Lifestyle Can Exert a Significant Impact on the Development of Metabolic Complications and Quality Life in Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Overweigh and Obesity in IBD Patients: Associated Factors
3.3. Metabolic Comorbidities of Overweight and Obesity in IBD Patients: Associated Factors
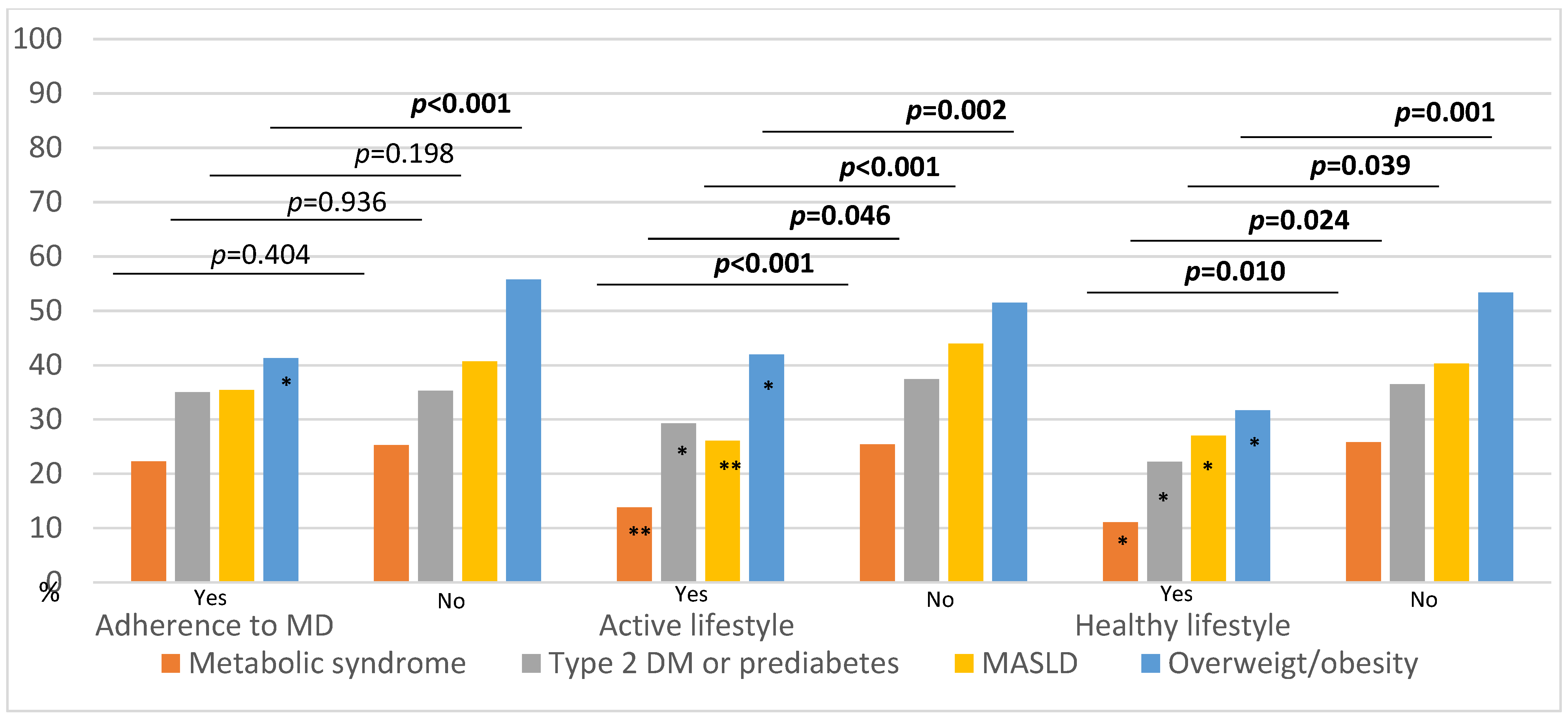
3.4. IBD Characteristics, Metabolic Comorbidities, and Their Relationship with Lifestyle
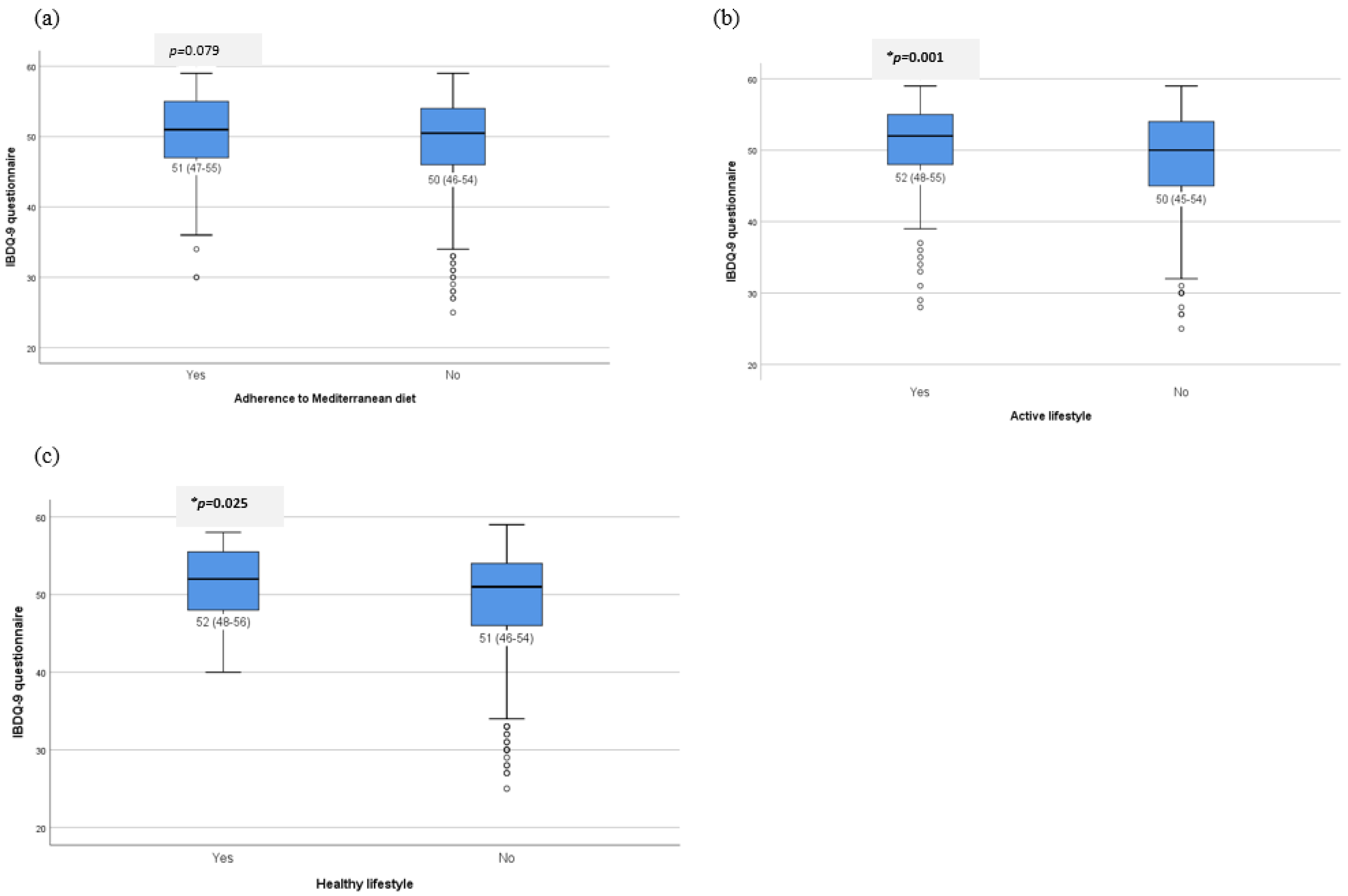
3.5. Impact of Lifestyle on the Quality of Life of Patients with IBD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Adherence to MD | p-Value | Active Lifestyle | p-Value | Healthy Lifestyle * | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 206) | No (n = 282) | Yes (n = 188) | No (n = 500) | Yes (n = 63) | No (n = 625) | ||||
| Sex; females n (%) | 124 (60.2) | 223 (46.3) | 0.001 | 93 (49.5) | 254 (50.8) | 0.756 | 36 (57.1) | 311 (49.8) | 0.264 |
| Age; m (IQR) | 53 (42–62) | 47 (38–58) | 0.001 | 47 (37–58.8) | 50 (40–59) | 0.161 | 53 (42–61) | 48 (39–59) | 0.133 |
| Smoker; yes n (%) | 39 (18.9) | 98 (20.3) | 0.674 | 31 (16.5) | 106 (21.2) | 0.168 | 11 (17.5) | 126 (20.2) | 0.609 |
| Former smoker; yes n (%) | 101 (49) | 212 (44) | 0.224 | 82 (43.6) | 231 (46.2) | 0.544 | 32 (50.8) | 281 (45) | 0.375 |
| WHR; high n (%) | 82 (39.8) | 262 (54.6) | <0.001 | 88 (47.3) | 256 (51.2) | 0.365 | 25 (39.7) | 319 (51.2) | 0.081 |
| Hypertension; yes n (%) | 40 (19.4) | 66 (13.7) | 0.057 | 23 (12.2) | 83 (16.6) | 0.157 | 11 (17.5) | 95 (15.2) | 0.636 |
| Renal disease; yes n (%) | 14 (7) | 51 (10.8) | 0.126 | 18 (9.8) | 47 (9.6) | 0.912 | 7 (11.3) | 58 (9.5) | 0.642 |
| Cerebrovascular disease, yes n (%) | 2 (1) | 8 (1.7) | 0.731 | 2 (1.1) | 8 (1.6) | 0.601 | 0 (0) | 10 (1.6) | 0.312 |
| CV disease; yes n (%) | 10 (4.9) | 15 (3.1) | 0.263 | 5 (2.7) | 20 (4) | 0.402 | 2 (3.2) | 23 (3.7) | 0.838 |
| HDL cholesterol; low n (%) | 28 (13.6) | 86 (17.8) | 0.170 | 22 (11.7) | 92 (18.4) | 0.035 | 6 (9.5) | 108 (17.3) | 0.115 |
