muscle amino acid and adenine nucleotide metabolism during exercise and in liver cirrhosis: Speculations on how to reduce the harmful effects of ammonia. Metabolites 2022, 12, 971. [Google Scholar] [CrossRef]
- Newsholme, E.; Hardy, G. Supplementation of diets with nutritional pharmaceuticals. Nutrition 1997, 13, 837–839. [Google Scholar] [CrossRef]
- Hankard, R.G.; Haymond, M.W.; Darmaun, D. Role of glutamine as a glucose precursor in fasting humans. Diabetes 1997, 46, 1535–1541. [Google Scholar] [CrossRef]
- Bröer, S. Amino acid transporters as modulators of glucose homeostasis. Trends Endocrinol. Metab. 2022, 33, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Skopec, F.; Skalská, H.; Sprongl, L. Effect of alanyl-glutamine on leucine and protein metabolism in endotoxemic rats. JPEN J. Parenter. Enter. Nutr. 2000, 24, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M.; Sispera, L.; Skalska, H. Enhanced glutamine availability exerts different effects on protein and amino acid metabolism in skeletal muscle from healthy and septic rats. JPEN J. Parenter. Enter. Nutr. 2015, 39, 847–854. [Google Scholar] [CrossRef]
- Holecek, M.; Sispera, L. Glutamine deficiency in extracellular fluid exerts adverse effects on protein and amino acid metabolism in skeletal muscle of healthy, laparotomized, and septic rats. Amino Acids 2014, 46, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Durán, R.V.; Oppliger, W.; Robitaille, A.M.; Heiserich, L.; Skendaj, R.; Gottlieb, E.; Hall, M. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 2012, 47, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Patneau, D.K.; Mayer, M.L. Structure-activity relationships for amino acid transmitter candidates acting at N-methyl-D-aspartate and quisqualate receptors. J. Neurosci. 1990, 10, 2385–2399. [Google Scholar] [CrossRef]
- Downing, J.A.; Joss, J.; Scaramuzzi, R.J. The effects of N-methyl-D,L-aspartic acid and aspartic acid on the plasma concentration of gonadotrophins, GH and prolactin in the ewe. J. Endocrinol. 1996, 149, 65–72. [Google Scholar] [CrossRef]
- Nadler, J.V. Aspartate release and signalling in the hippocampus. Neurochem. Res. 2011, 36, 668–676. [Google Scholar] [CrossRef]
- Wolosker, H.; D’Aniello, A.; Snyder, S.H. D-aspartate disposition in neuronal and endocrine tissues: Ontogeny, biosynthesis and release. Neuroscience 2000, 100, 183–189. [Google Scholar] [CrossRef]
- Hashimoto, A.; Kumashiro, S.; Nishikawa, T.; Oka, T.; Takahashi, K.; Mito, T.; Takashima, S.; Doi, N.; Mizutani, Y.; Yamazaki, T. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J. Neurochem. 1993, 61, 348–351. [Google Scholar] [CrossRef]
- Fujita, T.; Katsukawa, H.; Yodoya, E.; Wada, M.; Shimada, A.; Okada, N.; Yamamoto, A.; Ganapathy, V. Transport characteristics of N-acetyl-L-aspartate in rat astrocytes: Involvement of sodium-coupled high-affinity carboxylate transporter NaC3/NaDC3-mediated transport system. J. Neurochem. 2005, 93, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Tahay, G.; Wiame, E.; Tyteca, D.; Courtoy, P.J.; Van Schaftingen, E. Determinants of the enzymatic activity and the subcellular localization of aspartate N-acetyltransferase. Biochem. J. 2012, 441, 105–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinto, J.T.; Suffoletto, B.P.; Berzin, T.M.; Qiao, C.H.; Lin, S.; Tong, W.P.; May, F.; Mukherjee, B.; Heston, W.D. Prostate-specific membrane antigen: A novel folate hydrolase in human prostatic carcinoma cells. Clin. Cancer Res. 1996, 2, 1445–1451. [Google Scholar]
