Abstract
Breast cancer (BC) is a lethal malignancy with high morbidity and mortality but lacks effective treatments thus far. Despite the introduction of immune checkpoint inhibitors (ICIs) (including PD-1/PD-L1 inhibitors), durable and optimal clinical benefits still remain elusive for a considerable number of BC patients. To break through such a dilemma, novel ICI-based combination therapy has been explored for enhancing the therapeutic effect. Recent evidence has just pointed out that the HDAC2 inhibitor (HDAC2i), which has been proven to exhibit an anti-cancer effect, can act as a sensitizer for ICIs therapy. Simultaneously, dietary intervention, as a crucial supportive therapy, has been reported to provide ingredients containing HDAC2 inhibitory activity. Thus, the novel integration of dietary intervention with ICIs therapy may offer promising possibilities for improving treatment outcomes. In this study, we first conducted the differential expression and prognostic analyses of HDAC2 and BC patients using the GENT2 and Kaplan–Meier plotter platform. Then, we summarized the potential diet candidates for such an integrated therapeutic strategy. This article not only provides a whole new therapeutic strategy for an HDAC2i-containing diet combined with PD-1/PD-L1 inhibitors for BC treatment, but also aims to ignite enthusiasm for exploring this field.
1. Introduction
Breast cancer is one of the most common malignancies in women worldwide, which can occur in both men and women, and ranks as the second cancer-related cause of death worldwide [1,2]. Its development has been reported to be significantly associated with dietary habits (such as alcohol consumption, high intake of total fat, and low consumption of dietary fiber, etc.) [3]. Five molecular subtypes are classified based on the expression levels of the estrogen receptor (ER), progestogen receptor (PR), human epidermal growth factor receptor 2 (HER2) and Ki-67: Luminal A (ER positive and/or PR positive, HER2 negative, Ki-67 < 20%); Luminal B (HER2 negative/B1: ER positive and/or PR < 20%, HER2 negative, Ki-67 ≥ 20%; HER2 positive/B2: ER positive and/or PR positive, HER2 overexpression); HER2 positive type (HER2 positive, ER negative, PR negative); triple-negative (HER2 negative, ER negative, and PR negative) (TNBC); and other special types [1,2]. The recurrence and/or metastasis of breast cancer significantly contributes to breast cancer-specific mortality, as recurrent tumors tend to be more aggressive and often show resistance to currently available treatments [4]. Thus, to improve treatment outcomes, including overall survival (OS) and disease control, endocrine-, chemo-, targeted- and immuno-therapy alone or in combination have been studied extensively [5]. Immune checkpoint inhibitors (ICIs), which reactivate exhausted CD8+ T cells to kill tumor cells [6], have especially made significant progress in the clinical application of breast cancer treatment [5]. However, the benefit population of breast cancer from ICI monotherapy is limited, making the combined regimen a new research hotspot [5].
The usage of ICIs is to harness the body’s immune system to recognize and attack cancer cells more effectively. For instance, by blocking the interaction between immune checkpoints, such as programmed cell death protein 1 (PD-1) and its ligand (PD-L1), the “brakes” on the immune system could be released, allowing immune effector cells to recognize and attack cancer cells more effectively [5]. Fortunately, ICIs have demonstrated a certain efficacy in combination with chemotherapy in the treatment of both early- and late-stage triple-negative breast cancer (TNBC) [7]. The KEYNOTE-355 trial evaluated the efficacy of PD-1 antibody pembrolizumab in combination with chemotherapy for patients with metastatic TNBC. In the context of early-stage disease, the KEYNOTE-522 study demonstrated significant benefits by adding pembrolizumab to chemotherapy, irrespective of the PD-L1 status [7,8,9]. Furthermore, ongoing studies are currently exploring the application of ICI therapy for hormone receptor-positive and HER2-positive breast cancer [7]. Additionally, novel ICIs are being investigated for their potential across all breast cancer subtypes [7].
Despite these advancements, there are still important unresolved issues. For instance, determining the optimal partners for ICIs to mitigate ICI side effects, and predictive biomarkers to identify who benefit from ICI therapy [7,10]. Another issue that cannot be ignored is that ICIs caused loss of appetite, consequently reducing the patient’s nutritional intake [11], and aggravating the patient’s already damaged physique and immunity, forming a vicious circle.
Histone deacetylases 2 (HDAC2), as a member of HDACs, is a special protease involved in the tightening of the chromatin structure and suppression of gene transcription. Its expression level was significantly in positive correlation with the poor overall survival of patients with BC or hepatocellular carcinoma (HCC) and with the potential adverse effect of patients to PD-1 antibody therapy [12,13,14,15]. Moreover, breast cancer tissues show significantly higher HDAC2 expression than normal breast tissue [13,14,15]. HDAC2 inhibition is a potential anti-cancer agent against breast cancer [16,17,18]. HDAC2 inhibitors can exert a synergistic effect with ICIs [18,19]. Given that short-chain fatty acids (SCFA, e.g., butyric acid) are the fermentation products of dietary fibers, dietary intervention may provide a superior safety profile improving immunotherapy by functioning as HDAC2i [20]. Furthermore, SCFA-producer Lachnoclostridium in tumors was positively associated with infiltrating CD8+ T cells and chemokines of CXCL9 and CXCL10, as well as better survival in melanoma cancer [21].
Healthy eating is recommended for cancer patient survivors according to guidelines. Besides providing nutrition and energy intake, dietary intervention has been found to improve the body’s immunity and anti-cancer activity [22]. Therefore, based on our previous study and the latest findings, this review, for the first time, brings out a novel dietary intervention with potential synergistic effects for ICIs therapy by adding HDAC2i containing food, aiming to ignite enthusiasm to explore the potential application of an HDAC2i-containing diet combined with ICI for BC patients, and to eventually improve the clinical benefits for patients.
2. Materials and Methods
A comprehensive search of the literature was conducted to identify relevant reports from 2000 to 2023, using the PubMed and Web of Science databases. The search utilized various keywords, either individually or in combinations, including PD1, PDL1, breast cancer, HDAC2 inhibitor, immune checkpoint inhibitors, dietary intervention, herbal remedy, neoadjuvant, and immunotherapy sensitizer. The identified articles were thoroughly reviewed, with preference given to the most recent ones. Additional sources were extracted from the reference lists of selected papers. Priority was primarily assigned to original research and review articles based on animal studies and clinical trials.
Differential expression and prognostic analysis. The differential expression analysis of HDAC2 across cancer and normal tissues were obtained from the GENT2 platform (http://gent2.appex.kr/gent2/, accessed on 11 July 2023). The prognostic analysis was performed by applying the Kaplan–Meier plotter platform (https://kmplot.com/analysis/index.php?p=background, accessed on 11 July 2023). BC patients with high or low HDAC2 protein (or gene) expression were divided by median expression level or by best cutoff value. Cutoff values used in analysis for HDAC2 gene and protein were 1568 and 3, respectively. The total amount of samples for analyzing the HDAC2 gene figure was 4929 (high 2465 vs. low 2464), its counterpart for HDAC2 protein was 65 (high 47 vs. low 18).
3. Results
The HDAC2 expression profile across cancer and normal tissues was detected by using the GENT2 platform. Significant difference in HDAC2 expression was displayed between breast cancer tissue and normal breast tissue (p < 0.001, Log2FC, 0.122) (Figure 1A). (Significant test results by Two-sample t-test for the HDAC2 expression profile is displayed in Supplementary Materials). As shown in Kaplan–Meier survival curves (Figure 1B,C), BC Patients with a high expression of the HDAC2 gene [hazard ratio (HR), 1.65; p < 1 × 10−16; Figure 1B] or high expression level of the HDAC2 protein (HR, 2; p = 0.059; Figure 1C) exhibited a less favorable prognosis compared with patients with low expression levels. However, a significant difference has not been detected in the group of HDAC2 proteins.
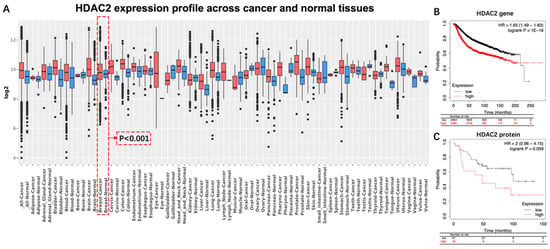
Figure 1.
Differential expression and prognostic significance of HDAC2 in breast cancer. (A) Significant difference existed between normal breast tissue and breast cancer tissue (p < 0.001, Log2FC = 0.122): (B) BC patients with either high HDAC2 level of gene, or (C) high HDAC2 level of protein exhibited unfavorable overall survival. HR, hazard ratio; BC, breast cancer.
4. Discussion
4.1. HDAC2: A Potential Index of Aggressiveness and a Therapeutic Target against BC
HDAC2 is an enzyme involved in the tightening of the chromatin structure and suppression of gene transcription. Evidence has indicated that HDAC2 is overexpressed in breast cancer cells compared to normal breast tissue. Higher levels of HDAC2 have also been associated with more aggressive tumor characteristics, such as increased cell proliferation, invasion, and metastasis [12,13,14,15]. For instance, a clinical study that included 226 BC patients reported that expression of HDAC2 protein is significantly higher in breast cancer than in benign tumors and indicates that HDAC2 may be involved in invasion, metastasis, anthracyclines therapy resistance, and poor prognosis of sporadic breast cancer (Table 1) [14]. Another study, conducted by Afroditi Nonni’s group, also examined the expression of HDAC2 in 118 deceased sporadic BC patients and its correlation with clinicopathological characteristics of the tumor and the prognosis of the patient. It was found that high expression of the HDAC2 protein is associated with higher histological grade, stage of disease, and worse prognosis (Table 1) [12]. As for the expression of the HDAC2 gene, evidence has also reported that HDAC2 is one of the most commonly amplified genes in aggressive basal-like breast cancer. Additionally, overexpression of HDAC2 was significantly correlated with high tumor grade, positive lymph node status, and poor prognosis [13]. Those results are also consistent with the outcomes of our analysis. For instance, our results indicated that expression of HDAC2 in BC tissue was significantly higher than that in breast normal tissue (p < 0.001) (Figure 1). Moreover, the trend can be observed that BC patients with high expression of HDAC2 gained worse prognosis than those without (Figure 1C). However, a significant difference was been detected (p = 0.059) (Figure 1). We speculated that the reason may be due to an insufficient sample size (n = 75).