| Age at diagnosis, mean (IQR) | 36 (28–48) | 34 (25–44) | 0.003 | 34 (25–45) | 36 (27–45) | 0.260 | 36 (29–49) | 35 (26–45) | 0.088 |
| More than 10 years of evolution; Yes, n (%) | 122 (59.2) | 272 (56.5) | 0.516 | 107 (56.9) | 287 (57.5) | 0.887 | 35 (55.6) | 359 (57.5) | 0.762 |
| IBD type; UC n (%) | 118 (57.3) | 251 (52.1) | 0.210 | 109 (58) | 260 (52) | 0.161 | 41 (65.1) | 328 (52.5) | 0.056 |
| Location; n (%) Proctitis (UC) Left side (UC) Pancolitis (UC) Ileal (L1, CD) Ileocecal (L3, CD) Colonic (L2, CD) | 35 (17) 37 (18) 47 (22.8) 41 (19.9) 32 (15.5) 14 (6.8) | 56 (11.6) 100 (20.7) 94 (19.5) 83 (17.2) 118 (24.5) 31 (6.4) | 0.065 | 31 (16.5) 37 (19.7) 41 (21.8) 30 (16) 40 (21.3) 9 (4.8) | 60 (12) 100 (20) 100 (20) 94 (18.8) 110 (22) 36 (7.2) | 0.525 | 12 (19) 15 (32.8) 14 (22.2) 8 (12.7) 12 (19) 2 (3.2) | 79 (12.6) 122 (19.5) 127 (20.3) 116 (18.6) 138 (22.1) 43 (6.9) | 0.423 |
| Behavior (CD); n (%) Inflammatory Stricturing Penetrating | 62 (70.5) 19 (21.6) 7 (8) | 132 (57.1) 64 (27.7) 35 (15.2) | 0.071 | 53 (67.1) 17 (21.5) 9 (11.4) | 141 (58.8) 66 (27.5) 33 (13.8) | 0.417 | 18 (81.8) 4 (18.2) 0 (0) | 176 (59.3) 79 (26.6) 42 (14.1) | 0.068 |
| Perianal disease; yes n (%) | 18 (20.5) | 68 (29.4) | 0.106 | 20 (25.3) | 66 (27.5) | 0.704 | 4 (18.2) | 82 (27.6) | 0.336 |
| Clinical remission; yes n (%) | 4 (4.5) | 14 (6.1) | 0.600 | 2 (2.5) | 16 (6.7) | 0.167 | 0 (0) | 18 (6.1) | 0.235 |
| Extraintestinal disease; yes n (%) | 37 (18) | 99 (20.5) | 0.437 | 34 (18.1) | 102 (20.4) | 0.497 | 9 (14.3) | 127 (20.3) | 0.252 |
| History of surgery; yes n (%) | 26 (12.6) | 107 (22.2) | 0.004 | 29 (15.4) | 104 (20.8) | 0.112 | 7 (11.1) | 126 (20.2) | 0.083 |
| Need of any biologic therapy; yes n (%) | 38 (22.4) | 125 (296) | 0.076 | 39 (23.2) | 124 (29.2) | 0.143 | 10 (18.9) | 153 (28.3) | 0.141 |
| Anti-TNF therapy; n (%) Yes | 38 (22.8) | 125 (30) | 0.076 | 39 (23.8) | 124 (29.6) | 0.160 | 10 (19.2) | 153 (28.8) | 0.142 |
