The Role of Diet and Nutrition in Allergic Diseases
Abstract
1. Introduction
2. Materials and Methods
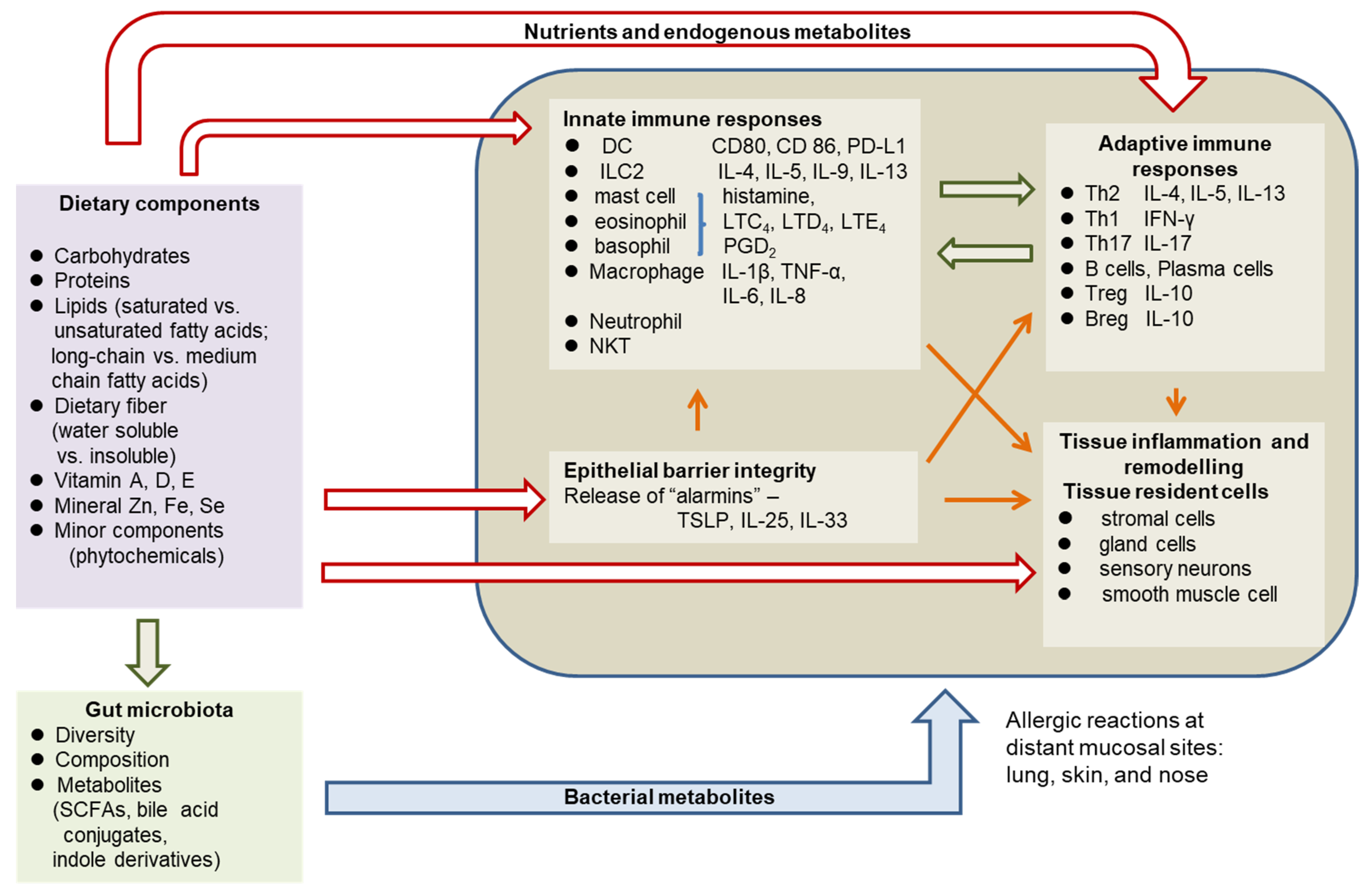
3. Pathophysiology of Allergic Diseases
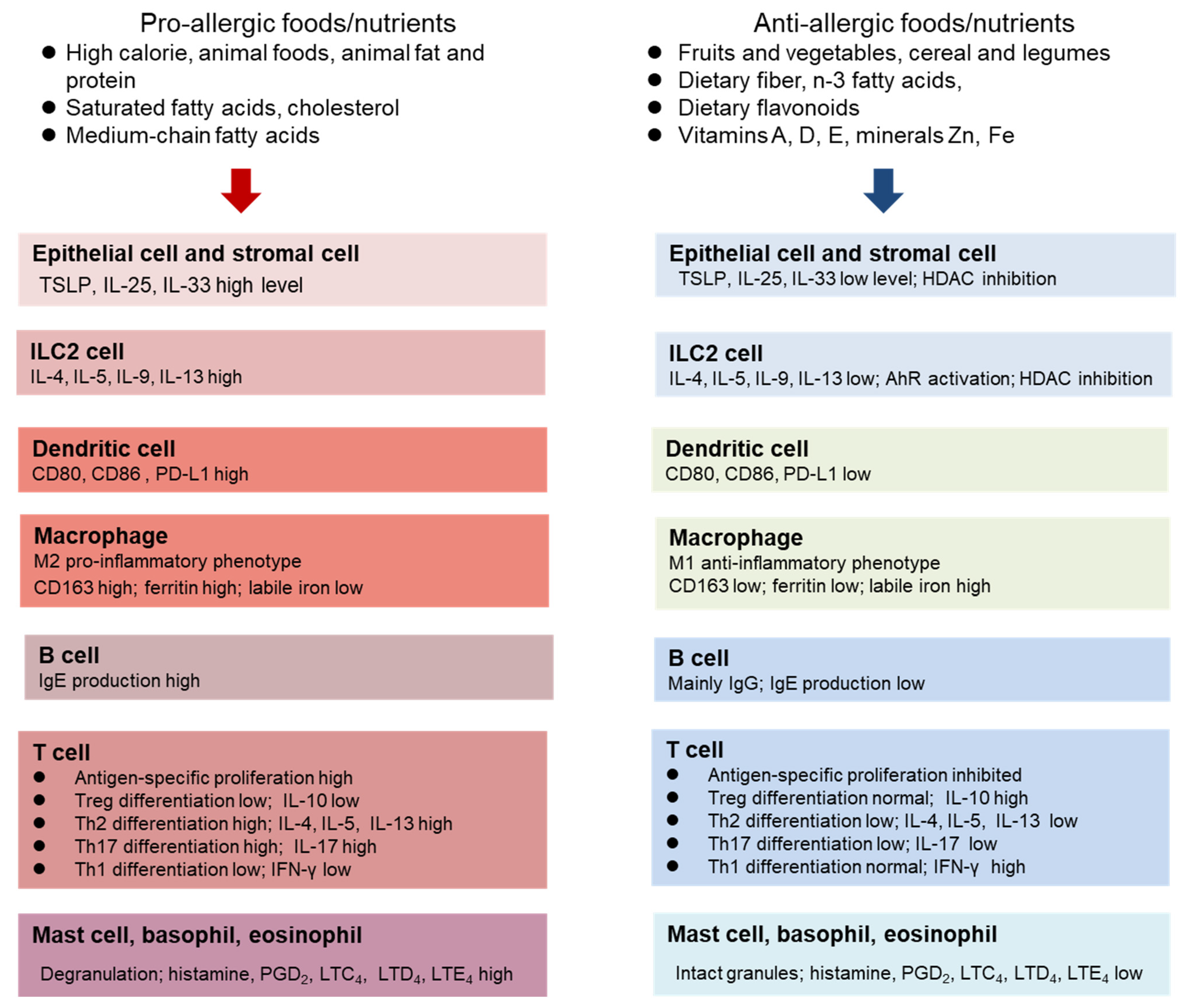
4. The Role of Diet and Nutritional Status in Allergy
4.1. Dietary protein, Amino Acids, and Energy
4.2. Dietary Lipids
4.3. Dietary Fiber
4.4. Dietary Flavonoids and Other Phytochemicals
| Flavonoids | Experimental Models | Results | Reference |
|---|---|---|---|
| Quercetin | OVA-induced AR in BALB/c mice 25 mg/kg dosage 5 d during challenge | Inhibited sneeze and nasal rubs | [99] |
| Suppressed angiogenic factors | |||
| and TNF-α, IL-6, IL-8 | |||
| Quercetin | Human HaCaT keratinocytes | Promoted wound repair | [20] |
| ↑ E-cadherin, Occludin, Twist, Snail | |||
| ↑ IL-10 at basal level | |||
| ↓ MMP1, MMP2, MMP9, ↓ TSLP | |||
| Kaempferol | DNCB/mite extract induced | ↓ ear thickness | [101] |
| dermatitis in BALB/c mice ear | ↓ Dermal and epidermal thickness | ||
| 15, 50 mg/kg 5 d on/2 d off | ↓ Mast cell infiltration | ||
| for 4 wks following 2nd DNCB | ↓ Serum IgE | ||
| ↓ mRNA of IL-4, IL-13, IFNγ | |||
| IL-17a, IL-6, IL-31, TSLP | |||
| in ear tissue | |||
| Jurkat cells | ↓ αCD3/CD28, PMA/A23187 | ||
| stimulated IL-2 production | |||
| ↓ AICD | |||
| Inhibited MRP-1 activity | |||
| Suppressed JNK phosphorylation | |||
| Kaempferol | OVA-induced allergic asthma | ↓ TGF-β production in the lung | [53] |
| in BALB/c mice | ↑ E-cadherin and epithelial thickening | ||
| 10, 20 mg/kg for 3 days | ↓ α-SMA, | ||
| during challenge | ↓ Collagen IV, ↓ MT1-MMP | ||
| ↓ Lung fibrosis | |||
| ↓ PAR1 signaling | |||
| Naringenin | OVA-induced AR in Sprague Dawley rats | Reduced nasal scratching and number of sneezing | [102] |
| 100 mg/kg 7 d during challenge | Decreased serum IL-4, IL-5 | ||
| Diosmetin | DNCB-induced AD | ↑ Skin barrier function | [103] |
| in SKH-1 hairless mice | ↓ Skin swelling, erythema | ||
| 5 mg/kg for 14 d | ↓ Skin erosion and dryness | ||
| during challenging period | ↓ Epidermal thickness | ||
| ↓ Mast cell infiltration in skin | |||
| ↓ Serum IgE and IL-4 | |||
| Baicalin | OVA-induced AR | Reduced inflammatory cells | [100] |
| in BALB/c mice | in nasal lavage fluid | ||
| L-Baicalin 50 mg/kg | ↓ Nasal symptoms | ||
| H-Baicalin 200 mg/kg | ↓ Thickness of nasal epithelium | ||
| 10 d following sensitization | ↓ Nasal mucus production | ||
| and 4 d before challenge | ↓ IL-17, ↑ IL-10 in nasal discharge | ||
| ↓ OVA-specific IgE, IgG1 antibodies | |||
| Inhibited autophagy in nasal mucosa | |||
| Baicalin | DNTB-induced AD | ↓ Dorsal skin thickness | [104] |
| in BALB/c mice | ↓ Trans-dermal water loss | ||
| 50, 100, 200 mg/kg | ↓ Epidermal thickness | ||
| 14-d following DNTB stimulation | ↑ Skin barrier function, ↓ TSLP | ||
| ↓ NF-κB signaling pathway in skin | |||
| ↓ JAK, STAT signaling pathway | |||
| ↑ Actinobacteria | |||
| Licoricidin | DNCB/mite induced atopic | ↓ Epidermal and dermal tissue | [105] |
| dermatitis in ear tissue | ↓ Infiltrating mast cells | ||
| in BALB/c mice | ↓ Serum IgE, IgG1, IgG2a | ||
| 50 mg/kg 5 d on/2 d off following | ↓ mRNA of IL-4, IL-5, | ||
| the 2nd DNCB for 4 wks | IL-6, IL-13 in ear tissue | ||
| ↓ Size and weight of draining | |||
| lymph nodes | |||
| ↓ T cells and Th2 cytokines in dLNs | |||
| ↑ T cell PTPN1 phosphorylation in dLNs | |||
| ↓ DC activation through | |||
| antagonizing PTPN1 | |||
| Resveratrol | 3-month repeated OVA | ↓ Airway hyperresponsiveness | [52] |
| exposure induced chronic | ↓ Inflammatory cells, IL-4, Il-5, Il-13 | ||
| asthma in BALB/c mice | in BAL fluid | ||
| ↓ Lung infiltration of inflammatory cells | |||
| ↓ Goblet cell number | |||
| ↓ Peribronchial α-SMA | |||
| ↓ Collagen amount in lung tissue | |||
| SDG | OVA-induced AR | Ameliorated sneezing number | [106] |
| in BALB/c mice | Decreased eosinophil and neutrophil | ||
| 100 mg/kg 3 times a week for | infiltration | ||
| 4 wks before initial sensitization | Enhanced β-glucuronidase | ||
| activity and increased | |||
| ED levels in nasal passage |
4.5. Vitamins and Minerals
5. Obesity and Allergy
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Undem, B.J.; Taylor-Clark, T. Mechanisms underlying the neuronal-based symptoms of allergy. J. Allergy Clin. Immunol. 2014, 133, 1521–1534. [Google Scholar] [CrossRef]