Table 1.
Correlation between HDAC2 protein expression and breast cancer.
4.2. HDAC2 Inhibition for Treating Breast Cancer
HDAC2 has been identified as a crucial regulator of epigenetic control in BC and HDAC2 suppression has been further proved to be an effective approach to treating BC by numerous studies [24,25,26]. For instance, HDAC2 inhibition has been observed to inhibit cellular proliferation in a p53-dependent manner in BC cells [27]. MiR-155 can also decrease the expression of erythroblastic oncogene B by targeting HDAC2 [28]. Moreover, evidence has indicated that PELP1 (proline, glutamate, and leucine-rich protein 1) can bind to miR-200a and miR-141 promoter sequences and modulate the expression of these miRNAs by recruiting HDAC2; therefore, regulating tumorigenic and metastatic potential of BC cells [29]. Thus, a molecular network involving HDAC2 has been considered to serve as a target for developing anti-cancer drugs [29].
Inhibition of HDAC2 has also been found to exert a synergistic effect in BC treatment. For instance, evidence indicates that depleting HDAC2 can sensitize breast cancer cells to apoptosis induced by epirubicin. This finding highlighted the potential of HDAC2 as a therapeutic target and a biomarker in treating breast cancer [30]. Moreover, the modulation of estrogen receptor (ER) signaling is a promising therapeutic approach in ER-expressing breast cancers, and the progesterone receptor (PR) also plays a critical role in this process [31]. Interestingly, selective inhibition of HDAC2, has been found to enhance the apoptotic effects of tamoxifen (a commonly used drug for ER/PR-positive breast cancer) and has demonstrated significant antitumor activity [31]. Additionally, HDAC2 inhibition has also been found to strongly restrain the multidrug-resistance of BC cells induced by the SRGN (serglycin)–YAP (YES-associated protein) axis, therefore enhancing the therapeutic effect of chemotherapy [32].
Taken together, these findings suggested that HDAC2-targeting intervention could represent an effective approach for breast cancer control.
4.3. HDAC2 Inhibition Enhances the Therapeutic Effect of ICIs in BC Treatment
The emergence of immune checkpoint inhibitors (ICI) has revolutionized the treatment of breast cancer [33,34,35,36,37,38,39,40]. Adjuvant or neoadjuvant immune checkpoint blockades are used for metastatic breast cancer [34]. Despite ICI therapy being promising, breast cancer cells often find ways to evade the host’s immune system, necessitating combination therapies to overcome these limitations. HDAC inhibitors (HDACi) have demonstrated potent immunomodulatory activity, making them a rational choice for cancer immunotherapies.
4.3.1. HDAC2 Regulates PD-L1 Nuclear Translocation
As a ligand of PD-1, high PD-L1 levels indicate tumor progression and are associated with poor prognosis in immunotherapy-treated human cancer [35]. PD-L1 nuclear translocation has been identified as a key mechanism underlying the immune evasion of BC cells, hindering PD-1 inhibitors [36]. Both cytoplasmic and nuclear PD-L1 can exert immunosuppressive functions on BC cells [37]. Nuclear PD-L1 has been linked to various cellular processes, for example, increasing the anti-apoptotic capacity of tumor cells, promoting mTOR activity, and upregulating glycolytic metabolism [36]. A study has shown that the level of nuclear PD-L1 expression was positively correlated with immune response-related transcription factors, such as STAT3, RelA (p65), and c-Jun [19]. Nuclear PD-L1 interacts with transcription factors, such as RelA and the IFN regulatory factor (IRF), influencing antitumor immunity. Inhibition of nuclear PD-L1 expression led to downregulation of genes involved in evading immune surveillance, such as PDCD1LG2 (encoding PD-L2), VSIR (encoding VISTA), and CD276 (encoding B7-H3), which enhance cytotoxic T-lymphocyte depletion and promote tumor aggressiveness, distant metastasis, and resistance to PD-L1/PD-1 blockade therapy [18,19,38] (Figure 2).
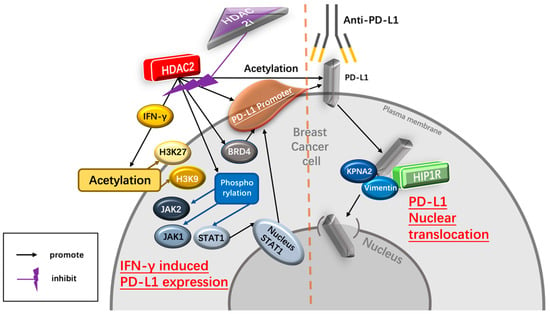
Figure 2.
Potential mechanism of HDAC2i enhancing the therapeutic effect of the PD-1/PD-L1 inhibitor. HDAC2i suppresses IFNγ-induced PD-L1 expression regulated by HDAC2, therefore decreasing the immune escape of BC cells mediated by PD-L1. In addition, HDAC2i inhibits the process of PD-L1 nuclear translocation regulated by HDAC2/HIP1R axis, hence enhancing the therapeutic effect of PD-1 blockade treatment. Abbreviations: IFN-γ, interferon-γ; H3K27, histone 3 lysine 2; H3K9, histone 3 lysine 9; JAK2, Janus kinase 2; JAK1, Janus kinase 1; stat1, signal transducer and activator of transcription 1; BRD4, Bromodomain-containing protein 4; KPNA2, karyopherin α-2; HIP-1R, Huntingtin-interacting protein 1-related.
Several recent studies have reported that HDAC2-associated deacetylation promotes the nuclear translocation of PD-L1, leading to tumor immune evasion in BC cells [18,19]. HDAC2 decreases the acetylation level of Lys 263 in the C-tail of PD-L1, resulting in PD-L1 nuclear translocation. In contrast, depletion of endogenous HDAC2 using siRNA, shRNA, or CRISPR-Cas9 increases PD-L1 acetylation [19]. Selective HDAC2is, such as Santacruzamate A (SCA) and ACY957, increase the acetylation of PD-L1, blocking the PD-L1 nuclear translocation. Clathrin-dependent endocytosis is involved in PD-L1 nuclear translocation, and HIP1R initiates this process by interacting with PD-L1’s C-tail. Lys 263 acetylation directly blocks the interaction between HIP1R and PD-L1. Overall, HDAC2 inhibition disrupts PD-L1 nuclear translocation, potentially enhancing the therapeutic efficacy of immune checkpoint inhibitors and boosting antitumor immune responses for BC [19].
4.3.2. HDAC2 Regulates IFN-γ- Induced PD-L1 Expression
- a.
- IFN-γ upregulates the expression of PD-L1
Evidence has revealed that high expression of PD-L1 is correlated with advanced histology and lymph node metastasis in TNBC and HER2+ subtypes, indicating a poor prognosis biomarker [39]. Moreover, PD-L1 knockdown has been found to inhibit the proliferation and migration of TNBC cells [40]. To date, the clinical trials of immunotherapies based on the PD-1/PD-L1 antagonists have shown a notable and durable response in TNBC patients, indicating PD-L1 as a crucial therapeutic target [41,42].
Interferon-γ (IFN-γ) is a crucial cytokine in both innate and adaptive immunity and should be considered as an important driving force for PD-L1 expression in tumor microenvironment. It is able to induce PD-L1 expression on BC cells and increase the apoptosis of antigen-specific T cells, such a process is referred to as “adaptive resistance” [43]. Depleting IFN-γ receptor 1 has been reported to decrease the expression of PD-L1 expression in BC cells, increase the amount of tumor-infiltrating CD8+ lymphocytes or CTLs, and to inhibit the development of BC cells [44]. In addition, injection of IFN-γ into subcutaneous BC cells induced PD-L1 expression and promoted the growth of BC cells. Inversely, PD-L1 depletion completely abrogated the growth of BC cells induced by IFN-γ injection [44] (Figure 2).
- b.
- HDAC2i affects IFN-γ induced PD-L1
HDAC2 can promote PD-L1 induced via IFN-γ stimulation in BC cells [18,19]. Specifically, IFN-γ can induce gene transcription involving STAT1 binding to the gamma interferon activation site (GAS), recruiting HAT and HDAC for chromatin remodeling [45]. Following IFN-γ stimulation, BRD4 is rapidly recruited to the PD-L1 locus, accompanied by increased H3K27ac and RNA Polymerase II (RNA Pol II) occupancy in cancer cells [45]. A ChIP-qPCR assay confirmed enhanced HDAC2 binding to the PD-L1 promoter after IFN-γ treatment. HDAC2 knockout led to reduced STAT1 occupancy and bromodomain-containing 4 (BRD4) recruitment to the PD-L1 promoter, attenuated H3K27ac and H3K9ac (markers of active transcription in the PD-L1 promoter) upregulation induced by IFN-γ, highlighting HDAC2’s role in activating PD-L1 expression through IFN-γ induced signaling pathways [18]. Moreover, upon the binding of IFN-γ to interferon receptors, it transduces signal through Janus kinases (JAKs), signal transducer and activators of transcriptions (STATs) [36]. The JAK/STAT1 pathway activated by IFN-γ positively correlates with PD-L1 expression and plays a critical role in breast cancer immune escape [46]. IFN-γ treatment induces phosphorylation of JAK1, JAK2, and STAT1 in TNBC cells, leading to upregulated STAT1 and PD-L1 expression. However, HDAC2 knockdown inhibits the phosphorylation of JAK1, JAK2, and STAT1, resulting in decreased IFN-γ-induced PD-L1 expression on the TNBC cell surface. HDAC2 knockout also hinders the translocation of STAT1 to the nucleus and inhibits intracellular PD-L1 expression stimulated by IFN-γ, indicating HDAC2’s promotion of IFN-γ induced PD-L1 expression in TNBC cells via JAK-STAT1 pathway activation [18] (Figure 2).