| Steroid dependence; yes n (%) | 51 (25) | 154 (32.4) | 0.056 | 47 (25.1) | 158 (32) | 0.079 | 13 (21) | 192 (31.1) | 0.099 |
| Need of any steroid therapy in the last 5 years. Yes n (%) | 70 (34.1) | 190 (40) | 0.149 | 70 (37.8) | 190 (38.4) | 0.896 | 21 (33.9) | 239 (38.7) | 0.458 |
References
- Chaparro, M.; Garre, A.; Ortiz, A.N.; Palomares, M.T.D.-L.; Rodríguez, C.; Riestra, S.; Vela, M.; Benítez, J.M.; Salgado, E.F.; Rodríguez, E.S.; et al. Incidence, clinical characteristics and management of inflammatory bowel disease in spain: Large-scale epidemiological study. J. Clin. Med. 2021, 10, 2885. [Google Scholar] [CrossRef] [PubMed]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Andersen, N.N.; Jess, T.; Schwarzinger, M. Increased risk of acute arterial events in young patients and severely active IBD: A nationwide French cohort study. Gut 2018, 67, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Bhuket, T.; Torres, S.; Liu, B.; Wong, R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA 2015, 313, 1973–1974. [Google Scholar] [CrossRef] [PubMed]
- Mahfood Haddad, T.; Hamdeh, S.; Kanmanthareddy, A.; Alla, V.M. Nonalcoholic fatty liver disease and the risk of clinical cardiovascular events: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2017, 11, S209–S216. [Google Scholar] [CrossRef]
- Rodriguez-Duque, J.C.; Calleja, J.L.; Iruzubieta, P.; Hernández-Conde, M.; Rivas-Rivas, C.; Vera, M.I.; Garcia, M.J.; Pascual, M.; Castro, B.; García-Blanco, A.; et al. Increased risk of MAFLD and Liver Fibrosis in Inflammatory Bowel Disease Independent of Classic Metabolic Risk Factors. Clin. Gastroenterol. Hepatol. 2022, 21, 406–414.e7. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Singh, S.; Dulai, P.S.; Zarrinpar, A.; Ramamoorthy, S.; Sandborn, W.J. Obesity in IBD: Epidemiology, Pathogenesis, Disease Course and Treatment Outcomes; Nature Reviews Gastroenterology and Hepatology; Nature Publishing Group: New York, NY, USA, 2017; Volume 14, pp. 110–121. [Google Scholar]