- Holgate, S.T.; Wenzel, S.; Postma, D.S.; Weiss, S.T.; Renz, H.; Sly, P.D. Asthma. Nat. Rev. Dis. Primers 2015, 1, 15025. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Anto, J.M.; Bachert, C.; Baiardini, I.; Bosnic-Anticevich, S.; Canonica, C.W.; Melén, E.; Palomares, O.; Scadding, G.K.; Togias, A.; et al. Allergic rhinitis. Nat. Rev. Dis. Primers 2020, 6, 95. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Julia, V.; Macia, L.; Dombrowicz, D. The impact of diet on asthma and allergic diseases. Nature 2015, 15, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Kao, Y.C.; Pan, W.H.; Yang, Y.H.; Chen, Y.C.; Lee, Y.L. Associations between respiratory diseases and dietary patterns derived by factors analysis and reduced rank regression. Ann. Nutr. Metab. 2016, 68, 306–314. [Google Scholar] [CrossRef]
- Netting, M.J.; Middleton, P.F.; Markrides, M. Does maternal diet during pregnancy and lactation affect outcomes in offspring? A systemic review of food-based approaches. Nutrition 2014, 30, 1225–1241. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Longo, M.N.L.; Luerigo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and allergic diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar]
- Sugihara, K.; Kamada, N. Diet-microbiota interactions in inflammatory bowel disease. Nutrients 2021, 13, 1533. [Google Scholar] [CrossRef]
- Zhang, P. Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fiber promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Pernot, J.; Perdijk, O.; Alqahtani, R.A.A.; Domingo, J.S.; Camacho-Muñoz, D.; Wong, N.C.; Kendall, A.C.; Wiederkehr, A.; Nicod, L.P.; et al. Gut-derived short-chain fatty acids modulate skin barrier integrity by promoting keratinocyte metabolism and differentiation. Mucosal Immunol. 2022, 15, 908–926. [Google Scholar] [CrossRef] [PubMed]
- Palomares, O.; Akdis, M.; Martin-Frontecha, M.; Akdis, C.A. Mechanisms of immune regulation in allergic diseases: The role of regulatory T and B cells. Immunol. Rev. 2017, 278, 219–236. [Google Scholar]
- Roan, F.; Obata-Ninomiya, K.; Ziegler, S.F. Epithelial cell-derived cytokines: More than just signaling the alarm. J. Clin. Investig. 2019, 129, 1441–1451. [Google Scholar] [PubMed]
- Dahlgren, M.W.; Jones, S.W.; Cautivo, K.M.; Dubinin, A.; Oritiz-Carpena, J.F.; Farhat, S.; Yu, K.S.; Lee, K.; Wang, C.Q.; Molofsky, A.V.; et al. Adventitial stromal cells define group 2 innate lymphoid cell tissue niches. Immunity 2019, 50, 702–722. [Google Scholar] [CrossRef]
- Moon, P.D.; Han, N.R.; Kim, H.M.; Jeong, H.J. High-fat diet exacerbates dermatitis through up-regulation of TSLP. J. Investig. Dermatol. 2019, 139, 1198–1201. [Google Scholar] [CrossRef]
- Han, S.C.; Kang, G.J.; Ko, Y.J.; Kang, H.K.; Moon, S.W.; Ann, Y.S.; Yoo, E.S. Fermented fish oil suppresses T helper 1/2 cell response in a mouse model of AD via generation of CD4+CD25+Foxp3+ T cells. BMC Immunol. 2012, 13, 44. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin improves inflammation, oxidative stress, and impaired would healing in AD model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Artis, D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 2020, 30, 475–491. [Google Scholar]
- Kiss, E.A.; Vonarbourg, C.; Kopfmann, S.; Hobeika, E.; Finke, D.; Esser, C.; Diefenbach, A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bostick, J.W.; Ye, J.; Qiu, J.; Zhang, B.; Urban, J.F.; Auram, D.; Zhou, L. Aryl hydrocarbon receptor signaling cell intrinsically inhibits intestinal group innate lymphoid cell function. Immunity 2018, 49, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Thio, C.L.P.; Chi, P.Y.; Lai, A.C.Y.; Chang, Y.J. Regulation of type 2 innate lymphoid cell-dependent airway hyperreactivity by butyrate. J. Allergy Clin. Immunol. 2018, 142, 1867–1883. [Google Scholar]
- Van der Marel, A.P.J.; Samsom, J.N.; Greuter, M.; van Berkel, L.A.; O’Toole, T.; Kraal, G.; Mebius, R.E. Blockade of IDO inhibits nasal tolerance induction. J. Immunol. 2007, 179, 894–900. [Google Scholar] [CrossRef]
- Ünüvar, S.; Erge, D.; Kiliçarslan, B.; Bağ, H.G.G.; Çatal, F.; Girgin, G.; Baydar, T. Neopterin levels and indoleamine 2,3-dioxygenase activity as biomarkers of immune system activation and childhood allergic diseases. Ann. Lab. Med. 2019, 39, 284–290. [Google Scholar] [PubMed]