4.4. Dietary Intervention Is Important for Breast Cancer Patients Receiving Anti-Cancer Immunotherapy
A healthy diet rich in vitamins, minerals, antioxidants, and phytochemicals, provides essential ingredients for building and strengthening a healthy immune system. Thus, the American Cancer Society (ACS) releases the Nutrition and Physical Activity Guideline for Cancer Survivors, which enhances immune function by supporting essential nutrients [47,48,49,50], helping to optimize the effectiveness of immunotherapy and improve treatment outcomes. Moreover, anti-cancer immunotherapy also possesses toxicities and can lead to various adverse effects on breast cancer patients, such as fatigue, nausea, loss of appetite, and gastrointestinal issues [51]. However, proper dietary interventions have been found to help alleviate these side effects by providing proper nutrition, promoting hydration, and supporting gastrointestinal health [52,53,54]. In addition (Reducing Inflammation), chronic inflammation has been found to be associated with cancer development and progression. Certain foods, such as fruits, vegetables, whole grains, and omega-3 fatty acids, possess anti-inflammatory properties [55,56,57]. Including these foods in the diet can help reduce inflammation, potentially benefiting breast cancer patients undergoing immunotherapy.
Furthermore (Maintaining Weight and Nutritional Status), cancer and its treatment can lead to weight loss, malnutrition, and muscle wasting, which can further weaken the body and hinder treatment effectiveness [58]. Dietary intervention aims to provide adequate calories, protein, and other essential nutrients to maintain a healthy weight and preserve nutritional status during immunotherapy [59,60]. Additionally, (Enhancing Overall Well-Being), a nutritious diet can contribute to overall well-being by promoting energy levels, reducing fatigue, improving mood, and supporting mental health [61,62]. Breast cancer patients undergoing immunotherapy may experience physical and emotional challenges [63,64] and a healthy diet can play a role in supporting their overall quality of life (Figure 1).
4.5. Dietary HDAC2i
Compared to commonly used anti-cancer therapies, dietary interventions are safer and more cost-effective [65,66]. Even if dietary interventions cannot be considered a replacement for conventional cancer treatments, their role in improving the outcomes of cancer treatment also cannot be ignored [67,68] (Figure 3).
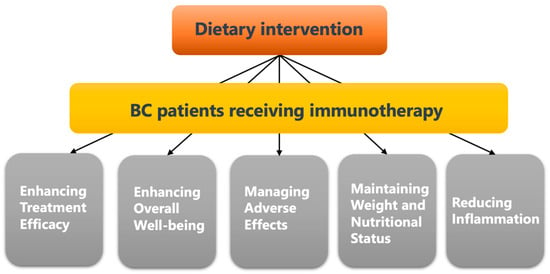
Figure 3.
Potential advantages of applying proper dietary intervention in BC patients who receive ICIs treatment.
One advantage of dietary intervention is the minimal side effects compared to anti-cancer therapies [69,70]. Immunotherapy, chemotherapy, and radiation therapy can often cause adverse reactions such as fatigue, arthralgia, rash, pruritus, pneumonitis, acute kidney injury, and also a weakened immune system [71,72,73,74]. In contrast, dietary changes usually focus on incorporating natural, nutrient-rich foods, which are less likely to cause significant side effects [70].
Anti-cancer therapies often involve powerful and aggressive drugs that can be toxic to healthy and normal cells in the human body [75]. Such toxicity can lead to additional health complications and adversely affect a patient’s well-being [75]. Dietary interventions, on the other hand, prioritize whole foods, fruits, vegetables, and other plant-based sources that provide essential nutrients without the toxic effects associated with some medical treatments [76].
Cancer patients who undergo multiple treatments or take various medications simultaneously will face the increased risk of adverse effects due to drug interactions or unwanted chiral compounds [77]. Dietary interventions generally use natural foods rather than pharmacological compounds, which have both left- and right-hand chiral compounds in balance [78].
Dietary interventions can be customized to suit each individual’s needs and medical conditions [60]. This adaptability allows dietary plans to be tailored based on any pre-existing conditions, allergies, or specific dietary restrictions of each patient, ensuring a safer approach [60].
As a long-term lifestyle change, dietary interventions offer the advantage of being sustainable even after the initial treatment period. This sustainable approach helps maintain the patient’s overall health and reduces the risk of developing long-term side effects associated with certain anti-cancer therapies [79].
Dietary interventions can complement anti-cancer therapies by providing a supportive role [78]. A healthy diet can help strengthen the immune system, improve overall health, and enhance the body’s ability to cope with cancer treatments.
Certain dietary strategies, such as adopting a balanced and nutrient-rich diet, regular physical activity, and weight management, have the potential to reduce the risk of developing cancer [68,80,81,82,83,84]. Prevention is a crucial aspect of cancer management, and dietary interventions can also play a significant role in reducing the occurrence of cancer [78].
4.6. Selected Candidates of HDAC2i
Dietary compounds which possess HDAC2 inhibitory activity offer a new strategy for BC prevention and treatment. These potential candidates may enhance the anti-cancer effect of PD-1 inhibitors (Table 2).

Table 2.
Examples of dietary compounds identified as inhibiting HDAC2 activity.
4.6.1. Genistein (GE)
GE is the most predominant bioactive isoflavone found mainly in soybean products and other food sources such as lupin, fava beans, kudzu, and psoralea [84]. GE has been reported to act as a potent chemo-preventive and therapeutic agent against various types of cancers including breast, prostate, and lung cancer [85]. For instance, GE suppresses the growth of BC cells in patient-derived tumor xenograft (PDX) [85]. Moreover, GE has been reported to modify the expression levels and activities of key epigenetic-associated genes, including HDAC2, DNA methyltransferases (DNMT3b) and ten-eleven translocation (TET3) methylcytosine dioxygenases. These genes are involved in epigenetic modifications, such as DNA methylation and histone methylation, which can regulate gene expression and impact cellular behavior [85]. By modulating the activities of these epigenetic regulators, GE may influence the epigenetic landscape of breast cancer cells, leading to changes in gene expression patterns that can affect cancer-related pathways.
4.6.2. Sulforaphane (SFN)
SFN is a natural compound and is abundant in cruciferous vegetables such as broccoli sprouts (BSp) and kale [111]. It has gained attention for its potential health benefits, including its ability to inhibit HDAC2 activity [112,113]. In the context of breast cancer, a dietary regimen of genistein and BSp in combination has been shown effective in reducing mammary tumor incidence and delaying tumor latency in a spontaneous breast cancer mouse model [86]. The combination of GE and SFN downregulated HDAC2 protein levels in breast cancer cells. This suggests that the combined action of GE and SFN can influence gene expression in breast cancer cells by modulating HDAC2 activity, thereby affecting immune response [86].
4.6.3. Chrysin and Its Analogues
Chrysin and its analogues are a group of polyphenolic compounds found in various dietary sources such as fruits, vegetables, olive oil, tea, and red wine [114]. The cytotoxic effects of chrysin have been shown against a wide range of cancer cell lines, including BC (MCF-7, MDA-MB-231), colon cancer (Lovo, DLD-1), and prostate cancer cells [87,88]. It is able to induce G1 cell cycle arrest and inhibit the activity of HDACs, specifically HDAC2 [89]. Moreover, polyphenolic compounds could promote the growth of SCFA-producer Lachnoclostridium [21], consequently modulating immune response.
4.6.4. Resveratrol (RSV)
RSV is also a polyphenol abundant in grape skin and seeds. It also presents in other food sources such as apples, blueberries, mulberries, peanuts, pistachios, plums, and red wine [90]. RSV has numerous beneficial properties of anti-glycosylation, anti-inflammation, anti-neurodegeneration, and antioxidation in various types of cancer [91]. One intriguing aspect of RSV is its proposed potential as a pan-HDAC inhibitor [92]. Studies have shown that RSV can inhibit the growth of BC cells (MCF-7 and MDA-MB-231) by inhibiting the activity of HDAC2 in a dose-dependent manner [93].
4.6.5. Oleuropein (OLE)
OLE is a polyphenolic compound in virgin olive oil with antineoplastic properties and it is well tolerated by humans [94]. Studies have shown that OLE can reduce progression, invasion, and proliferation of breast cancer cells by suppressing the activity of both HDAC2 and HDAC3 [95]. However, OLE exhibits little negative effect on normal breast epithelial cells, suggesting a potential selectivity towards BC cells and its potential for BC patients receiving ICIs therapy [95].
4.6.6. Curcumin
Curcumin, a lipophilic polyphenol derived from turmeric (Curcuma longa), has been extensively studied for its diverse health-promoting properties, including antioxidant, anti-inflammatory, hepatoprotective, anti-atherosclerotic, and antidiabetic effects [96]. Moreover, curcumin has been investigated for its potential as an HDAC inhibitor in MCF-7 and MDA-MB-231 cells [97], showing inhibitive effects on both HDAC activity and the expression of HDAC 1 and 2 in a dose-dependent manner [97].
4.6.7. Valeric Acid
Valerian (Valeriana officinalis) is a medicated diet that has been commonly used in cooking soup by some ethnic minorities in China for hundreds of years for restoring and balancing body energy [98]. Valeric acid, a major active component of valerian, has been identified as a potential HDAC inhibitor with anti-cancer effects on liver and breast cancer [98]. A study reported that valeric acid significantly decreases HDAC2 activity in treated breast cancer cells and may lead to alterations of DNA methylation [99].
4.6.8. Rh4
Ginseng is also a typical medicated diet item and is commonly used for making cuisine mainly in Asia. It is also a traditional Chinese herb with multiple biological effects. One of its components, Rh4, has been identified as a rare ginsenoside with potential inhibitive effects on the development of various cancers [100]. Rh4 can inhibit the expression of PD-L1 by regulating HDAC2-mediated JAK/STAT in breast cancer cells [101]. In addition, a study of the binding of Rh4 and HDAC2 suggests a high binding affinity existing between Rh4 and HDAC2, indicating the potential of ginseng as a dietary intervention for BC patients [101].
4.6.9. Butyrate (NaB)
NaB, a short-chain fatty acid generated via the fermentation of dietary fiber by the colonic microbiota, has shown anticancer activities mediated through HDACi [102]. NaB is primarily derived from undigested dietary carbohydrates, such as resistant starch and dietary fiber and, to a lesser extent, from dietary and endogenous proteins [103,104]. Studies have demonstrated that treatment with NaB, when combined with retinoids, enhances the inhibition of breast cancer cell proliferation [105]. Furthermore, the combination of butyrate with tumor necrosis factor-α (TNF-α), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), and anti-Fas agonist has been found to strongly induce apoptosis, leading to a significant decrease in the viability of breast cancer cells [106]. The action of NaB is often mediated through Sp1/Sp3 binding sites (e.g., p21 (Waf1/Cip1)). Both Sp1 and Sp3 were associated with HDAC activity in human breast cancer cells. And Sp1 and Sp3 recruit HDAC1 and HDAC2, with the latter being phosphorylated by protein kinase CK2 [115]. CK2 is upregulated in several cancers including breast cancer, which may promote breast cancer by deregulating key transcription processes [115].