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohns Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef]
- Spekhorst, L.M.; Visschedijk, M.C.; Alberts, R.; Festen, E.A.; van der Wouden, E.J.; Dijkstra, G.; Weersma, R.K. Performance of the Montreal classification for inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 15374–15381. [Google Scholar] [CrossRef]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef]
- Duan, D.; Kengne, A.P.; Echouffo-Tcheugui, J.B. Screening for Diabetes and Prediabetes; Endocrinology and Metabolism Clinics of North America; W.B. Saunders: Philadelphia, PA, USA, 2021; Volume 50, pp. 369–385. [Google Scholar]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-Society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature Authors. 2023. Available online: http://journals.lww.com/hep (accessed on 24 June 2023).
- Tilg, H.; Effenberger, M. From NAFLD to MAFLD: When pathophysiology succeeds. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 387–388. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.S.; Cherubini, P.A.; de Oliveira, M.A.; Bianchini, L.; Torres, C.M.; Bianchin, M.M. Diagnostic yield and accuracy of different metabolic syndrome criteria in adult Patients with epilepsy. Front. Neurol. 2017, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Gómez-gracia, E.; Ruiz-gutiérrez, V.; Fiol, M. Primary prevention of cardiovascular disease with a mediterranean diet. Z. Fur Gefassmedizin. 2013, 10, 886. [Google Scholar]
- Amireault, S.; Godin, G. The godin-shephard leisure-time physical activity questionnaire: Validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Ski. 2015, 120, 604–622. [Google Scholar] [CrossRef]
- Obesity: Preventing and Managing the Global Epidemic, Report of a WHO Consultation; World Health Organization Technical Report series; World Health Organization: Geneva, Switzerland, 2000; Volume 894.