- Shibata, K.; Imanishi, D.; Abe, K.; Suzuki, M.; Takahashi, S.; Kera, Y. d-Aspartate N-methyltransferase catalyzes biosynthesis of N-methyl-d-aspartate (NMDA), a well-known selective agonist of the NMDA receptor, in mice. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140527. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.J.; Du, J.; Sloat, S.R.; Contreras, L.; Linton, J.D.; Turner, S.J.; Sadilek, M.; Satrústegui, J.; Hurley, J.B. Pyruvate kinase and aspartate-glutamate carrier distributions reveal key metabolic links between neurons and glia in retina. Proc. Natl. Acad. Sci. USA 2014, 111, 15579–15584. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Martinez-Hernandez, A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979, 161, 303–310. [Google Scholar] [CrossRef]
- Daikhin, Y.; Yudkoff, M. Compartmentation of brain glutamate metabolism in neurons and glia. J. Nutr. 2000, 130, 1026S–1031S. [Google Scholar] [CrossRef]
- McKenna, M.C. The glutamate-glutamine cycle is not stoichiometric: Fates of glutamate in brain. J. Neurosci. Res. 2007, 85, 3347–3358. [Google Scholar] [CrossRef]
- Broeks, M.H.; van Karnebeek, C.D.M.; Wanders, R.J.A.; Jans, J.J.M.; Verhoeven-Duif, N.M. Inborn disorders of the malate aspartate shuttle. J. Inherit. Metab. Dis. 2021, 44, 792–808. [Google Scholar] [CrossRef]
- Dahlin, M.; Martin, D.A.; Hedlund, Z.; Jonsson, M.; von Döbeln, U.; Wedell, A. The ketogenic diet compensates for AGC1 deficiency and improves myelination. Epilepsia 2015, 56, e176–e181. [Google Scholar] [CrossRef]
- Saheki, T.; Moriyama, M.; Funahashi, A.; Kuroda, E. AGC2 (citrin) deficiency-from recognition of the disease till construction of therapeutic procedures. Biomolecules 2020, 10, 1100. [Google Scholar] [CrossRef]
- Arai-Ichinoi, N.; Kikuchi, A.; Wada, Y.; Sakamoto, O.; Kure, S. Hypoglycemic attacks and growth failure are the most common manifestations of citrin deficiency after 1 year of age. J. Inherit. Metab. Dis. 2021, 44, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Yazaki, M.; Tanaka, N.; Sano, K.; Hashimoto, E.; Takei, Y.; Song, Y.Z.; Tanaka, E.; Kiyosawa, K.; Saheki, T.; et al. Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Tavoulari, S.; Lacabanne, D.; Thangaratnarajah, C.; Kunji, E.R.S. Pathogenic variants of the mitochondrial aspartate/glutamate carrier causing citrin deficiency. Trends Endocrinol. Metab. 2022, 33, 539–553. [Google Scholar] [CrossRef]
- Chang, K.W.; Chen, H.L.; Chien, Y.H.; Chen, T.C.; Yeh, C.T. SLC25A13 gene mutations in Taiwanese patients with non-viral hepatocellular carcinoma. Mol. Genet. Metab. 2011, 103, 293–296. [Google Scholar] [CrossRef]
- Tsai, C.W.; Yang, C.C.; Chen, H.L.; Hwu, W.L.; Wu, M.Z.; Liu, K.L.; Wu, M.S. Homozygous SLC25A13 mutation in a Taiwanese patient with adult-onset citrullinemia complicated with steatosis and hepatocellular carcinoma. J. Formos. Med. Assoc. 2006, 105, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, N.; Sekijima, Y.; Takei, Y.; Ikeda, S.; Kawasaki, S.; Kobayashi, K.; Saheki, T. Hepatocellular carcinoma in a case of adult-onset type II citrullinemia. Intern. Med. 2003, 42, 978–982. [Google Scholar] [CrossRef][Green Version]
- Ito, T.; Shiraki, K.; Sekoguchi, K.; Yamanaka, T.; Sugimoto, K.; Takase, K.; Tameda, Y.; Nakano, T. Hepatocellular carcinoma associated with adult-type citrullinemia. Dig. Dis. Sci. 2000, 45, 2203–2206. [Google Scholar] [CrossRef]
- Infantino, V.; Dituri, F.; Convertini, P.; Santarsiero, A.; Palmieri, F.; Todisco, S.; Mancarella, S.; Giannelli, G.; Iacobazzi, V. Epigenetic upregulation and functional role of the mitochondrial aspartate/glutamate carrier isoform 1 in hepatocellular carcinoma. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 38–47. [Google Scholar] [CrossRef]
- Mention, K.; Joncquel Chevalier Curt, M.; Dessein, A.F.; Douillard, C.; Dobbelaere, D.; Vamecq, J. Citrin deficiency: Does the reactivation of liver aralar-1 come into play and promote HCC development? Biochimie 2021, 190, 20–23. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Andring, J.T.; McKenna, R.; Kilberg, M.S. Asparagine synthetase: Function, structure, and role in disease. J. Biol. Chem. 2017, 292, 19952–19958. [Google Scholar] [CrossRef]