- Lau, H.X.; EI-Heis, S.; Yap, Q.V.; Chan, Y.H.; Tan, C.P.T.; Karnani, N.; Tan, K.M.L.; Tham, E.H.; Goh, A.E.N.; Teoh, O.H.; et al. Role of maternal tryptophan metabolism in allergic diseases in the offspring. Clin. Exp. Allergy 2021, 51, 1346–1360. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Doherty, T.A.; Karta, M.R.; Das, S.; Baum, R.; Rosenthal, P.; Beppu, A.; Miller, M.; Kurten, R.; Broide, D.H. Regulatory B cells and T follicular helper cells are reduced in AR. J. Allergy Clin. Immunol. 2016, 138, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Wiest, M.; Upchurch, K.; Hasan, M.M.; Cardenas, J.; Lanier, B.; Millard, M.; Turner, J.; Oh, S.; Joo, H. Phenotypic and functional alterations of regulatory B cell subsets in adult asthma patients. Clin. Exp. Allergy 2019, 49, 1214–1224. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Ishiuji, Y.; Yoshizaki, A.; Kurita, M.; Hayashi, M.; Ishiji, T.; Nakagawa, H.; Asahina, A.; Yanaba, K. IL-10-producing regulatory B cells are decreased in patients with AD. J. Investig. Dermatol. 2019, 139, 475–478. [Google Scholar] [CrossRef]
- Liu, J.Q.; Geng, X.R.; Hu, T.Y.; Mo, L.M.; Luo, X.Q.; Qiu, S.Y.; Liu, D.B.; Liu, Z.G.; Shao, J.B.; Liu, Z.Q.; et al. Glutaminolysis is required in maintaining immune regulatory functions in B cells. Mucosal Immunol. 2022, 15, 268–278. [Google Scholar] [PubMed]
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal Th17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.W.; Hwang, K.A.; Shin, M.S.; Lee, S.H.; Kim, W.U.; Kang, I. 1,25-dihyroxyvitamin D-3 promotes foxp3 expression via binding to vitamin D response elements in its conserved sequence region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef]
- Yokota-Nakatsuma, A.; Takeuchi, H.; Ohoka, Y.; Kato, C.; Song, S.Y.; Hoshino, T.; Yagita, H.; Ohteki, T.; Iwata, M. Retinoic acid prevents mesenteric lymph node dendritic cells from inducing IL-13-producing inflammatory Th2 cells. Mucosal Immunol. 2014, 7, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Vasiliou, J.E.; Lui, S.; Walker, S.A.; Chohan, V.; Xystrkis, E.; Bush, A.; Hawrylowicz, C.M.; Saglani, S.; Lloyd, C.M. Vitamin D deficiency induces Th2 skewing and eosiphilia in neonatal allergic airway disease. Allergy 2014, 69, 1380–1389. [Google Scholar] [CrossRef]
- Maywald, M.; Meurer, S.K.; Weiskirchen, R.; Rink, L. Zinc supplementation augments TGF-β1-depedent regulatory T cell induction. Mol. Nutr. Food Res. 2017, 61, 1600493. [Google Scholar] [CrossRef]
- Rosenkranz, E.; Hilgers, R.D.; Uciechowski, P.; Petersen, A.; Plümäkers, B.; Rink, L. Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. Eur. J. Nutr. 2017, 56, 557–567. [Google Scholar] [CrossRef]
- Vaidyanathan, B.; Chaudhy, A.; Yewdell, W.T.; Angeletti, D.; Yen, W.F.; Wheatley, A.K.; Bradfield, C.A.; McDermott, A.B.; Yewdell, J.W.; Rudensky, A.Y.; et al. The aryl hydrocarbon receptor controls cell-fate decisions in B cells. J. Exp. Med. 2017, 214, 197–208. [Google Scholar]
- Barroso, A.; Mahler, J.V.; Fonseca-Castro, P.H.; Quintana, F.J. Therapeutic induction of tolerogenic dendritic cells via aryl hydrocarbon receptor signaling. Curr. Opin. Immunol. 2021, 70, 33–39. [Google Scholar]
- Ye, J.; Qiu, J.; Bostick, J.W.; Ueda, A.; Schjerven, H.; Li, S.Y.; Jobin, C.; Chen, Z.M.E.; Zhou, L. The aryl hydrocarbon receptor preferentially marks and promotes gut regulatory T cells. Cell Rep. 2017, 21, 2277–2290. [Google Scholar] [CrossRef]
- Piper, C.J.M.; Rosser, E.C.; Oleinika, K.; Nistala, K.; Krausgruber, T.; Rendeiro, A.P.F.; Banos, A.; Drozdov, I.; Villa, M.; Thomson, S.; et al. Aryl Hydrocarbon Receptor Contributes to the Transcriptional Program of IL-10-Producing Regulatory B Cells. Cell Rep. 2019, 29, 1878–1892. [Google Scholar] [CrossRef]
- Afify, S.M.; Regner, A.; Pacios, L.F.; Blokhuis, B.R.; Jensen, S.A.; Redegeld, F.A.; Pali-Schöll, I.; Hufnagl, K.; Bianchini, R.; Guethoff, S.; et al. Micronutritional supplementation with a holoBLG-based FSMP (food for special medical purposes)-lozenge alleviates allergic symptoms in BALB/c mice: Imitating the protective farm effect. Clin. Exp. Allergy 2022, 52, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F.; Afify, S.M.; Pacios, L.F.; Blokhuis, B.R.; Redegeld, F.; Regner, A.; Petje, L.M.; Flocchi, A.; Untersmayr, E.; Dvorak, Z.; et al. Cow’s milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells. J. Allergy Clin. Immunol. 2021, 147, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. AR and its impact on asthma (ARIA) 2008 update. Allergy 2008, 63 (Suppl. S86), 8–160. [Google Scholar] [PubMed]
- Zheng, M.; Wang, X.; Bo, M.; Wang, K.; Zhao, Y.; He, F.; Cao, F.; Zhang, L.; Bachert, C. Prevalence of AR among Adults in Urban and Rural Areas of China: A Population-Based Cross-Sectional Survey. Allergy Asthma Immunol. Res. 2015, 7, 148–157. [Google Scholar] [CrossRef]
- Urrutia-Pereira, M.; Mocelin, L.P.; Ellwood, P.; Garcia-Marcos, L.; Simon, L.; Rinelli, P.; Chong-Neto, H.J.; Solé, D. Prevalence of rhinitis and associated factors in adolescents and adults: A global asthma network study. Rev. Paul. Pediatr. 2023, 41, e2021400. [Google Scholar] [CrossRef]