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Surmiak, M.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Danielak, A.; Ptak-Belowska, A.; et al. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules 2019, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Lomer, M.C.E.; Cahill, O.; Baschali, A.; Sarathy, P.P.; Sarantidou, M.; Mantzaris, G.J.; Gaya, D.R.; Katsanos, K.; Christodoulou, D.K.; Gerasimidis, K. A multicentre study of nutrition risk assessment in adult patients with inflammatory bowel disease attending outpatient clinics. Ann. Nutr. Metab. 2019, 74, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Pringle, P.L.; Stewart, K.O.; Peloquin, J.M.; Sturgeon, H.C.; Nguyen, D.D.; Sauk, J.; Garber, J.; Yajnik, V.; Ananthakrishnan, A.N.; Chan, A.T.; et al. Body Mass Index, Genetic Susceptibility, and Risk of Complications among Individuals with Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 2304–2310. [Google Scholar] [CrossRef]
- Seminerio, J.L.; Koutroubakis, I.E.; Ramos-Rivers, C.; Hashash, J.G.; Dudekula, A.; Regueiro, M.; Baidoo, L.; Barrie, A.; Swoger, J.; Schwartz, M.; et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2857–2863. [Google Scholar] [CrossRef]
- Tian, J.; Venn, A.; Otahal, P.; Gall, S. The association between quitting smoking and weight gain: A systemic review and meta-analysis of prospective cohort studies. Obes. Rev. 2015, 16, 883–901. [Google Scholar] [CrossRef]
- Farraye, F.A.; Melmed, G.Y.; Lichtenstein, G.R.; Kane, S.V. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2017, 112, 241–258. [Google Scholar] [CrossRef]
- Berthon, B.S.; MacDonald-Wicks, L.K.; Wood, L.G. A systematic review of the effect of oral glucocorticoids on energy intake, appetite, and body weight in humans. Nutr. Res. 2014, 34, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of malnutrition and nutritional characteristics of patients with inflammatory bowel disease. J. Crohns Colitis. 2017, 11, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.; Grossmann, M. Obesity, type 2 diabetes, and testosterone in ageing men. Rev. Endocr. Metab. Disord. 2022, 23, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Gish, R.G.; Geier, A. MAFLD and Cardiovascular Events: What Does the Evidence Show? Clin. Gastroenterol. Hepatol. 2021, 19, 2025–2028. [Google Scholar] [CrossRef]
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Sokic-Milutinovic, A.; Milovanovic, T.; Lukic, S.; Milosavljevic, T.; Drazilov, S.S.; Klaassen, K.; Pavlovic, S.; et al. Metabolic Syndrome in Inflammatory Bowel Disease: Association with Genetic Markers of Obesity and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 31–38. [Google Scholar] [CrossRef]
- Romero-Gómez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Kim, M.-H.; Sung, J.-H.; Jin, M.-N.; Jang, E.; Yu, H.T.; Kim, T.-H.; Pak, H.-N.; Lee, M.-H.; Lip, G.Y.H.; Yang, P.-S.; et al. Impact of Physical Activity on All-Cause Mortality According to Specific Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 811058. [Google Scholar] [CrossRef]
- Vogelaar, L.; Van Den Berg-Emons, R.; Bussmann, H.; Rozenberg, R.; Timman, R.; Van Der Woude, C.J. Physical fitness and physical activity in fatigued and non-fatigued inflammatory bowel disease patients. Scand. J. Gastroenterol. 2015, 50, 1357–1367. [Google Scholar] [CrossRef]