- Ruzzo, E.K.; Capo-Chichi, J.M.; Ben-Zeev, B.; Chitayat, D.; Mao, H.; Pappas, A.L.; Hitomi, Y.; Lu, Y.F.; Yao, X.; Hamdan, F.F. Deficiency of asparagine synthetase causes congenital microcephaly and a progressive form of encephalopathy. Neuron 2013, 80, 429–441. [Google Scholar] [CrossRef]
- Sacharow, S.J.; Dudenhausen, E.E.; Lomelino, C.L.; Rodan, L.; El Achkar, C.M.; Olson, H.E.; Genetti, C.A.; Agrawal, P.B.; McKenna, R.; Kilberg, M.S. Characterization of a novel variant in siblings with asparagine synthetase deficiency. Mol. Genet. Metab. 2018, 123, 317–325. [Google Scholar] [CrossRef]
- Alrifai, M.T.; Alfadhel, M. Worsening of seizures after asparagine supplementation in a child with asparagine synthetase deficiency. Pediatr. Neurol. 2016, 58, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar] [PubMed]
- Peghini, P.; Janzen, J.; Stoffel, W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997, 16, 3822–3832. [Google Scholar] [CrossRef]
- Yahyaoui, R.; Pérez-Frías, J. Amino acid transport defects in human inherited metabolic disorders. Int. J. Mol. Sci. 2019, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Gorgoglione, R.; Impedovo, V.; Riley, C.L.; Fratantonio, D.; Tiziani, S.; Palmieri, L.; Dolce, V.; Fiermonte, G. Aspartate biosynthesis in cancer cells: Role of mitochondrial transporters and new therapeutic perspectives. Cancers 2022, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Y.; Wu, Y.; Zheng, M.; Sun, D.; Li, H.; Chen, L. Glutamic oxaloacetic transaminase 1 as a potential target in human cancer. Eur. J. Pharmacol. 2022, 917, 174754. [Google Scholar] [CrossRef]
- Miyo, M.; Konno, M.; Nishida, N.; Sueda, T.; Noguchi, K.; Matsui, H.; Colvin, H.; Kawamoto, K.; Koseki, J.; Haraguchi, N. Metabolic adaptation to nutritional stress in human colorectal cancer. Sci. Rep. 2016, 6, 38415. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Punzi, G.; Obre, E.; Lacombe, D.; De Grassi, A.; Pierri, C.L.; Rossignol, R. AGC1/2, the mitochondrial aspartate-glutamate carriers. Biochim. Biophys. Acta 2016, 1863, 2394–2412. [Google Scholar] [CrossRef]
- Lv, Y.; Yuan, C.H.; Han, L.Y.; Huang, G.R.; Ju, L.C.; Chen, L.H.; Han, H.Y.; Zhang, C.; Zeng, L.H. The overexpression of SLC25A13 predicts poor prognosis and is correlated with immune cell infiltration in patients with skin cutaneous melanoma. Dis. Markers 2022, 2022, 4091978. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Gui, D.Y.; Hosios, A.M.; Bush, L.N.; Freinkman, E.; Vander Heiden, M.G. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015, 162, 552–563. [Google Scholar] [CrossRef]
- Broome, J.D. Studies on the mechanism of tumor inhibition by L-asparaginase. Effects of the enzyme on asparagine levels in the blood, normal tissues, and 6C3HED lymphomas of mice: Differences in asparagine formation and utilization in asparaginase-sensitive and -resistant lymphoma cells. J. Exp. Med. 1968, 127, 1055–1072. [Google Scholar] [PubMed]
- Egler, R.A.; Ahuja, S.P.; Matloub, Y. L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J. Pharmacol. Pharmacother. 2016, 7, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Dufour, E.; Gay, F.; Aguera, K.; Scoazec, J.Y.; Horand, F.; Lorenzi, P.L.; Godfrin, Y. Pancreatic tumor sensitivity to plasma L-asparagine starvation. Pancreas 2012, 41, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Phillips, C.; Trillo, L.; De Miguel, Z.; Das, D.; Salehi, A. The role of NMDA receptors in the pathophysiology and treatment of mood disorders. Neurosci. Biobehav. Rev. 2014, 47, 336–358. [Google Scholar] [CrossRef]