- Eifan, A.O.; Durham, S.R. Pathogenesis of rhinitis. Clin. Exp. Allergy 2016, 46, 1139–1151. [Google Scholar] [CrossRef]
- Sahoyama, Y.; Hamazato, F.; Shiozawa, M.; Nakagawa, T.; Suda, W.; Ogata, Y.; Hachiya, T.; Kawakami, E.; Hattori, M. Multiple nutritional and gut microbial factors associated with AR: The Hitachi Health Study. Sci. Rep. 2022, 12, 3359. [Google Scholar] [CrossRef]
- Bartosik, T.; Jensen, S.A.; Afify, S.M.; Bianchini, R.; Hufnagl, K.; Hofstetter, G.; Berger, M.; Bastl, M.; Berger, U.; Rivelles, E.; et al. Ameliorating atopy by compensating micronutrional deficiencies in immune cells: A double-blinded placebo-controlled pilot study. J. Allergy Clin. Immunol. 2022, 10, 1889–1902. [Google Scholar] [CrossRef]
- Peroni, D.G.; Hufnagl, K.; Comberiati, P.; Roth-Walter, F. Lack of iron, zinc, and vitamins as contributor to the etiology of atopic diseases. Front. Nutr. 2023, 9, 1032481. [Google Scholar] [CrossRef]
- Arpornchayanon, W.; Klinprung, S.; Chansakaow, S.; Hanprasertpong, N.; Chaiyasate, S.; Tokuda, M.; Tamura, H. Antiallergic activities of shallot (Allium ascalonicum L.) and its therapeutic effects in AR. Asian Pac. J. Allergy Immunol. 2022, 40, 393–400. [Google Scholar]
- Lee, H.Y.; Kim, I.K.; Yoon, H.K.; Kwon, S.S.; Rhee, C.K.; Lee, S.Y. Inhibitory effects of resveratrol on airway remodeling by transforming growth factor-β/smad signaling pathway in chronic asthma model. Allergy Asthma Immunol. Res. 2017, 9, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.H.; Cho, I.H.; Shin, D.; Han, S.Y.; Park, S.H.; Kang, Y.H. Inhibition of airway epithelial-to-mesenchymal transition and fibrosis in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab. Investig. 2014, 94, 297–308. [Google Scholar]
- The role of vitamin D supplementation on airway remodeling in asthma: A systemic review. Nutrients 2023, 15, 2477. [CrossRef]
- Leung, D.Y.M.; Berdyshev, E.; Gloeva, E. Cutaneous barrier dysfunction in allergic diseases. J. Allergy Clin. Immnol. 2020, 145, 1485–1497. [Google Scholar]
- Kraft, M.T.; Prince, B.T. AD is a barrier issue, not an allergy issue. Immunol. Allergy Clin. N. Am. 2019, 39, 507–519. [Google Scholar] [CrossRef]
- Khan, A.; Adalsteinsson, J.; Whitaker-Worth, D.L. AD and nutrition. Clin. Dermatol. 2022, 40, 135–144. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fiber intake and asthma (symptoms and control) results from the French national e-cohort NutriNet-Santé. Brit. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Cazzoletti, L.; Zanolin, M.E.; Speita, F.; Bono, R.; Chamitava, L.; Cerveri, I.; Garcia-Larsen, V.; Grosso, A.; Mattioli, V.; Pirina, P.; et al. Dietary fats, olive oil and respiratory diseases in Italian adults: A population-based study. Clin. Exp. Allergy 2019, 49, 799–807. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Li, L.S.; Sun, J.L.; Guan, K.; Wei, J.F. 1H NMR-based metabolomics study of metabolic profiling for pollinosis. World Allergy Org. J. 2019, 12, 100005. [Google Scholar] [CrossRef]
- Ma, G.C.; Wang, T.S.; Wang, J.; Ma, Z.J.; Pu, S.B. Serum metabolomics of patients with AR. Biomed. Chromatogr. 2020, 34, e4739. [Google Scholar] [CrossRef]
- Yoshino, K.; Sakai, K.; Okada, H.; Sakai, T.; Yamamoto, S. IgE responses in mice fed moderate protein deficient and high protein diets. J. Nutr. Sci. Vitaminol. 2003, 49, 172–178. [Google Scholar] [CrossRef][Green Version]
- Fan, W.Y.; Kouda, K.; Nakamura, H.; Takeuchi, H. Effects of dietary restriction on spontaneous dermatitis in NC/Nga mice. Exp. Biol. Med. 2001, 226, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Kositz, C.; Schroecksnadel, K.; Grander, G.; Schennach, H.; Kofler, H.; Fuchs, D. Serum tryptophan concentration in patients predicts outcome of specific immunotherapy with pollen extracts. Int. Arch. Allergy Immunol. 2008, 147, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Licari, A.; Fuchs, D.; Marseglia, G.; Ciprandi, G. Tryptophan metabolic pathway and neopterin in asthmatic children in clinical practice. Ital. J. Pediatr. 2019, 45, 11. [Google Scholar] [CrossRef] [PubMed]
- Luukkainen, A.; Karjalainen, J.; Hurme, M.; Paavonen, T.; Toppila-salmi, S. Relationships of indoleamine 2,3-dioxygenase activity and cofactors with asthma and nasal polyps. Am. J. Rhinil. Allergy 2014, 28, e5–e10. [Google Scholar] [CrossRef] [PubMed]