4.6.10. Other Potential Candidates
Some other dietary compounds have also been identified as HDAC2i in other cancer types. For instance, green tea and its bioactive components, especially polyphenols, possess many health-promoting and disease-preventing benefits with anti-inflammatory, antimutagenic, antioxidant, and anticancer properties, but have no significant toxicity on normal cells in vivo. It has the potential as an effective chemotherapeutic agent for cancer prevention and treatment through various cellular, molecular, and biochemical mechanisms [116]. The major polyphenol components of green tea are (-)-epigallocatechin-3-gallate (EGCG), (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG) and (-)-epicatechin (EC) [116]. One of the molecular mechanisms underlying the anticancer effects of green tea polyphenols (GTPs) is HDAC2 inhibition [107]. Another study presented GTPs suppressed melanoma via regulating the circ_MITF/miR-30e-3p/HDAC2 axis [108]. Rosmarinic acid (RA), a main phenolic compound in rosemary, presents anti-inflammatory, anti-oxidant, and anti-cancer effects [109]. RA induced cell cycle arrest and apoptosis through modulation of HDAC2 expression in prostate cancer [109]. In addition, ursolic acid (UA) is a well-known natural triterpenoid abundant in apple peels, basil (Ocimum basilicum), blueberries (Vaccinium spp.), cranberries (Vaccinium macrocarpon), heather flowers (Calluna vulgaris), Labrador tea (Ledum groenlandicum Retzius), olives (Olea europaea), pears (Pyrus pyrifolia), and rosemary (Rosmarinus officinalis). A study reported that UA reduced the expression of epigenetic modifying enzymes, including DNA methyltransferases DNMT1 and DNMT3a and histone deacetylases (HDACs) HDAC1, HDAC2, HDAC3 and HDAC8 (Class I), and HDAC6 and HDAC7 (Class II), and HDAC activity [110]. Given that fungal Neurospora crassa contains enriched retinal, and flavin adenine dinucleotide (FAD) or flavin mononucleotide (FMN), showing tumor growth inhibition of breast cancer [117,118], it will be interesting to explore whether N. crassa has potential as an adjuvant of ICIs. Overall, these dietary compounds mentioned above can also be considered as potential candidates. However, their HDAC2 inhibitory effects should be further confirmed and evaluated in breast cancer cells.
4.7. Potential Approaches of Taking Bioactive Compound
For enhancing absorption efficiency and therefore improving the potential health benefits and biological activities of certain bioactive compounds, many means of application have been developed [119]. Some of them might provide a better way for BC patients receiving ICIs to gain HDAC2i efficiently. However, it is important to emphasize that thorough exploration in this area is still a pressing necessity.
- a.
- Nutraceuticals and Dietary Supplements
Many bioactive compounds with antioxidant or anti-inflammatory properties, found in certain fruits or vegetables, have been produced as nutraceuticals and dietary supplements for taking them more conveniently and easily [65]. Moreover, nutraceuticals and dietary supplements have been found to maintain excellent safety levels [120]. For instance, anthocyanins from berries, flavonols from dark chocolate, and resveratrol from red grapes have been widely used as consumed nutraceuticals [121]. Hence, dietary HDAC2i can be potentially produced as nutraceuticals and dietary supplements for clinical use.
- b.
- Nanotechnology and Drug Delivery
Bioactive compounds can also be incorporated into well-designed nanoparticles for targeted drug delivery, enhancing drug efficacy and reducing side effects [122]. For instance, theracurmin, a curcumin formulation consisting of dispersed curcumin with colloidal nanoparticles, possesses significantly improved bioavailability and therapeutic efficacy for treating osteoarthritis, compared to turmeric powder monotherapy [123,124,125]. More specifically, by adding nanoparticles, theracurmin was shown to have greater bioavailability than turmeric powder by 40 fold in rats and 27 fold higher in humans [125], and to have fewer side effects [123]. Moreover, many nanoparticles have already been developed for specific targeted delivery to breast cancer cells with excellent safety, such as Cur-Dox-NPs (selective co-delivery of doxorubicin and curcumin), FeAC-DOX@PC-HCQ NPs, DHAPN, and Opaxio™ [126,127,128]. Thus, this approach has potential for widespread utilization among BC patients seeking HDAC2 inhibitors. Nevertheless, it is essential to emphasize that substantial research is imperative to substantiate its feasibility and efficacy.
- c.
- Pharmaceuticals and Medicinal Products
Some bioactive compounds can be isolated and developed into pharmaceutical drugs to efficiently improve their therapeutic effect [129]. For instance, curcumin, a bioactive compound that has been found to possess multiple biological regulatory functions, has been successfully isolated from plant curcuma aromatica salisb for treating different types of cancer, including BC [130,131]. Moreover, paclitaxel, an efficient anti-cancer tricyclic diterpenoid compound, was also originally isolated from the plant Taxus brevifolia and subsequently synthesized for cancer treatment [132]. Therefore, plants containing HDAC2i may also be generated as pharmaceuticals and medicinal products.
- d.
- Phytotherapy and Traditional Medicine
Phytotherapy and traditional medicine have been widely applied in treating various of diseases [133,134]. They are natural, with relatively low irritation and side effects on the human body, and can also be utilized in combination with other treatment [135,136]. Moreover, evidence has proven that these therapeutic approaches can help to enhance the therapeutic effect of anti-cancer treatment, such as chemotherapy [137]. Normally, patients can achieve certain active ingredients of nutrients by decocting herbal plants. For instance, valeric acid, the dietary HDAC2i mentioned above can be obtained by a traditional Chinese medicine decoction containing the valerian herb [98,99]. Thus, phytotherapy (or traditional medicine) seems to be a reliable way for patients to take HDAC2i. However, the effectiveness of these methods for absorbing HDAC2i needs more evaluation.
4.8. Nutrients That May Impair the Therapeutic Effect of ICIs
Even some dietary items that contain HDAC2i may improve the efficiency of ICIs, while other nutrients of diets that can potentially hamper the therapeutic effect of such therapy should also be noted [138]. Hence, those nutrients have been summarized below in order to emphasize the potential risks and provoke further exploration on their specific mechanisms and exact interactions.
- a.
- Omega-3 Fatty Acids
Omega-3 fatty acids, commonly present in fish oil and certain plant sources, possess anti-inflammatory properties and are essential for synthesizing hormones and endogenous substances [139]. Natural killer (NK) cells are innate lymphocytes responsible for orchestrating immune responses against tumors and viruses [140]. Fish oil supplementation was found to decrease NK cell activity, which rebounded after supplementation ceased [141]. Notably, the age of individuals might influence the impact of omega-3 supplementation on NK cells [141]. Therefore, excessive consumption of omega-3 fatty acids has been considered to potentially hamper the normal function of immunity, which might further dampen the efficacy of ICIs. Thus, an appropriate amount of omega-3 intake is important for BC patients receiving ICIs. Of course, the exact mechanisms and interactions should be further explored.
- b.
- Vitamins
Vitamins are a type of trace organic substance obtained from food that can maintain normal physiological functions in humans [142]. Vitamins participate in the biochemical reactions of the human body and regulate metabolic functions, including immunity [143]. Deficiency or over intake of certain vitamins has been found to impair anti-cancer immunity, therefore affecting the efficiency of ICIs [138]. For instance, vitamin D has shown the ability to elevate the T-regulatory (Treg)/T-helper 17 (Th-17) cell ratio, leading to immune suppression and contributing to the onset of immune-related adverse events (irAEs), indicating its potential risk for patients receiving ICI therapy [144,145]. Moreover, vitamin A has also been reported to suppress the expression of PD-L1 causing cancer resistance to PD-1/PD-L1 blockade therapy [146,147]. In addition, evidence has shown that vitamin B6 can suppress PD-L1 expression and block the PD-1/PD-L1 signaling pathway [148]. Thus, extra attention is required when administrating vitamin supplementation for breast cancer patients receiving ICIs. More research is also indispensable in this field.
- c.
- Probiotics
Probiotics, including bacteria and yeast, are living microorganisms [149,150]. Some of them have been commonly utilized to promote gut health, closely intertwined with immune function [149,150]. Recent evidence has newly pointed out that an excessive immune response in the gut induced by overconsumption of probiotics might constrain the systemic immune reaction necessary for the optimal efficacy of ICIs [151,152]. Briefly, a clinical study involving 46 melanoma patients indicated that taking over-the-counter probiotic supplements (for unrelated conditions) was linked to a 70% reduction in response rate to ICI treatment [152]. Therefore, probiotics should be approached cautiously in BC patients undergoing immunotherapy.
- d.
- High-Fiber Diets
Fiber-rich diets primarily comprise two essential elements: soluble fiber and insoluble fiber. These vital components are found in an array of plant-based foods, including legumes, whole grains, cereals, vegetables, fruits, nuts, and seeds. Dietary fiber is composed of non-starch polysaccharides and various plant constituents like cellulose, resistant starch, and resistant dextrins [153]. High-fiber diets have been considered to modulate the gut microbiota and influence immune responses [154]. While a diverse gut microbiome is generally associated with better health, certain bacterial metabolites produced from high-fiber diets could potentially hamper the efficiency of ICIs [155]. More specifically, evidence has shown that, in non-small cell lung cancer patients, notably increased serum indoleamine-2,3-dioxygenase (IDO) levels, which were potentially produced by high-fiber diets, induced primary resistance to ICI treatment [155]. Thus, such bacterial metabolites might play a pivotal role in ICI resistance [155]. Therefore, the overall benefits of a high-fiber diet should be considered in balance.
- e.
- Ketogenic diet
The ketogenic diet (KD) is characterized by high fat, low to moderate protein, and very low carbohydrate intake [156]. Evidence has shown that KD can lead to a downregulation of CTLA-4 and PD-1 expression on tumor-infiltrating lymphocytes (TILs), as well as PD-L1 expression on glioblastoma cells in animal models [157]. Also, it has been observed that the ketogenic diet KD can lead to the downregulation of cell membrane-associated PD-L1 [158]. Therefore, KD has the potential to reduce the effectiveness of PD-1/PD-L1 blockade therapy and should be avoided by patients using PD-1/PD-L1 inhibitors.