- Gatt, K.; Schembri, J.; Katsanos, K.H.; Christodoulou, D.; Karmiris, K.; Kopylov, U.; Pontas, C.; Koutroubakis, I.E.; Foteinogiannopoulou, K.; Fabian, A.; et al. Inflammatory bowel disease [IBD] and physical activity: A study on the impact of diagnosis on the level of exercise amongst patients with IBD. J. Crohns Colitis 2019, 13, 686–692. [Google Scholar] [CrossRef]
| General Characteristic, Comorbidities, and Anthropometric Variables | Total (n = 688) | Clinical Variables Related to the Disease | Total (n = 688) |
|---|---|---|---|
| Sex: male n (%) | 341 (49.6) | Age of diagnosis, mean (IQR) | 35 (26–45) |
| Age, median (IQR) | 49 (39–59) | Years of evolution of the disease, mean (IQR) | 11 (5–20) |
| Current smokers: yes, n (%): | 137 (80.1) | More than 10 years of evolution, n (%) | 394 (57.4) |
| Former smokers, yes, n (%): | 313 (45.5) | IBD type and Location, n (%) UC Proctitis (UC) Left side colitis (UC) Extensive colitis (UC) CD Ileal (CD, L1) Ileocolonic (CD, L3) Colonic (CD, L2) | 369 (53.6) 91 (24.6) 137 (37.1) 141 (38.2) 319 (46.4) 124 (38.8) 150 (47) 45 (14.1) |
| Chronic renal disease: yes, n (%): | 65 (9.4) | Behaviour (EC), n (%) Inflammatory (B1) Stricturing (B2) Penetrating (B3) | 194 (60.8) 85 (26.6) 43 (13.5) |
| Cerebrovascular disease: yes, n (%) Cardiovascular disease: yes, n (%) | 10 (1.5) 25 (3.6) | Perianal disease, yes n (%) | 86 (12.5) |
| SBP, median (IQR) DBP, median (IQR) Hypertension: yes, n (%): | 132 (121–148) 80 (72–86) 106 (15.4) | Extraintestinal disease, yes n (%) | 136 (19.8) |
| Body mass Index, n (%): Low weight Normal weight Overweight Obesity | 15 (2.2) 319 (46.4) 238 (34.6) 116 (16.9) | Need for surgery, yes n (%) | 133 (19.3) |
| Waits to hip ratio: high n (%): | 344 (50) | Threartment, n (%) None 5 ASA Anti TNF Anti TNF and IMS IMS Vedolizumab Ustekinumab Tofacitinib Esteroids | 95 (13.8) 233 (33.9) 155 (22.5) 34 (4.9) 75 (10.9) 32 (4.7) 49 (7.1) 5 (0.7) 10 (1.5) |
| Metabolic syndrome, yes n (%) | 168 (24.4) | Steroid dependence, yes, n (%) | 208 (29.8) |
| MASLD, yes, (%) | 269 (39.1) | Systemic steroids in the last year, yes (n%) | 99 (14.4) |
| DM2 or pre-DM: yes, n (%): Insulin, m (IQR) | 242 (35.2) 6.95 (4.1–12.3) | Systemic steroids in the last 5 years, yes (n%) | 247 (35.9) |
| High total cholesterol, n (%): | 188 (165–210) | Topical steroids in the last year, yes (n%) | 8 (1.2) |
| Low HDL, n (%): | 114 (16.6) | Topical steroids in the last 5 years, yes (n%) | 25 (3.6) |
| Triglycerides: high, n (%): | 132 (19.2) | Disease activity, yes, n (%) | 41 (6) |
| No Overweight/Obesity (n = 234) | Overweight Yes (n = 238) | p-Value * | Obesity Yes (n = 116) | p-Value * | |
|---|---|---|---|---|---|
| Sex; females n (%) | 200 (59.9) | 94 (39.5) | <0.001 | 53 (45.7) | 0.008 |
| Age; m (IQR) | 46 (36–57) | 53 (41–61.5) | <0.001 | 52.5 (44–60) | 0.001 |
| Smoker; n (%) | 77 (23.1) | 39 (16.4) | 0.051 | 21 (18.1) | 0.266 |
| Former smoker; n (%) | 127 (38) | 123 (51.7) | 0.001 | 63 (54.3) | 0.002 |
| High WHR; n (%) | 120 (36.1) | 151 (63.4) | 0.001 | 73 (62.9) | 0.001 |
| Age at diagnosis, m (IQR) | 31.5 (24–42) | 37 (29–47) | <0.001 | 37 (29–48) | 0.001 |
| More than 10 years of evolution; Yes, n (%) | 189 (56.6) | 141 (59.4) | 0.488 | 64 (55.2) | 0.791 |