- Gostner, J.M.; Becker, K.; Kofler, H.; Strasser, B.; Fuchs, D. Tryptophan metabolism in allergic disorders. Int. Arch. Allergy Immunol. 2016, 169, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newshoime, P. Glutamine: Metabolism and immune function, supplementation and clinical transition. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Xiao, Y.C.; Zhou, X.F.; Chang, J.H.; Chang, M.; Cheng, X.H.; Blonska, M.; Lin, X.; Sun, S.C. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity 2014, 40, 692–705. [Google Scholar] [CrossRef]
- Huang, S.L.; Pan, W.H. Dietary fats and asthma in teenagers: Analyses of the first nutrition and health survey in Taiwan (NAHSIT). Clin. Exp. Allergy 2001, 31, 1875–1880. [Google Scholar] [CrossRef]
- Jena, P.K.; Sheng, L.; McNeil, K.; Chau, T.Q.; Yu, S.; Kiuru, M.; Fung, M.A.; Hwang, S.T.; Wan, Y.J.Y. Long-term western diet intake leads to dysregulated bile acid signaling and dermatitis with Th2 and Th17 pathway features in mice. J. Dermatol. Sci. 2019, 95, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Tang, L.; de Villiers, W.J.S.; Cohen, D.; Woodward, J.; Finkelman, F.D.; Eckhardt, E.R.M. Dietary medium-chain triglycerides promote oral allergic sensitization and orally induced anaphylaxis to peanut protein in mice. J. Allergy Clin. Immunol. 2013, 131, 442–450. [Google Scholar] [CrossRef]
- Iwamoto, A.; Hamajima, H.; Tsuge, K.; Tsuruta, Y.; Nagata, Y.; Yotsumoto, H.; Yanagita, T. Inhibitory effects of green asparagus extract, especially phospholipids, on allergic responses in vitro and in vivo. J. Agric. Food Chem. 2020, 68, 15199–15207. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Rinaldi, A.O.; Sözener, Z.Ç.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The influence of dietary fatty acids on immune responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef]
- Fujii, M.; Nakashima, J.; Tomozawa, J.; Shimazaki, Y.; Ohyanagi, N.; Kawaguchi, S.; Ohya, S.; Kohno, S.; Nabe, T. Deficiency of n-6 polyunsaturated fatty acids is mainly responsible for AD-like pruritic skin inflammation in special diet-fed hairless mice. Exp. Dermatol. 2013, 22, 272–277. [Google Scholar] [CrossRef]
- Sawane, K.; Nagatake, T.; Hosomi, K.; Hirata, S.; Adachi, J.; Abe, Y.; Isoyama, J.; Suzuki, H.; Matsunaga, A.; Kunisawa, J.; et al. Dietary omega-3 fatty acid dampens AR via eosinophilic production of the anti-allergic lipid mediator 15-hydroxyeicosapentaenoic acid in mice. Nutrients 2019, 11, 2868. [Google Scholar] [CrossRef]
- Van den Elsen, L.W.; Nusse, Y.; Balvers, M.; Redegeld, F.A.; Knol, E.F.; Garssen, J.; Willemsen, L.E.M. n-3 long-chain PUFA reduce allergy-related mediator release by human mast cells in vitro via inhibition of reactive oxygen species. Br. J. Nutr. 2013, 109, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Ohyanagi, C.; Kawaguchi, N.; Matsuda, H.; Miyamoto, Y.; Ohya, S.; Nabe, T. Eicosapentaenoic acid ethyl ester ameliorates AD-like symptoms in special diet-fed hairless mice, partly by restoring covalently bound ceramides in the stratum corneum. Exp. Dermatol. 2018, 27, 837–840. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, M.; Li, D.; Li, J.; Guo, Z.; Liu, Y.; Wan, S.; Liu, Y. Olive oil ameliorate allergic response in ovalbumin-induced food allergy mouse by promoting intestinal mucosal immunity. Food Sci. Hum. Wellness 2023, 12, 801–808. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J.; Guo, Y.; Ma, L.; Liu, Y.; Kuang, H.; Han, B.; Xiao, Y.; Wang, Y. Dietary olive oil enhances the oral tolerance of the food allergen ovalbumin in mice by regulating intestinal microecological homeostatis. J. Food Biochem. 2022, 46, e14297. [Google Scholar] [CrossRef] [PubMed]
- Agra, L.C.; Lins, M.P.; da Silva Marques, P.; Smaniotto, S.; de Melo, C.B.; Lagente, V.; Barreto, E. Uvaol attenuates pleuritis and eosinophilic inflammation in ovalbumin-induced allergy in mice. Eur. J. Pharmacol. 2016, 780, 232–242. [Google Scholar]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Harris, N.L.; Marsland, B.J.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [PubMed]
- Cait, A.; Huges, M.R.; Antignano, F.; Cait, T.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yoo, Y.; Hwang, J.; Na, Y.C.; Kim, H.S. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying AD. J. Allergy Clin. Immunol. 2016, 137, 852–860. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Cheng, M.L.; Chiang, M.H.; Kuo, Y.L.; Tsai, M.H.; Chiu, C.C.; Lin, G. gut microbial-derived butyrate is inversely associated with IgE responses to allergens in childhood asthma. Pediatr. Allergy Immunol. 2019, 30, 689–697. [Google Scholar] [CrossRef]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D.; Cait, J.; Sbihi, H.; Stiemsma, L.; Subbarao, P.; Mandhane, P.J.; et al. Reduced genetic potential for butyrate fermentation in the gut microbiome of infants who develop allergic sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647. [Google Scholar]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldafferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, G.P. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar]