- f.
- Protein-restricted diet
A low-protein diet serves as a therapeutic approach for managing inherited metabolic disorders like phenylketonuria and homocystinuria. Additionally, it can be employed in the treatment of kidney or liver ailments. Furthermore, a reduced intake of protein has been observed to potentially lower the risk of bone fractures, likely due to alterations in calcium [159]. Notably, recent studies have found that the deprivation of glutamine, a building block of proteins, can reduce PD-1 expression, indicating the potential to suppress the efficiency of PD-1 inhibitors [160].
5. Conclusions
According to recent data, the number of new cases of BC has accounted for about 11.7% of all malignant tumors. It has surpassed lung cancer as the most common malignant tumor, and its mortality rate has ranked among the top five of all malignant tumors [118]. In an effort to cure BC patients, ICIs (e.g., PD-1/PD-L1 inhibitors) have been introduced. However, it is still a challenge to provide durable and ideal clinical benefits and cure BC patients. Thus, novel ICI-based therapeutic strategies in combination have been studied to further improve the effect of treatment, such as HDAC2i plus a PD-1/PD-L1 inhibitor.
Dietary intervention, as a supportive therapy, is able to function as an HDAC2i through daily intake for cancer patients. An HDAC2i can suppress IFN-γ-induced PD-L1 expression and inhibit the process of PD-L1 nuclear translocation. Thus, a diet-derived HDAC2i has the potential to improve the clinical outcomes of BC patients, especially for those who are taking PD-1/PD-L1 inhibitors. Furthermore, to the best of our knowledge, there are still no relevant clinical guidelines on the application of an HDAC2i-containing diet for patients, especially for BC patients receiving ICIs. For such a group of patients, this novel dietary therapy can not only provide new dietary options but also may improve their clinical outcomes.
To sum up, multiple anti-cancer and immunomodulatory effects of HDAC2i guarantee future research into investigating such a novel dietary intervention for BC patients receiving ICIs. The potential of diet to enhance the therapeutic effect of ICIs is still to be fully evaluated. Certainly, further research is also required to identify more HDAC2i-containing foods for more dietary choices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15183984/s1.
Author Contributions
R.H. independently created the original concept of this paper; R.H. and Y.W. completed the original draft; R.H. conducted the analysis and created the figures; H.R. and Y.W. created the tables; R.H. reviewed and edited the manuscript with the help of L.L., C.L. and P.Z.; visualization, R.H.; supervision, R.H.; project administration, R.H.; funding acquisition, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “National Natural Science Foundation of China (Youth foundation) (No.82204864)” project (to Rui Han), and “Basic and Applied Basic Research on Municipal School (College) Joint Funding Projects (Guangzhou municipal science and technology bureau) (No.202201020252)” project (to Rui Han).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tao, X.; Li, T.; Gandomkar, Z.; Brennan, P.C.; Reed, W.M. Incidence, mortality, survival, and disease burden of breast cancer in China compared to other developed countries. Asia-Pac. J. Clin. Oncol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Bazzi, T.; Al-Husseini, M.; Saravolatz, L.; Kafri, Z. Trends in Breast Cancer Incidence and Mortality in the United States From 2004-2018: A Surveillance, Epidemiology, and End Results (SEER)-Based Study. Cureus 2023, 15, e37982. [Google Scholar] [CrossRef] [PubMed]
- Pedersini, R.; di Mauro, P.; Bosio, S.; Zanini, B.; Zanini, A.; Amoroso, V.; Turla, A.; Vassalli, L.; Ardine, M.; Monteverdi, S.; et al. Changes in eating habits and food preferences in breast cancer patients undergoing adjuvant chemotherapy. Sci. Rep. 2021, 11, 12975. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.; McGale, P.; Probert, J.; Broggio, J.; Charman, J.; Darby, S.C.; Kerr, A.J.; Whelan, T.; Cutter, D.J.; Mannu, G.; et al. Breast cancer mortality in 500 000 women with early invasive breast cancer in England, 1993–2015: Population based observational cohort study. BMJ 2023, 381, e074684. [Google Scholar] [CrossRef]
- Bertucci, F.; Gonçalves, A. Immunotherapy in Breast Cancer: The Emerging Role of PD-1 and PD-L1. Curr. Oncol. Rep. 2017, 19, 64. [Google Scholar] [CrossRef]
- Lu, L.; Bai, Y.; Wang, Z. Elevated T cell activation score is associated with improved survival of breast cancer. Breast Cancer Res. Treat. 2017, 164, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.L.; Huppert, L.A.; Rugo, H.S. Role of Immunotherapy in Breast Cancer. JCO Oncol. Pract. 2023, 19, 167–179. [Google Scholar] [CrossRef]
- Hattori, M.; Masuda, N.; Takano, T.; Tsugawa, K.; Inoue, K.; Matsumoto, K.; Ishikawa, T.; Itoh, M.; Yasojima, H.; Tanabe, Y.; et al. Pembrolizumab plus chemotherapy in Japanese patients with triple-negative breast cancer: Results from KEYNOTE-355. Cancer Med. 2023, 12, 10280–10293. [Google Scholar] [CrossRef]
- Downs-Canner, S.; Mittendorf, E.A. Correction: Preoperative Immunotherapy Combined with Chemotherapy for Triple-Negative Breast Cancer: Perspective on the KEYNOTE-522 Study. Ann. Surg. Oncol. 2023, 30, 3286. [Google Scholar] [CrossRef]
- Khalid, A.B.; Calderon, G.; Jalal, S.I.; Durm, G.A. Physician Awareness of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Breast Cancer Res. Treat 2022, 20, 1316–1320. [Google Scholar] [CrossRef]
- Gumusay, O.; Callan, J.; Rugo, H.S. Immunotherapy toxicity: Identification and management. Breast Cancer Res. Treat. 2022, 192, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, S.; Dong, Y.; Wang, T.; Niu, X.; Zhao, L.; Wang, G. Antitumor activity and mechanism of resistance of the novel HDAC and PI3K dual inhibitor CUDC-907 in pancreatic cancer. Cancer Chemother. Pharmacol. 2021, 87, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Garmpis, N.; Damaskos, C.; Dimitroulis, D.; Kouraklis, G.; Garmpi, A.; Sarantis, P.; Koustas, E.; Patsouras, A.; Psilopatis, I.; Antoniou, E.A.; et al. Clinical Significance of the Histone Deacetylase 2 (HDAC-2) Expression in Human Breast Cancer. J. Pers. Med. 2022, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Jiang, Y.; Yu, H.; Huang, Q.; Liu, L.; Guo, X.; Li, L.; Mi, Q.; Zhang, K.; Yang, Z. HDAC2 overexpression correlates with aggressive clinicopathological features and DNA-damage response pathway of breast cancer. Am. J. Cancer Res. 2017, 7, 1213–1226. [Google Scholar]
- Zhao, H.; Yu, Z.; Zhao, L.; He, M.; Ren, J.; Wu, H.; Chen, Q.; Yao, W.; Wei, M. HDAC2 overexpression is a poor prognostic factor of breast cancer patients with increased multidrug resistance-associated protein expression who received anthracyclines therapy. Jpn. J. Clin. Oncol. 2016, 46, 893–902. [Google Scholar] [CrossRef]
- Maccallini, C.; Ammazzalorso, A.; De Filippis, B.; Fantacuzzi, M.; Giampietro, L.; Amoroso, R. HDAC Inhibitors for the Therapy of Triple Negative Breast Cancer. Pharmaceuticals 2022, 15, 667. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Xu, P.; Xiong, W.; Lin, Y.; Fan, L.; Pan, H.; Li, Y. Histone deacetylase 2 knockout suppresses immune escape of triple-negative breast cancer cells via downregulating PD-L1 expression. Cell Death Dis. 2021, 12, 779. [Google Scholar] [CrossRef]
- Gao, Y.; Nihira, N.T.; Bu, X.; Chu, C.; Zhang, J.; Kolodziejczyk, A.; Fan, Y.; Chan, N.T.; Ma, L.; Liu, J.; et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020, 22, 1064–1075. [Google Scholar] [CrossRef]
- Bassett, S.A.; Barnett, M.P.G. The Role of Dietary Histone Deacetylases (HDACs) Inhibitors in Health and Disease. Nutrients 2014, 6, 4273–4301. [Google Scholar] [CrossRef]
- Zhu, G.; Su, H.; Johnson, C.H.; Khan, S.A.; Kluger, H.; Lu, L. Intratumour microbiome associated with the infiltration of cytotoxic CD8+ T cells and patient survival in cutaneous melanoma. Eur. J. Cancer 2021, 151, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dietary Guidelines for Breast Cancer Patients: A Critical Review. Adv. Nutr. Int. Rev. J. 2017, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Min, S.K.; Park, H.-R.; Kim, D.H.; Kwon, M.J.; Kim, L.S.; Ju, Y.-S. Expression of Histone Deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in Invasive Ductal Carcinomas of the Breast. J. Breast Cancer 2014, 17, 323–331. [Google Scholar] [CrossRef]
- Muller, B.M.; Jana, L.; Kasajima, A.; Lehmann, A.; Prinzler, J.; Budczies, J.; Winzer, K.-J.; Dietel, M.; Weichert, W.; Denkert, C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer—Overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 2013, 13, 215. [Google Scholar] [CrossRef]
- Long, M.; Hou, W.; Liu, Y.; Hu, T. A Histone Acetylation Modulator Gene Signature for Classification and Prognosis of Breast Cancer. Curr. Oncol. 2021, 28, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Hwang, C.Y.; Lee, J.; Cho, K.-H. Network Analysis Identifies Regulators of Basal-Like Breast Cancer Reprogramming and Endocrine Therapy Vulnerability. Cancer Res. 2022, 82, 320–333. [Google Scholar] [CrossRef]