| IBD type; UC n (%) | 176 (52.7) | 133 (55.9) | 0.451 | 60 (51.7) | 0.857 |
| Location; n (%) Proctitis (UC) Left side colitis (UC) Pancolitis (UC) Ileal (L1, CD) Ileocecal (L3, CD) Colonic (L2, CD) | 42 (12.6) 65 (19.5) 69 20.7) 64 (19.2) 71 (21.3) 23 (6.9) | 35 (14.7) 46 (19.3) 52 (21.8) 43 (18.1) 51 (21.4) 11 (4.6) | 0.869 | 14 (12.1) 26 (22.4) 20 (17.2) 17 (14.7) 28 (24.1) 11 (9.5) | 0.706 |
| Behavior (CD); n (%) Inflammatory (B1) Stricturing (B2) Penetrating (B3) | 103 (65.2) 35 (22.2) 20 (12.7) | 57 (54.3) 35 (33.3) 13 (12.4) | 0.122 | 34 (60.7) 13 (23.2) 9 (16.1) | 0.777 |
| Perianal disease; yes n (%) | 42 (26.6) | 25 (23.8) | 0.613 | 19 (33.9) | 0.295 |
| Clinical remission; yes n (%) | 13 (3.9) | 16 (6.7) | 0.128 | 12 (10.3) | 0.009 |
| Extraintestinal disease; yes n (%) | 70 (21) | 43 (18.1) | 0.392 | 23 (19.8) | 0.796 |
| History of surgery; yes n (%) | 62 (18.6) | 43 (18.1) | 0.880 | 28 (24.1) | 0.196 |
| More than two surgeries, n (%) | 32 (9.6) | 15 (6.3) | 0.159 | 9 (7.8) | 0.557 |
| Need of any biologic therapy; yes n (%) | 78 (27.4) | 56 (26.9) | 0.913 | 29 (29) | 0.754 |
| Steroid dependence; yes n (%) | 105 (31.8) | 65 (27.7) | 0.288 | 35 (30.4) | 0.783 |
| Need of any steroid therapy in the last 5 years, n (%) | 130 (39.4) | 81 (34.5) | 0.233 | 49 (42.6) | 0.545 |
| IBDQ-9 | 51 (45–54.3) | 51 (47–55) | 0.412 | 50.5 (46–54) | 0.860 |
| Adherence to MD, n (%) | 121 (36.2) | 59 (24.8) | 0.004 | 26 (22.4) | 0.006 |
| Active lifestyle, n (%) | 109 (32.6) | 59 (24.8) | 0.042 | 20 (17.2) | 0.002 |
| Healthy lifestyle, n (%) | 43 (12.9) | 15 (6.3) | 0.010 | 5 (4.3) | 0.010 |
| Metabolic Syndrome | p-Value | Type 2 DM or Prediabetes | p-Value | MASLD | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 520) | Yes (n = 168) | No (n = 446) | Yes (n = 242) | No (n = 419) | Yes (n = 269) | ||||
| Sex; females n (%) | 274 (52.7) | 73 (43.5) | 0.037 | 258 (57.8) | 89 (36.8) | <0.001 | 252 (60.1) | 95 (35.3) | <0.001 |
| Age; m (IQR) | 46 (37–57) | 58 (50–65.8) | <0.001 | 46 (36.5–56) | 55 (46–64) | <0.001 | 46 (36–57) | 54 (44.5–62.5) | <0.001 |
| Smoker; yes n (%) | 105 (20.2) | 32 (19) | 0.747 | 95 (21.3) | 42 (17.4) | 0.216 | 88 (21) | 49 (18.2) | 0.372 |
| Former smoker; yes n (%) | 221 (42.5) | 92 (54.8) | 0.006 | 185 (41.5) | 128 (52.9) | 0.004 | 178 (57.5) | 135 (50.2) | 0.048 |
| Overweight or obesity (BMI); n (%) Yes | 207 (39.8) | 147 (87.5) | <0.001 | 186 (41.7) | 168 (69.4) | <0.001 | 143 (34.1) | 211 (78.4) | <0.001 |
| Waist hip ratio; high n (%) | 230 (44.4) | 114 (67.9) | <0.001 | 183 (41.1) | 161 (66.8) | <0.001 | 155 (37.2) | 189 (70.3) | <0.001 |
| Age at diagnosis, mean (IQR) | 33 (25–42) | 42 (32.3–50.8) | <0.001 | 32 (25–42) | 38 (30–49) | <0.001 | 32 (25–43) | 37 (29.5–47) | <0.001 |
| More than 10 years of evolution; Yes, n (%) | 285 (54.9) | 109 (64.9) | 0.023 | 237 (53.3) | 157 (64.9) | 0.003 | 223 (53.3) | 171 (63.6) | 0.008 |
| IBD type; UC n (%) | 285 (54.8) | 84 (50) | 0.277 | 232 (52) | 137 (56.6) | 0.249 | 231 (55.1) | 138 (51.3) | 0.326 |
| Location; n (%) Proctitis (UC) Left side (UC) Pancolitis (UC) Ileal (CD) Ileocecal (CD) Colonic (CD) | 70 (13.5) 101 (19.4) 115 (22.1) 97 (18.7) 108 (20.8) 29 (5.6) | 21(12.5) 36 (21.4) 26 (15.5) 27 (16.1) 42 (25) 16 (9.5) | 0.179 | 58 (13) 81 (18.2) 93 (20.9) 87 (19.5) 101 (22.6) 26 (5.8) | 33 (13.6) 56 (23.1) 48 (19.8) 37 (15.3) 49 (20.2) 19 (7.9) | 0.413 | 61 (14.6) 83 (19.8) 88 (21) 74 (17.7) 88 (21) 25 (6) | 30 (11.2) 54 (20.1) 53 (19.7) 50 (18.6) 62 (23) 20 (7.4) | 0.775 |