- Li, X.; Guo, J.; Ji, K.; Zhang, P. Bamboo shoot fiber prevents obesity in mice by modulating the gut microbiota. Sci. Rep. 2016, 6, 32953. [Google Scholar] [CrossRef]
- Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Cerrudo, V.; Audebert, M.; Dumont, F.; Mancano, G.; Khodorova, N.; Andriamihaja, M.; et al. Quantity and source of dietary protein influence metabolic production by gut microbiota and rectal mucosa gene expression: A randomized, parallel, double-blind trail in overweight humans. Am. J. Clin. Nutr. 2017, 106, 1005–1019. [Google Scholar] [PubMed]
- Chiu, C.Y.; Chan, Y.L.; Tsai, M.T.; Wang, C.J.; Chiang, M.H.; Chiu, C.C. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ. J. 2019, 12, 100021. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S.S. Diet, Microbiota and gut-lung connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, R.; Berthon, B.S.; Rogers, G.B.; Baines, K.J.; Leong, L.E.X.; Gibson, P.G.; Williams, E.J.; Wood, L.G. soluble fiber supplementation with and without a probiotic in adults with asthma: A 7-day randomized, double-blind, three way cross-over trial. eBioMedicine 2019, 46, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Venter, C.; Meyer, R.W.; Greenhawt, M.; Pali-Schöll, I.; Nwaru, B.; Roduit, C.; Untersmayr, E.; Adel-Patient, K.; Agache, I.; Agostoni, C.; et al. Role of dietary fiber in promoting immune health-an EAACI position paper. Allergy 2022, 77, 3185–3198. [Google Scholar] [CrossRef]
- Wang, J.; Wen, L.; Wang, Y.; Chen, F. Therapeutic effect of histone deacetylase inhibitor, sodium butyrate, on AR in vivo. DNA Cell Biol. 2016, 35, 203–208. [Google Scholar] [CrossRef]
- Geraghty, A.A.; Lindsay, K.L.; Alberdi, G.; McAuliffe, F.M.; Gibney, E.R. Nutrition during pregnancy impacts offspring’s epigenetic status-evidence from human and animal studies. Nutr. Metab. Insights 2015, 8 (Suppl. S1), 41–47. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Jafarinia, M.; Hosseini, M.S.; Kasiri, N.; Fazel, N.; Fathi, F.; Hakemi, M.G.; Eskandari, N. Quercetin with the potential on allergic diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef]
- Okumo, T.; Furuta, A.; Kimura, T.; Yusa, K.; Asano, K.; Sunagawa, M. Inhibition of angiogenic factor productions by quercetin in vitro and in vivo. Medicines 2021, 8, 22. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Liu, X.; Ma, Z.; Li, Y. Baicalin regulates Treg/Th17 cell imbalance by inhibiting autophagy in AR. Mol. Immunol. 2020, 125, 162–171. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, G.S. Therapeutic effect of kaempferol on AD by attenuation of T cell activity via interaction with multidrug-associated protein. Br. J. Pharmacol. 2021, 178, 1772–1788. [Google Scholar] [CrossRef]
- Sahin, A.; Sakat, M.S.; Kilic, K.; Aktan, B.; Yildirim, S.; Kandemir, F.M.; Dortbudak, M.B.; Kucukler, S. The protective effect of naringenin against ovalbumin-induced AR in rats. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 4839–4846. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bong, S.K.; Lee, J.W.; Park, N.J.; Choi, Y.; Kim, S.M.; Yang, M.H.; Kim, Y.K.; Kim, S.N. Diosmetin and its glycoside, diosmin, improves AD-like lesions in 2,4-dinitrochlorobenzene-induced murine models. Biomol. Ther. 2020, 28, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xian, Y.F.; Loo, S.K.F.; Ip, S.P.; Yang, W.; Chan, W.Y.; Lin, Z.X.; Wu, J.C.Y. Baicalin ameliorates 2, 4-dinitrochlorobenzene-induced AD-like skin lesions in mice through modulating skin barrier function, gut microbiota and JAK/STAT pathway. Bioorg. Chem. 2022, 119, 105538. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, J.; Choi, H.G.; Kim, E.K.; Jun, C.D. Licoricidin abrogates T-cell activation by modulating PTPN1 activity and attenuates AD in vivo. J. Investig. Dermatol. 2021, 141, 2490–2498. [Google Scholar] [CrossRef]
- Sawane, K.; Nagatake, T.; Hosomi, K.; Kunisawa, J. Anti-allergic property of dietary phytoestrogen secoisolariciresinol diglucoside through microbial and β-glucuronidase-mediated metabolism. J. Nutr. Biochem. 2023, 112, 109219. [Google Scholar] [CrossRef] [PubMed]