- Harms, K.L.; Chen, X. Histone Deacetylase 2 Modulates p53 Transcriptional Activities through Regulation of p53-DNA Binding Activity. Cancer Res. 2007, 67, 3145–3152. [Google Scholar] [CrossRef]
- He, X.-H.; Zhu, W.; Yuan, P.; Jiang, S.; Li, D.; Zhang, H.-W.; Liu, M.-F. miR-155 downregulates ErbB2 and suppresses ErbB2-induced malignant transformation of breast epithelial cells. Oncogene 2016, 35, 6015–6025. [Google Scholar] [CrossRef]
- Jo, H.; Shim, K.; Kim, H.-U.; Jung, H.S.; Jeoung, D. HDAC2 as a target for developing anti-cancer drugs. Comput. Struct. Biotechnol. J. 2023, 21, 2048–2057. [Google Scholar] [CrossRef]
- Biçaku, E.; Marchion, D.C.; Schmitt, M.L.; Münster, P.N. Selective Inhibition of Histone Deacetylase 2 Silences Progesterone Receptor–Mediated Signaling. Cancer Res. 2008, 68, 1513–1519. [Google Scholar] [CrossRef]
- Marchion, D.C.; Bicaku, E.; Turner, J.G.; Schmitt, M.L.; Morelli, D.R.; Munster, P.N. HDAC2 regulates chromatin plasticity and enhances DNA vulnerability. Mol. Cancer Ther. 2009, 8, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qiu, N.; Yin, J.; Zhang, J.; Liu, H.; Guo, W.; Liu, M.; Liu, T.; Chen, D.; Luo, K.; et al. SRGN crosstalks with YAP to maintain chemoresistance and stemness in breast cancer cells by modulating HDAC2 expression. Theranostics 2020, 10, 4290–4307. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, E.; Tahaghoghi-Hajghorbani, S.; Jafarzadeh, L.; Sanaei, M.-J.; Pourbagheri-Sigaroodi, A.; Bashash, D. The application of immune checkpoint blockade in breast cancer and the emerging role of nanoparticle. J. Control. Release 2021, 340, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.; Anders, C.; McArthur, H.; Force, J. Biomarkers of Immune Checkpoint Blockade Response in Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2021, 22, 38. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Risch, E.; Halaban, R.; Zhen, P.; Bacchiocchi, A.; Risch, H.A. Dynamic changes of circulating soluble PD-1/PD-L1 and its association with patient survival in immune checkpoint blockade-treated melanoma. Int. Immunopharmacol. 2023, 118, 110092. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wu, C.; Chen, M.; Jiang, Y.; Wu, Y.; Mao, R.; Fan, Y. The generation of PD-L1 and PD-L2 in cancer cells: From nuclear chromatin reorganization to extracellular presentation. Acta Pharm. Sin. B 2022, 12, 1041–1053. [Google Scholar] [CrossRef]
- Xiong, W.; Gao, Y.; Wei, W.; Zhang, J. Extracellular and nuclear PD-L1 in modulating cancer immunotherapy. Trends Cancer 2021, 7, 837–846. [Google Scholar] [CrossRef]
- Koh, Y.W.; Han, J.-H.; Haam, S.; Lee, H.W. HIP1R Expression and Its Association with PD-1 Pathway Blockade Response in Refractory Advanced NonSmall Cell Lung Cancer: A Gene Set Enrichment Analysis. J. Clin. Med. 2020, 9, 1425. [Google Scholar] [CrossRef]
- Muenst, S.; Schaerli, A.R.; Gao, F.; Däster, S.; Trella, E.; Droeser, R.A.; Muraro, M.G.; Zajac, P.; Zanetti, R.; Gillanders, W.E.; et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res. Treat. 2014, 146, 15–24. [Google Scholar] [CrossRef]
- Lotfinejad, P.; Kazemi, T.; Safaei, S.; Amini, M.; Baghbani, E.; Shotorbani, S.S.; Niaragh, F.J.; Derakhshani, A.; Shadbad, M.A.; Silvestris, N. PD-L1 silencing inhibits triple-negative breast cancer development and upregulates T-cell-induced pro-inflammatory cytokines. Biomed. Pharmacother. 2021, 138, 111436. [Google Scholar] [CrossRef]
- Setordzi, P.; Chang, X.; Liu, Z.; Wu, Y.; Zuo, D. The recent advances of PD-1 and PD-L1 checkpoint signaling inhibition for breast cancer immunotherapy. Eur. J. Pharmacol. 2021, 895, 173867. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Minn, A.J.; Wherry, E.J. Combination Cancer Therapies with Immune Checkpoint Blockade: Convergence on Interferon Signaling. Cell 2016, 165, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Abiko, K.; Matsumura, N.; Hamanishi, J.; Horikawa, N.; Murakami, R.; Yamaguchi, K.; Yoshioka, Y.; Baba, T.; Konishi, I.; Mandai, M. IFN-γ from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 2015, 112, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Bouhet, S.; Lafont, V.; Billard, E.; Gross, A.; Dornand, J. The IFNgamma-induced STAT1-CBP/P300 association, required for a normal response to the cytokine, is disrupted in Brucella-infected macrophages. Microb. Pathog. 2009, 46, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Mimura, K.; Tamaki, T.; Shiraishi, K.; Kua, L.-F.; Koh, V.; Ohmori, M.; Kimura, A.; Inoue, S.; Okayama, H.; et al. Phospho-STAT1 expression as a potential biomarker for anti-PD-1/anti-PD-L1 immunotherapy for breast cancer. Int. J. Oncol. 2019, 54, 2030–2038. [Google Scholar] [CrossRef]
- Calder, P.C. Foods to deliver immune-supporting nutrients. Curr. Opin. Food Sci. 2022, 43, 136–145. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.G.; Gombart, A.F.; Eggersdorfer, M. Reply to Comment on: Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Chen, O.; Mah, E.; Dioum, E.; Marwaha, A.; Shanmugam, S.; Malleshi, N.; Sudha, V.; Gayathri, R.; Unnikrishnan, R.; Anjana, R.M.; et al. The Role of Oat Nutrients in the Immune System: A Narrative Review. Nutrients 2021, 13, 1048. [Google Scholar] [CrossRef]
- Noor, S.; Piscopo, S.; Gasmi, A. Nutrients Interaction with the Immune System. Arch. Razi. Inst. 2021, 76, 1579–1588. [Google Scholar] [CrossRef]
- Kichloo, A.; Albosta, M.; Dahiya, D.; Guidi, J.C.; Aljadah, M.; Singh, J.; Shaka, H.; Wani, F.; Kumar, A.; Lekkala, M. Systemic adverse effects and toxicities associated with immunotherapy: A review. World J. Clin. Oncol. 2021, 12, 150–163. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Chiussi, G.; Castaldo, G.; Guerra, A.; Meschi, T. Calcium Oxalate Nephrolithiasis and Gut Microbiota: Not just a Gut-Kidney Axis. A Nutritional Perspective. Nutrients 2020, 12, 548. [Google Scholar] [CrossRef]
- Taraszewska, A. Risk factors for gastroesophageal reflux disease symptoms related to lifestyle and diet. Rocz Panstw Zakl Hig 2021, 72, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, A.; Bajaj, A.; Xiao, Y.; Markandey, M.; Ahuja, V.; Kashyap, P.C. Role of Diet–Microbiome Interaction in Gastrointestinal Disorders and Strategies to Modulate Them with Microbiome-Targeted Therapies. Annu. Rev. Nutr. 2023, 43, 355–383. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.E.; Dijkstra, G. Food and Food Groups in Inflammatory Bowel Disease (IBD): The Design of the Groningen Anti-Inflammatory Diet (GrAID). Nutrients 2021, 13, 1067. [Google Scholar] [CrossRef] [PubMed]
- Tolkien, K.; Bradburn, S.; Murgatroyd, C. An anti-inflammatory diet as a potential intervention for depressive disorders: A systematic review and meta-analysis. Clin. Nutr. 2019, 38, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- De Groot, S.; Lugtenberg, R.T.; Cohen, D.; Welters, M.J.P.; Ehsan, I.; Vreeswijk, M.P.G.; Smit, V.T.H.B.M.; de Graaf, H.; Heijns, J.B.; Portielje, J.E.A.; et al. Fasting mimicking diet as an adjunct to neoadjuvant chemotherapy for breast cancer in the multicentre randomized phase 2 DIRECT trial. Nat. Commun. 2020, 11, 3083. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Baracos, V.; Bertz, H.; Bozzetti, F.; Calder, P.C.; Deutz, N.E.P.; Erickson, N.; Laviano, A.; Lisanti, M.P.; Lobo, D.N.; et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin. Nutr. 2017, 36, 1187–1196. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, J.; Zhang, L.; Wu, J.; Zhan, Z. Enteral immunonutrition versus enteral nutrition for gastric cancer patients undergoing a total gastrectomy: A systematic review and meta-analysis. BMC Gastroenterol. 2018, 18, 11. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Wyatt, M.; Johnson, A.J.; Toy, E.P.; Khan, J.M.; Dunn, K.; Clegg, D.J.; Reddy, S. Diet-microbiome interactions in cancer treatment: Opportunities and challenges for precision nutrition in cancer. Neoplasia 2022, 29, 100800. [Google Scholar] [CrossRef]
- Owen, L.; Corfe, B. The role of diet and nutrition on mental health and wellbeing. Proc. Nutr. Soc. 2017, 76, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. Int. Rev. J. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Carreira, H.; Williams, R.; Müller, M.; Harewood, R.; Stanway, S.; Bhaskaran, K. Associations Between Breast Cancer Survivorship and Adverse Mental Health Outcomes: A Systematic Review. J. Natl. Cancer Inst. 2018, 110, 1311–1327. [Google Scholar] [CrossRef] [PubMed]
- Fanakidou, I.; Zyga, S.; Alikari, V.; Tsironi, M.; Stathoulis, J.; Theofilou, P. Mental health, loneliness, and illness perception outcomes in quality of life among young breast cancer patients after mastectomy: The role of breast reconstruction. Qual. Life Res. 2018, 27, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Garay, C.; Djouder, N. Dietary interventions and precision nutrition in cancer therapy. Trends Mol. Med. 2023, 29, 489–511. [Google Scholar] [CrossRef]