- Errico, F.; Napolitano, F.; Squillace, M.; Vitucci, D.; Blasi, G.; de Bartolomeis, A.; Bertolino, A.; D’Aniello, A.; Usiello, A. Decreased levels of D-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. J. Psychiatr. Res. 2013, 47, 1432–1437. [Google Scholar] [CrossRef]
- Paslakis, G.; Träber, F.; Roberz, J.; Block, W.; Jessen, F. N-acetyl-aspartate (NAA) as a correlate of pharmacological treatment in psychiatric disorders: A systematic review. Eur. Neuropsychopharmacol. 2014, 24, 1659–1675. [Google Scholar] [CrossRef]
- Xu, J.; Jakher, Y.; Ahrens-Nicklas, R.C. Brain branched-chain amino acids in maple syrup urine disease: Implications for neurological disorders. Int. J. Mol. Sci. 2020, 21, 7490. [Google Scholar] [CrossRef]
- Holeček, M. Why are branched-chain amino acids increased in starvation and diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. The role of skeletal muscle in the pathogenesis of altered concentrations of branched-chain amino acids (valine, leucine, and isoleucine) in liver cirrhosis, diabetes, and other diseases. Physiol. Res. 2021, 70, 293–305. [Google Scholar] [CrossRef]
- Holeček, M.; Vodeničarovová, M.; Fingrová, R. Dual effects of beta-hydroxy-beta-methylbutyrate (HMB) on amino acid, energy, and protein metabolism in the liver and muscles of rats with streptozotocin-induced type 1 diabetes. Biomolecules 2020, 10, 1475. [Google Scholar] [CrossRef]
- Brosnan, J.T.; Man, K.C.; Hall, D.E.; Colbourne, S.A.; Brosnan, M.E. Interorgan metabolism of amino acids in streptozotocin-diabetic ketoacidotic rat. Am. J. Physiol. 1983, 244, E151–E158. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, T.; Alvarez, B.; Busquets, S.; Carbó, N.; López-Soriano, F.J.; Argilés, J.M. The increased skeletal muscle protein turnover of the streptozotocin diabetic rat is associated with high concentrations of branched-chain amino acids. Biochem. Mol. Med. 1997, 61, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Role of impaired glycolysis in perturbations of amino acid metabolism in diabetes mellitus. Int. J. Mol. Sci. 2023, 24, 1724. [Google Scholar] [CrossRef]
- Hayashi, M.; Ohnishi, H.; Kawade, Y.; Muto, Y.; Takahashi, Y. Augmented utilization of branched-chain amino acids by skeletal muscle in decompensated liver cirrhosis in special relation to ammonia detoxication. Gastroenterol. Jpn. 1981, 16, 64–70. [Google Scholar] [CrossRef]
- Holecek, M.; Sprongl, L.; Tichý, M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism 2000, 49, 1330–1334. [Google Scholar] [CrossRef]
- Holecek, M.; Kandar, R.; Sispera, L.; Kovarik, M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: Different sensitivity of red and white muscle. Amino Acids 2011, 40, 575–584. [Google Scholar] [CrossRef]
- Rodney, S.; Boneh, A. Amino acid profiles in patients with urea cycle disorders at admission to hospital due to metabolic decompensation. JIMD Rep. 2013, 9, 97–104. [Google Scholar] [PubMed]
- Holeček, M.; Vodeničarovová, M. Muscle wasting and branched-chain amino acid, alpha-ketoglutarate, and ATP depletion in a rat model of liver cirrhosis. Int. J. Exp. Pathol. 2018, 99, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.P.; Li, Y.; Liu, Y.; Wang, H.L. Relationship between the incidence of non-hepatic hyperammonemia and the prognosis of patients in the intensive care unit. World J. Gastroenterol. 2020, 26, 7222–7231. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.; Davuluri, G.; Hill, E.A.; Moyer, M.; Runkana, A.; Prayson, R.; van Lunteren, E.; Dasarathy, S. Hyperammonemia results in reduced muscle function independent of muscle mass. Am. J. Physiol. 2016, 310, G163–G170. [Google Scholar] [CrossRef]