- Civelek, M.; Bilotta, S.; Lorentz, A. Resveratrol attenuates mast cell mediated allergic reactions: Potential for use as a nutraceutical in allergic diseases. Mol. Nutr. Food Res. 2022, 66, 2200170. [Google Scholar] [CrossRef] [PubMed]
- Che, D.N.; Cho, B.O.; Shin, J.Y.; Kang, H.J.; Kim, J.; Choi, J.; Jang, S. Anti-AD effects of hydrolyzed celery extract in mice. J. Food Biochem. 2020, 44, e13198. [Google Scholar] [CrossRef]
- Dorjsemble, B.; Nho, C.W.; Choi, Y.; Kim, J.C. Extract from black soybean cultivar A63 extract ameliorates AD-like skin inflammation in an oxazolone-induced murine model. Molecules 2022, 27, 2751. [Google Scholar] [CrossRef]
- Gadelha, F.A.A.F.; Cavalcanti, R.F.P.; Vieira, G.C.; Ferrira, L.K.D.P.; de Sousa, G.R.; Filho, J.M.B.; Barbosa, M.A.; dos Santos, S.G.; Piuvezam, M.R. Immunomodulatory properties of Musa papadisiaca L. inflorescence in combined AR and asthma syndrome (CARAS) model towards NFκB pathway inhibiton. J. Funct. Food 2021, 83, 104540. [Google Scholar] [CrossRef]
- Bui, T.T.; Piao, C.H.; Hyeon, E.; Fan, Y.; Nguyen, T.V.; Jung, S.Y.; Choi, D.W.; Lee, S.; Shin, H.S.; Song, C.H.; et al. The protective role of Piper nigrun fruit extract in an ovalbumin-induced AR by targeting of NFκBp65 and STAT3 signaling. Biomed. Pharmacother. 2019, 109, 1015–1923. [Google Scholar] [CrossRef] [PubMed]
- Ishida, M.; Miyagawa, F.; Nishi, R.; Sugahara, T. Aqueous extract from Cuminum cyminum L. seed alleviates ovalbumin-induced AR in mouse via balancing of helper T cells. Foods 2022, 11, 3224. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Shirai, M.; Inaba, Y.; Takara, T. Effects of repeated oral intake of a quercetin-containing supplement on allergic reaction: A randomized, placebo-controlled, double-blind parallel-group study. Eur. Rev. Med. Pharmacol. 2022, 26, 4331–4345. [Google Scholar]
- Derakhshan, A.; Khodadoost, M.; Ghanei, M.; Gachkar, L.; Hajimahdipour, H.; Taghipour, A.; Yousefi, J.; Khoshkhui, M.; Azad, F.J. Effects of a novel barley-based formulation on AR: A randomized controlled trial. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1224–1231. [Google Scholar] [CrossRef]
- Sanchez-Armendariz, K.; Garcia-Gil, A.; Romero, C.A.; Contreras-Ruiz, J.; Karam-Orante, M.; Balcazar-Antonio, D.; Dominguez-Cherit, J. Oral vitamin D3 5000 IU/day as an adjuvant in the treatment of AD: A randomized control trial. Int. J. Dermatol. 2018, 57, 1516–1520. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Juhasz, M.L.W.; Mesinkovska, N.A. The role of oral vitamins and supplements in the management of AD: A systematic review. Int. J. Dermatol. 2019, 58, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Petje, L.M.; Jensen, S.A.; Szikora, S.; Sulzbacher, M.; Bartosik, T.; Pjevac, P.; Hausmann, B.; Hufnagi, K.; Untersmayr, E.; Fischer, L.; et al. Functional iron-deficiency in women with AR is associated with symptoms after nasal provocation and lack of iron-sequestering microbes. Allergy 2021, 76, 2882–2923. [Google Scholar] [CrossRef]
- Jiang, J.; Nasab, E.M.; Athari, S.M.; Athari, S.S. Effects of vitamin E and selenium on AR and asthma pathophysiology. Respir. Physiol. Neurobiol. 2021, 286, 103614. [Google Scholar] [CrossRef]
- Wu, G.; Zhu, H.; Wu, X.; Liu, L.; Ma, X.; Yuan, Y.; Fu, X.; Zhang, L.; Lv, Y.; Li, D.; et al. Anti-allergic function of α-Tocopherol is mediated by suppression of PI3K-PKB activity in mast cells in mouse model of AR. Allergol. Immnopathol. 2020, 48, 395–400. [Google Scholar] [CrossRef]
- Truong-Tran, A.Q.; Ruffin, R.E.; Foster, P.S.; Koskinen, A.M.; Coyle, P.; Philox, J.C.; Rofe, A.M.; Zalewski, P.D. Altered zinc homeostasis and caspase-3 activity in murine allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2002, 27, 286–296. [Google Scholar] [CrossRef]
- Kulik, L.; Maywald, M.; Kloubert, V.; Wessels, I.; Rink, L. Zinc deficiency drives Th17 polarization and promotes loss of Treg cell function. J. Nutr. Biochem. 2019, 63, 11–18. [Google Scholar] [CrossRef]
- Tsai, Y.L.; Ko, W.S.; Hsino, J.L.; Pan, H.H.; Chiou, Y.L. zinc sulfate improved the unbalanced T cell profiles in Der p-allergic asthma: An ex vivo study. Clin. Respir. J. 2018, 12, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Roth-Walter, F. Iron-deficiency in atopic diseases: Innate immune priming by allergens and siderophores. Front. Allergy 2022, 3, 859922. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Podlecka, D.; Jerzynska, J.; Sanad, K.; Polanska, K.; Bobrow-Korzeniowska, M.; Stelmach, I.; Brzozowska, A. Micronutrients and the risks of allergic diseases in school children. Intl. J. Environ. Res. Public Health 2022, 19, 12187. [Google Scholar] [CrossRef] [PubMed]