- Vernieri, C.; Casola, S.; Foiani, M.; Pietrantonio, F.; De Braud, F.; Longo, V. Targeting Cancer Metabolism: Dietary and Pharmacologic Interventions. Cancer Discov. 2016, 6, 1315–1333. [Google Scholar] [CrossRef]
- Bose, S.; Allen, A.E.; Locasale, J.W. The Molecular Link from Diet to Cancer Cell Metabolism. Mol. Cell 2020, 78, 1034–1044. [Google Scholar] [CrossRef]
- Locasale, J.W. Diet and Exercise in Cancer Metabolism. Cancer Discov. 2022, 12, 2249–2257. [Google Scholar] [CrossRef]
- Morita, M.; Kudo, K.; Shima, H.; Tanuma, N. Dietary intervention as a therapeutic for cancer. Cancer Sci. 2021, 112, 498–504. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H. Neurological adverse events associated with PD-1/PD-L1 immune checkpoint inhibitors. Front. Neurosci. 2023, 17, 1227049. [Google Scholar] [CrossRef] [PubMed]
- Alturki, N.A. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J. Clin. Med. 2023, 12, 4301. [Google Scholar] [CrossRef] [PubMed]
- Zraik, I.M.; Heß-Busch, Y. Management of chemotherapy side effects and their long-term sequelae. Urologe A 2021, 60, 862–871. [Google Scholar] [CrossRef]
- Buchholz, T.A. Radiation Therapy for Early-Stage Breast Cancer after Breast-Conserving Surgery. New Engl. J. Med. 2009, 360, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Gao, Z.-J.; Yu, X.; Wang, P. Dietary regulation in health and disease. Signal Transduct. Target. Ther. 2022, 7, 252. [Google Scholar] [CrossRef]
- Prado, C.M.; Antoun, S.; Sawyer, M.B.; Baracos, V.E. Two faces of drug therapy in cancer: Drug-related lean tissue loss and its adverse consequences to survival and toxicity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 250–254. [Google Scholar] [CrossRef]
- Soldati, L.; Di Renzo, L.; Jirillo, E.; Ascierto, P.A.; Marincola, F.M.; De Lorenzo, A. The influence of diet on anti-cancer immune responsiveness. J. Transl. Med. 2018, 16, 75. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Gan, R.-Y.; Zhao, C.-N.; Meng, X.; Li, H.-B. Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 60, 1025–1037. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Harrigan, M.; Cartmel, B.; Chagpar, A.; Bai, Y.; Li, F.-Y.; Rimm, D.L.; Pusztai, L.; Lu, L.; Sanft, T.; et al. Impact of a randomized weight loss trial on breast tissue markers in breast cancer survivors. npj Breast Cancer 2022, 8, 29. [Google Scholar] [CrossRef]
- Puklin, L.; Cartmel, B.; Harrigan, M.; Lu, L.; Li, F.-Y.; Sanft, T.; Irwin, M.L. Randomized trial of weight loss on circulating ghrelin levels among breast cancer survivors. npj Breast Cancer 2021, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.A.; Alvarez-Reeves, M.; Lu, L.; Yu, H.; Irwin, M.L. Effect of Exercise on Metabolic Syndrome Variables in Breast Cancer Survivors. Int. J. Endocrinol. 2013, 2013, 168797. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Irwin, M.L.; Lu, L.; Risch, H.; Mayne, S.; Mu, L.; Deng, Q.; Scarampi, L.; Mitidieri, M.; Katsaros, D.; et al. Physical activity and breast cancer survival: An epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res. Treat. 2012, 133, 127–135. [Google Scholar] [CrossRef]
- Garbiec, E.; Cielecka-Piontek, J.; Kowalówka, M.; Hołubiec, M.; Zalewski, P. Genistein—Opportunities Related to an Interesting Molecule of Natural Origin. Molecules 2022, 27, 815. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Arora, I.; Chen, M.; Wu, H.; Crowley, M.R.; Tollefsbol, T.O.; Li, Y. Therapeutic Effects of Dietary Soybean Genistein on Triple-Negative Breast Cancer via Regulation of Epigenetic Mechanisms. Nutrients 2021, 13, 3944. [Google Scholar] [CrossRef]
- Paul, B.; Li, Y.; Tollefsbol, T.O. The Effects of Combinatorial Genistein and Sulforaphane in Breast Tumor Inhibition: Role in Epigenetic Regulation. Int. J. Mol. Sci. 2018, 19, 1754. [Google Scholar] [CrossRef]
- Chang, H.; Mi, M.; Ling, W.; Zhu, J.; Zhang, Q.; Wei, N.; Zhou, Y.; Tang, Y.; Yuan, J. Structurally related cytotoxic effects of flavonoids on human cancer cells in vitro. Arch. Pharmacal Res. 2008, 31, 1137–1144. [Google Scholar] [CrossRef]
- Androutsopoulos, V.; Papakyriakou, A.; Vourloumis, D.; Spandidos, D.A. Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. Bioorg. Med. Chem. 2011, 19, 2842–2849. [Google Scholar] [CrossRef]
- Pal-Bhadra, M.; Ramaiah, M.J.; Reddy, T.L.; Krishnan, A.; Pushpavalli, S.; Babu, K.S.; Tiwari, A.K.; Rao, J.M.; Yadav, J.S.; Bhadra, U. Plant HDAC inhibitor chrysin arrest cell growth and induce p21 WAF1 by altering chromatin of STAT response element in A375 cells. BMC Cancer 2012, 12, 180. [Google Scholar] [CrossRef]
- Sedlak, L.; Wojnar, W.; Zych, M.; Wyględowska-Promieńska, D.; Mrukwa-Kominek, E.; Kaczmarczyk-Sedlak, I. Effect of Resveratrol, a Dietary-Derived Polyphenol, on the Oxidative Stress and Polyol Pathway in the Lens of Rats with Streptozotocin-Induced Diabetes. Nutrients 2018, 10, 1423. [Google Scholar] [CrossRef]
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef]
- Venturelli, S.; Berger, A.; Böcker, A.; Busch, C.; Weiland, T.; Noor, S.; Leischner, C.; Schleicher, S.; Mayer, M.; Weiss, T.S.; et al. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS ONE 2013, 8, e73097. [Google Scholar] [CrossRef]
- Izquierdo-Torres, E.; Hernández-Oliveras, A.; Meneses-Morales, I.; Rodríguez, G.; Fuentes-García, G.; Zarain-Herzberg, Á. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Z.; Yang, X.; Liu, L.; Ahn, K.S. An updated review on the potential antineoplastic actions of oleuropein. Phytother. Res. 2022, 36, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Derakhshan, S.M.; Derakhshan, N.M.; Khaniani, M.S.; Alivand, M.R. Downregulation of HDAC2 and HDAC3 via oleuropein as a potent prevention and therapeutic agent in MCF-7 breast cancer cells. J. Cell. Biochem. 2019, 120, 9172–9180. [Google Scholar] [CrossRef] [PubMed]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Q.; Yu, K.; Yan, Q.-X.; Xing, C.-Y.; Chen, Y.; Yan, Z.; Shi, Y.-F.; Zhao, K.-W.; Gao, S.-M. Pure curcumin increases the expression of SOCS1 and SOCS3 in myeloproliferative neoplasms through suppressing class Ι histone deacetylases. Carcinogenesis 2013, 34, 1442–1449. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Li, Y.; Ling, C.; Lu, L. Valeric acid acts as a novel HDAC3 inhibitor against prostate cancer. Med. Oncol. 2022, 39, 213. [Google Scholar] [CrossRef]
- Shi, F.; Li, Y.; Han, R.; Fu, A.; Wang, R.; Nusbaum, O.; Qin, Q.; Chen, X.; Hou, L.; Zhu, Y. Valerian and valeric acid inhibit growth of breast cancer cells possibly by mediating epigenetic modifications. Sci. Rep. 2021, 11, 2519. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, Z.; Qu, L.; Zhang, Y.; Zhu, C.; Fan, D. Gastroprotective effects of ginsenoside Rh4 against ethanol-induced gastric mucosal injury by inhibiting the MAPK/NF-κB signaling pathway. Food Funct. 2023, 14, 5167–5181. [Google Scholar] [CrossRef]
- Dong, F.; Qu, L.; Duan, Z.; He, Y.; Ma, X.; Fan, D. Ginsenoside Rh4 inhibits breast cancer growth through targeting histone deacetylase 2 to regulate immune microenvironment and apoptosis. Bioorganic Chem. 2023, 135, 106537. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Nagamine, M.; De Conti, A.; Chaible, L.; Fontelles, C.; Junior, A.J.; Vannucchi, H.; Dagli, M.; Bassoli, B.; Moreno, F.; et al. Efficacy of the dietary histone deacetylase inhibitor butyrate alone or in combination with vitamin A against proliferation of MCF-7 human breast cancer cells. Braz. J. Med. Biol. Res. 2012, 45, 841–850. [Google Scholar] [CrossRef]
- Chopin, V.; Slomianny, C.; Hondermarck, H.; Le Bourhis, X. Synergistic induction of apoptosis in breast cancer cells by cotreatment with butyrate and TNF-alpha, TRAIL, or anti-Fas agonist antibody involves enhancement of death receptors’ signaling and requires P21(waf1). Exp. Cell Res. 2004, 298, 560–573. [Google Scholar] [CrossRef]
- Thakur, V.S.; Gupta, K.; Gupta, S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis 2012, 33, 377–384. [Google Scholar] [CrossRef]
- Wu, K.; Wei, Y.; Yu, Y.; Shan, M.; Tang, Y.; Sun, Y. Green tea polyphenols inhibit malignant melanoma progression via regulating circ_MITF/miR-30e-3p/HDAC2 axis. Biotechnol. Appl. Biochem. 2022, 69, 808–821. [Google Scholar] [CrossRef]
- Jang, Y.-G.; Hwang, K.-A.; Choi, K.-C. Rosmarinic Acid, a Component of Rosemary Tea, Induced the Cell Cycle Arrest and Apoptosis through Modulation of HDAC2 Expression in Prostate Cancer Cell Lines. Nutrients 2018, 10, 1784. [Google Scholar] [CrossRef]
- Kim, H.; Ramirez, C.N.; Su, Z.-Y.; Kong, A.-N.T. Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef]