- Kumar, A.; Davuluri, G.; Silva, R.N.E.; Engelen, M.P.K.J.; Ten Have, G.A.M.; Prayson, R.; Deutz, N.E.P.; Dasarathy, S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 2017, 65, 2045–2058. [Google Scholar] [CrossRef]
- Trudeau, F. Aspartate as an ergogenic supplement. Sports Med. 2008, 38, 9–16. [Google Scholar] [CrossRef]
- Ji, L.L.; Miller, R.H.; Nagle, F.J.; Lardy, H.A.; Stratman, F.W. Amino acid metabolism during exercise in trained rats: The potential role of carnitine in the metabolic fate of branched-chain amino acids. Metabolism 1987, 36, 748–752. [Google Scholar] [CrossRef]
- Adán, C.; Ardévol, A.; Rafecas, I.; Remesar, X.; Alemany, M.; Fernández-López, J.A. Amino acid nitrogen handling by hind leg muscle of the rat during exercise. Arch. Physiol. Biochem. 1997, 105, 478–486. [Google Scholar] [CrossRef]
- Bergström, J.; Fürst, P.; Hultman, E. Free amino acids in muscle tissue and plasma during exercise in man. Clin. Physiol. 1985, 5, 155–160. [Google Scholar] [CrossRef]
- Colombani, P.C.; Bitzi, R.; Frey-Rindova, P.; Frey, W.; Arnold, M.; Langhans, W.; Wenk, C. Chronic arginine aspartate supplementation in runners reduces total plasma amino acid level at rest and during a marathon run. Eur. J. Nutr. 1999, 38, 263–270. [Google Scholar] [CrossRef]
- Lancha, A.H., Jr.; Recco, M.B.; Abdalla, D.S.; Curi, R. Effect of aspartate, asparagine, and carnitine supplementation in the diet on metabolism of skeletal muscle during a moderate exercise. Physiol. Behav. 1995, 57, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Marquezi, M.L.; Roschel, H.A.; dos Santa Costa, A.; Sawada, L.A.; Lancham, A.H., Jr. Effect of aspartate and asparagine supplementation on fatigue determinants in intense exercise. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Ammonia removal by metabolic scavengers for the prevention and treatment of hepatic encephalopathy in cirrhosis. Drugs R D 2021, 21, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.; Anandh Babu, P.V.; Shyamaladevi, C.S. Protective effect of aspartate and glutamate on cardiac mitochondrial function during myocardial infarction in experimental rats. Chem. Biol. Interact. 2008, 176, 227–233. [Google Scholar] [CrossRef]
- Sivakumar, R.; Babu, P.V.; Shyamaladevi, C.S. Aspartate and glutamate prevents isoproterenol-induced cardiac toxicity by alleviating oxidative stress in rats. Exp. Toxicol. Pathol. 2011, 63, 137–142. [Google Scholar] [CrossRef]
- Hou, E.; Sun, N.; Zhang, F.; Zhao, C.; Usa, K.; Liang, M.; Tian, Z. Malate and aspartate increase L-arginine and nitric oxide and attenuate hypertension. Cell Rep. 2017, 19, 1631–1639. [Google Scholar] [CrossRef]
- Ichikawa, S.; Gohda, T.; Murakoshi, M.; Li, Z.; Adachi, E.; Koshida, T.; Suzuki, Y. Aspartic acid supplementation ameliorates symptoms of diabetic kidney disease in mice. FEBS Open Bio 2020, 10, 1122–1134. [Google Scholar] [CrossRef]
- Nofre, C.; Tinti, J.-M. Neotame: Discovery, properties, utility. Food Chem. 2000, 69, 245–257. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Burdock, G.A.; Doull, J.; Kroes, R.M.; Marsh, G.M.; Pariza, M.W.; Spencer, P.S.; Waddell, W.J.; Walker, R.; Williams, G.M. Aspartame: A safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Crit. Rev. Toxicol. 2007, 37, 629–727. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Lee, Y.Y. Neurophysiological symptoms and aspartame: What is the connection? Nutr. Neurosci. 2018, 21, 306–316. [Google Scholar] [CrossRef]
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R. Artificial sweeteners and risk of cardiovascular diseases: Results from the prospective NutriNet-Santé cohort. BMJ 2022, 378, e071204. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef]
- Naddaf, M. Aspartame is a possible carcinogen: The science behind the decision. Nature, 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W. Clinical and forensic toxicology of methanol. Forensic Sci. Rev. 2021, 33, 117–143. [Google Scholar] [PubMed]