| Behavior (CD); n (%) Inflammatory (B1) Stricturing (B2) Penetrating (B3) | 142 (60.4) 68 (28.9) 25 (10.6) | 52 (61.9) 15 (17.9) 17 (20.2) | 0.026 | 133 (62.1) 62 (29) 19 (8.9) | 61 (58.1) 21 (20) 23 (21.9) | 0.003 | 118 (62.8) 47 (25) 23 (12.2) | 76 (58) 36 (27.5) 19 (14.5) | 0.680 |
| Perianal disease; yes n (%) | 60 (25.5) | 26 (31) | 0.337 | 51 (23.8) | 35 (33.3) | 0.072 | 52 (27.7) | 34 (26) | 0.736 |
| Clinical remission; yes n (%) | 492 (94.6) | 155 (92.3) | 0.263 | 420 (94.2) | 227 (93.8) | 0.845 | 397 (94.7) | 250 (92.9) | 0.327 |
| Extraintestinal disease; yes n (%) | 103 (19.8) | 33 (19.6) | 0.963 | 89 (20) | 47 (19.4) | 0.867 | 82 (19.6) | 54 (20.1) | 0.871 |
| History of surgery; yes n (%) | 93 (17.9) | 40 (23.8) | 0.091 | 83 (18.6) | 50 (20.7) | 0.515 | 72 (17.2) | 61 (22.7) | 0.075 |
| Need of any biologic therapy; yes n (%) | 129 (28.2) | 34 (25) | 0.459 | 106 (27.7) | 57 (27) | 0.848 | 96 (26.3) | 67 (29.4) | 0.413 |
| Anti-TNF therapy; n (%) Yes | 129 (28.9) | 34 (25) | 0.380 | 106 (28.3) | 57 (27.3) | 0.783 | 96 (26.7) | 67 (29.9) | 0.407 |
| Steroid dependence; yes n (%) | 162 (31.5) | 43 (26.1) | 0.189 | 143 (32.4) | 62 (25.9) | 0.079 | 124 (29.7) | 81 (30.8) | 0.769 |
| Need of any steroid therapy in the last 5 years. Yes n (%) | 199 (38.6) | 61 (37) | 0.701 | 186 (42.2) | 74 (31) | 0.004 | 159 (38.2) | 101 (38.3) | 0.992 |
| IBQ-9; m(IQR) | 51 (46–54) | 51 (45–54) | 0.650 | 51 (46–54) | 51 (46–55) | 0903 | 51 (46–54) | 51 (46–54) | 0.593 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Mateo, S.; Martínez-Domínguez, S.J.; Gargallo-Puyuelo, C.J.; Arroyo Villarino, M.T.; Laredo De La Torre, V.; Gallego, B.; Alfambra, E.; Gomollón, F. Lifestyle Can Exert a Significant Impact on the Development of Metabolic Complications and Quality Life in Patients with Inflammatory Bowel Disease. Nutrients 2023, 15, 3983. https://doi.org/10.3390/nu15183983
García-Mateo S, Martínez-Domínguez SJ, Gargallo-Puyuelo CJ, Arroyo Villarino MT, Laredo De La Torre V, Gallego B, Alfambra E, Gomollón F. Lifestyle Can Exert a Significant Impact on the Development of Metabolic Complications and Quality Life in Patients with Inflammatory Bowel Disease. Nutrients. 2023; 15(18):3983. https://doi.org/10.3390/nu15183983
Chicago/Turabian StyleGarcía-Mateo, Sandra, Samuel J. Martínez-Domínguez, Carla Jerusalén Gargallo-Puyuelo, María Teresa Arroyo Villarino, Viviana Laredo De La Torre, Beatriz Gallego, Erika Alfambra, and Fernando Gomollón. 2023. "Lifestyle Can Exert a Significant Impact on the Development of Metabolic Complications and Quality Life in Patients with Inflammatory Bowel Disease" Nutrients 15, no. 18: 3983. https://doi.org/10.3390/nu15183983
APA StyleGarcía-Mateo, S., Martínez-Domínguez, S. J., Gargallo-Puyuelo, C. J., Arroyo Villarino, M. T., Laredo De La Torre, V., Gallego, B., Alfambra, E., & Gomollón, F. (2023). Lifestyle Can Exert a Significant Impact on the Development of Metabolic Complications and Quality Life in Patients with Inflammatory Bowel Disease. Nutrients, 15(18), 3983. https://doi.org/10.3390/nu15183983