- Çakır, I.; Pan, P.L.; Hadley, C.K.; El-Gamal, A.; Fadel, A.; Elsayegh, D.; Mohamed, O.; Rizk, N.M. Is a corresponding author Masoud Ghamari-Langroudi. Sulforaphane reduces obesity by reversing leptin resistance. Elife 2022, 11, e67368. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Bai, Q.; Zou, L.-Y.; Zhang, Q.-Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.-D.; Mi, M.-T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosom. Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, S.M.; Royce, S.G.; Licciardi, P.V.; Karagiannis, T.C. Dietary Sulforaphane in Cancer Chemoprevention: The Role of Epigenetic Regulation and HDAC Inhibition. Antioxid. Redox Signal. 2015, 22, 1382–1424. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Davie, J.R. Inhibition of Histone Deacetylase Activity by Butyrate. J. Nutr. 2003, 133 (Suppl. 7), 2485S–2493S. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Ling, C.; Lu, L. Neurospora crassa is a potential source of anti-cancer agents against breast cancer. Breast Cancer 2022, 29, 1032–1041. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Dima, C.; Assadpour, E.; Nechifor, A.; Dima, S.; Li, Y.; Jafari, S.M. Oral bioavailability of bioactive compounds; modulating factors, in vitro analysis methods, and enhancing strategies. Crit. Rev. Food Sci. Nutr. 2023, 1–39. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Juszczak, H.M.; Wong, M.A. Scoping review of the association of plant-based diet quality with health out-comes. Front. Nutr. 2023, 10, 1211535. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid Intake and Bone Health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Mukai, S.; Yamada, S.; Matsuoka, M.; Tarumi, E.; Hashimoto, T.; Tamura, C.; Imaizumi, A.; Nishihira, J.; Nakamura, T. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: A randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014, 19, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M.; Imaizumi, A.; Otsuka, Y.; Sasaki, H.; Hashiguchi, M.; Tsujiko, K.; Matsumoto, S.; Ishiguro, H.; Chiba, T. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2012, 69, 65–70. [Google Scholar] [CrossRef]
- Sasaki, H.; Sunagawa, Y.; Takahashi, K.; Imaizumi, A.; Fukuda, H.; Hashimoto, T.; Wada, H.; Katanasaka, Y.; Kakeya, H.; Fujita, M.; et al. Innovative Preparation of Curcumin for Improved Oral Bioavailability. Biol. Pharm. Bull. 2011, 34, 660–665. [Google Scholar] [CrossRef]
- Gao, C.; Tang, F.; Gong, G.; Zhang, J.; Hoi, M.P.M.; Lee, S.M.Y.; Wang, R. pH-Responsive prodrug nanoparticles based on a sodium alginate derivative for selective co-release of doxorubicin and curcumin into tumor cells. Nanoscale 2017, 9, 12533–12542. [Google Scholar] [CrossRef]
- Zhang, H.; Xue, Q.; Zhou, Z.; He, N.; Li, S.; Zhao, C. Co-delivery of doxorubicin and hydroxychloroquine via chitosan/alginate nanoparticles for blocking autophagy and enhancing chemotherapy in breast cancer therapy. Front. Pharmacol. 2023, 14, 1176232. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Y.; Li, Y.; Ma, X.; Zhang, S.; Yuan, Y.; Kohn, J.; Liu, C.; Qian, J. Synergistic Combination of Bioactive Hydroxyapatite Nanoparticles and the Chemotherapeutic Doxorubicin to Over-come Tumor Multidrug Resistance. Small 2021, 17, e2007672. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Lin, X.; Shen, L.; Feng, Y. Drug delivery for bioactive polysaccharides to improve their drug-like properties and curative efficacy. Drug Deliv. 2017, 24 (Suppl. 1), 70–80. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef]
- Passos, C.L.A.; Polinati, R.M.; Ferreira, C.; dos Santos, N.A.N.; Lima, D.G.V.; da Silva, J.L.; Fialho, E. Curcumin and melphalan cotreatment induces cell cycle arrest and apoptosis in MDA-MB-231 breast cancer cells. Sci. Rep. 2023, 13, 13446. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, L. Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 2019, 24, 40. [Google Scholar] [CrossRef] [PubMed]
- Nootim, P.; Kapol, N.; Bunchuailua, W.; Poompruek, P.; Tungsukruthai, P. Current state of cancer patient care incorporating Thai traditional medicine in Thailand: A qualitative study. J. Integr. Med. 2020, 18, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, N.; Salehi, A.; Vojoud, M.; Sharifi, M.H.; Hoseinkhani, A. Use of complementary and alternative medicine in pregnant women: A cross-sectional survey in the south of Iran. J. Integr. Med. 2019, 17, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Meng, Y.; Guo, G.; Zhao, C.; Zhou, X. Efficacy and safety of traditional Chinese medicine in the treatment of osteonecrosis of the femoral head. J. Orthop. Surg. Res. 2023, 18, 600. [Google Scholar] [CrossRef]
- Bu, Z.-J.; Liu, Y.-N.; Shahjalal, M.; Zheng, Y.-Y.; Liu, C.-J.; Ye, M.-M.; Xu, J.-Y.; Peng, X.-Y.; Wang, X.-H.; Chen, X.; et al. Comparative effectiveness and safety of Chinese medicine belly button application for childhood diarrhea: A Bayesian network meta-analysis of randomized controlled trials. Front. Pediatr. 2023, 11, 1180694. [Google Scholar] [CrossRef]
- Bushehri, R.H.; Navabi, P.; Saeedifar, A.M.; Keshavarzian, N.; Rouzbahani, N.H.; Mosayebi, G.; Ghazavi, A.; Ghorban, K.; Ganji, A. Integration of phytotherapy and chemotherapy: Recent advances in anticancer molecular pathways. Iran J. Basic Med. Sci. 2023, 26, 987–1000. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Lv, X.; Hu, L.; Li, W.; Zi, M.; He, Y. Impact of Diets on Response to Immune Checkpoint Inhibitors (ICIs) Therapy against Tumors. Life 2022, 12, 409. [Google Scholar] [CrossRef]
- Kromhout, D.; Yasuda, S.; Geleijnse, J.M.; Shimokawa, H. Fish oil and omega-3 fatty acids in cardiovascular disease: Do they really work? Eur. Heart J. 2012, 33, 436–443. [Google Scholar] [CrossRef]
- Björkström, N.K.; Strunz, B.; Ljunggren, H.-G. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2022, 22, 112–123. [Google Scholar] [CrossRef]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and Mineral Supplements in the Primary Prevention of Cardiovascular Disease and Cancer: An Updated Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef]
- Beck, K.L.; von Hurst, P.R.; O’Brien, W.J.; Badenhorst, C.E. Micronutrients and athletic performance: A review. Food Chem. Toxicol. 2021, 158, 112618. [Google Scholar] [CrossRef] [PubMed]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tobin, R.P.; Jordan, K.R.; Robinson, W.A.; Davis, D.; Borges, V.F.; Gonzalez, R.; Lewis, K.D.; McCarter, M.D. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipili-mumab. Int. Immunopharmacol. 2018, 63, 282–291. [Google Scholar] [CrossRef]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L.; et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018, 8, 1156–1175. [Google Scholar] [CrossRef]
- Yuan, J.; Li, J.; Shang, M.; Fu, Y.; Wang, T. Identification of vitamin B6 as a PD-L1 suppressor and an adjuvant for cancer immunotherapy. Biochem. Biophys. Res. Commun. 2021, 561, 187–194. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Bedada, T.L.; Feto, T.K.; Awoke, K.S.; Garedew, A.D.; Yifat, F.T.; Birri, D.J. Probiotics for cancer alternative prevention and treatment. BioMedicine 2020, 129, 110409. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.-Z.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.N.; Gopalakrishnan, V.; McQuade, J.; Andrews, M.C.; Helmink, B.; Khan, M.W.; Sirmans, E.; Haydu, L.; Cogdill, A.; Burton, E.; et al. Abstract 2838: The gut microbiome (GM) and immunotherapy response are influenced by host lifestyle factors. Cancer Res 2019, 79 (Suppl. 13), 2838. [Google Scholar] [CrossRef]
- Kuang, R.; Binion, D.G. Should high-fiber diets be recommended for patients with inflammatory bowel disease? Curr. Opin. Gas-Troenterol. 2022, 38, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.Z.; Nelson, E.; Chu, P.-Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Kocher, F.; Amann, A.; Zimmer, K.; Geisler, S.; Fuchs, D.; Pichler, R.; Wolf, D.; Kurz, K.; Seeber, A.; Pircher, A. High indoleamine-2,3-dioxygenase 1 (IDO) activity is linked to primary resistance to immunotherapy in non-small cell lung cancer (NSCLC). Transl. Lung Cancer Res. 2021, 10, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.D.; Aminzadeh-Gohari, S.; Tulipan, J.; Catalano, L.; Feichtinger, R.G.; Kofler, B. Ketogenic diet in the treatment of cancer—Where do we stand? Mol. Metab. 2020, 33, 102–121. [Google Scholar] [CrossRef]
- Lussier, D.M.; Woolf, E.C.; Johnson, J.L.; Brooks, K.S.; Blattman, J.N.; Scheck, A.C. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 2016, 16, 310. [Google Scholar] [CrossRef]
- Rom-Jurek, E.-M.; Kirchhammer, N.; Ugocsai, P.; Ortmann, O.; Wege, A.K.; Brockhoff, G. Regulation of Programmed Death Ligand 1 (PD-L1) Expression in Breast Cancer Cell Lines In Vitro and in Immunodeficient and Humanized Tumor Mice. Int. J. Mol. Sci. 2018, 19, 563. [Google Scholar] [CrossRef]
- Orillion, A.R.; Damayanti, N.P.; Shen, L.; Adelaiye-Ogala, R.; Affronti, H.C.; Elbanna, M.; Chintala, S.; Ciesielski, M.J.; Fontana, L.; Kao, C.; et al. Dietary Protein Restriction Reprograms Tumor-Associated Macrophages and Enhances Immunotherapy. Clin. Cancer Res. 2018, 24, 6383–6395. [Google Scholar] [CrossRef]
- Nabe, S.; Yamada, T.; Suzuki, J.; Toriyama, K.; Yasuoka, T.; Kuwahara, M.; Shiraishi, A.; Takenaka, K.; Yasukawa, M.; Yamashita, M. Reinforce the antitumor activity of CD8+ T cells via glutamine restriction. Cancer Sci. 2018, 109, 3737–3750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).