- Goran, M.I.; Plows, J.F.; Ventura, E.E. Effects of consuming sugars and alternative sweeteners during pregnancy on maternal and child health: Evidence for a secondhand sugar effect. Proc. Nutr. Soc. 2019, 78, 262–271. [Google Scholar] [CrossRef]
- Azad, M.B.; Sharma, A.K.; de Souza, R.J.; Dolinsky, V.W.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Lefebvre, D.L.; Sears, M.R.; et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr. 2016, 170, 662–670. [Google Scholar] [CrossRef]
- Zhu, Y.; Olsen, S.F.; Mendola, P.; Halldorsson, T.I.; Rawal, S.; Hinkle, S.N.; Yeung, E.H.; Chavarro, J.E.; Grunnet, L.G.; Granström, C.; et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: A prospective cohort study. Int. J. Epidemiol. 2017, 46, 1499–1508. [Google Scholar] [CrossRef]
- Wilk, K.; Korytek, W.; Pelczyńska, M.; Moszak, M.; Bogdański, P. The effect of artificial sweeteners use on sweet taste perception and weight loss efficacy: A review. Nutrients 2022, 14, 1261. [Google Scholar] [CrossRef]
- Roshanzamir, F.; Safavi, S.M. The putative effects of D-Aspartic acid on blood testosterone levels: A systematic review. Int. J. Reprod. Biomed. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Leutholtz, B. D-aspartic acid supplementation combined with 28 days of heavy resistance training has no effect on body composition, muscle strength, and serum hormones associated with the hypothalamo-pituitary-gonadal axis in resistance-trained men. Nutr. Res. 2013, 33, 803–810. [Google Scholar] [CrossRef]
- Melville, G.W.; Siegler, J.C.; Marshall, P.W.M. The effects of d-aspartic acid supplementation in resistance-trained men over a three month training period: A randomised controlled trial. PLoS ONE 2017, 12, e0182630. [Google Scholar] [CrossRef]
- D’Aniello, G.; Grieco, N.; Di Filippo, M.A.; Cappiello, F.; Topo, E.; D’Aniello, E.; Ronsini, S. Reproductive implication of D-aspartic acid in human pre-ovulatory follicular fluid. Hum. Reprod. 2007, 22, 3178–3183. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; Li, S.; Pi, D.; Zhu, H.; Hou, Y.; Shi, H.; Leng, W. Asparagine attenuates intestinal injury, improves energy status and inhibits AMP-activated protein kinase signalling pathways in weaned piglets challenged with Escherichia coli lipopolysaccharide. Br. J. Nutr. 2015, 114, 553–565. [Google Scholar] [CrossRef]
- Ahlborg, B.; Ekelund, L.G.; Nilsson, C.G. Effect of potassium-magnesium-aspartate on the capacity for prolonged exercise in man. Acta Physiol. Scand. 1968, 74, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Hagan, R.D.; Upton, S.J.; Duncan, J.J.; Cummings, J.M.; Gettman, L.R. Absence of effect of potassium-magnesium aspartate on physiologic responses to prolonged work in aerobically trained men. Int. J. Sports Med. 1982, 3, 177–181. [Google Scholar] [CrossRef]
- Maughan, R.J.; Sadler, D.J. The effects of oral administration of salts of aspartic acid on the metabolic response to prolonged exhausting exercise in man. Int. J. Sports Med. 1983, 4, 119–123. [Google Scholar] [CrossRef]
- Sen Gupta, J.; Srivastava, K.K. Effect of potassium-magnesium aspartate on endurance work in man. Indian J. Exp. Biol. 1973, 11, 392–394. [Google Scholar] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. https://doi.org/10.3390/nu15184023
Holeček M. Aspartic Acid in Health and Disease. Nutrients. 2023; 15(18):4023. https://doi.org/10.3390/nu15184023
Chicago/Turabian StyleHoleček, Milan. 2023. "Aspartic Acid in Health and Disease" Nutrients 15, no. 18: 4023. https://doi.org/10.3390/nu15184023
APA StyleHoleček, M. (2023). Aspartic Acid in Health and Disease. Nutrients, 15(18), 4023. https://doi.org/10.3390/nu15184023