- Norton, R.L.; Hoffmann, P.R. Selenium and Asthma. Mol. Asp. Med. 2012, 33, 98–106. [Google Scholar] [CrossRef]
- Gozzi-Silva, S.C.; Teixeira, F.M.E.; Duarte, A.J.S.; Sato, M.N.; de Oliveira, L.M. Immunomodulatory role of nutrients: How can pulmonary dysfunctions improve? Front. Nutr. 2021, 8, 674258. [Google Scholar] [CrossRef]
- Chen, M.; Sun, Y.; Wu, Y.L. Lower circulating zinc and selenium levels are associated with an increased risk of asthma: Evidence from a meta-analysis. Public Health Nutr. 2019, 23, 1555–1562. [Google Scholar] [CrossRef]
- Kuti, B.P.; Kuti, D.K.K.; Smith, O.S. Serum zinc, selenium and total antioxidant contents of Nigerian children with asthma: Association with disease severity and symptoms control. J. Trop. Pediatr. 2020, 66, 395–402. [Google Scholar] [CrossRef]
- Baumann, S.; Lorentz, A. Obesity—A promoter of allergy? Int. Arch. Allergy Immunol. 2013, 162, 205–213. [Google Scholar] [CrossRef]
- Sybilski, A.J.; Raciborski, F.; Lipiec, A.; Tomaszewska, A.; Lusawa, A.; Furmańczyk, K.; Krzych-Fałta, E.; Komorowski, J.; Samoliński, B. Obesity—A risk factor for asthma, but not for atopic dermatitis, allergic rhinitis and sensitization. Public Health Nutr. 2015, 18, 530–536. [Google Scholar] [CrossRef]
- Chang, C.L.; Ali, G.B.; Pham, J.; Dharmage, S.C.; Lodge, C.J.; Tang, M.K.; Lowe, A.J. Childhood body mass index trajectories and asthma and allergies: A sysmatic review. Clin. Exp. Allergy 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Cowan, D.C. Obesity, Inflammation, and Severe Asthma: An Update. Curr. Allergy Asthma Rep. 2021, 21, 46. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, F.; Han, Y.; Lou, H.; Tang, X.; Zhang, L. Obesity/overweight and risk of allergic rhinitis: A meta-analysis of observational studies. Allergy 2020, 75, 1272–1275. [Google Scholar] [CrossRef]
- Han, M.W.; Kim, S.H.; Oh, I.; Kim, Y.H.; Lee, J. Obesity can contribute to severe persistent allergic rhinitis in children through leptin and interleukin-1 beta. Int. Arch. Allergy. Immunol. 2021, 18, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhu, T.; Wakefield, J.S.; Mauro, T.M.; Elias, P.M.; Man, M.Q. Link between obesity and atopic dermatitis: Does obesity predispose to atopic dermatitis, or vice versa? Exp. Dermatol. 2023, 2, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Alwarith, J.; Kahleova, H.; Crosby, L.; Brooks, A.; Brandon, L.; Levin, S.M.; Barnard, N.D. The role of nutrition in asthma prevention and treatment. Nutr. Rev. 2020, 78, 928–938. [Google Scholar] [CrossRef]
- Jensen, M.E.; Gibson, P.G.; Collins, C.E.; Hilton, J.M.; Wood, L.G. Diet-induced weight loss in obese children with asthma: A randomized controlled trial. Clin. Exp. Allergy 2013, 43, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin. Exp. Allergy 2013, 43, 36–49. [Google Scholar] [CrossRef]
- Luna-Pech, J.A.; Torres-Mendoza, B.M.; Luna-Pech, J.A.; Garcia-Cobas, C.Y.; Navarrete-Navarro, S.; Elizalde-Lozano, A.M. Normocaloric diet improves asthma-related quality of life in obese pubertal adolescents. Int. Arch. Allergy Immunol. 2014, 163, 252–258. [Google Scholar] [CrossRef]
- Son, J.H.; Chung, B.Y.; Jung, M.J.; Choi, Y.W.; Kim, H.O.; Park, C.W. Influence of Weight Loss on Severity of Atopic Dermatitis in a 20-Year-Old Female with Atopic Dermatitis. Ann. Dermatol. 2018, 30, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Kim, H.R.; Kang, S.Y.; Kim, H.O.; Chung, B.Y.; Park, C.W. Effect of Weight Reduction on Treatment Outcomes for Patients with Atopic Dermatitis. Ann. Dermatol. 2020, 32, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Dort, S.; Holubkov, R.; Barnard, N.D. A Plant-Based High-Carbohydrate, Low-Fat Diet in Overweight Individuals in a 16-Week Randomized Clinical Trial: The Role of Carbohydrates. Nutrients 2018, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Turner-McGrievy, G.; Barnard, N.D.; Scialli, A.R.; Lanou, A.J. Effects of a low-fat vegan diet and a Step II diet on macro- and micronutrient intakes in overweight postmenopausal women. Nutrition 2004, 20, 738–746. [Google Scholar]
- Ivanova, S.; Delattre, C.; Karcheva-Bahchevanska, D.; Benbasat, N.; Nalbantova, V.; Ivanov, K. Plant-Based Diet as a Strategy for Weight Control. Foods 2021, 10, 3052. [Google Scholar] [CrossRef]
- Lindahl, O.; Lindwall, L.; Spångberg, A.; Stenram, A.; Ockerman, P.A. Vegan regimen with reduced medication in the treatment of bronchial asthma. J. Asthma 1985, 22, 45–55. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouda, K.; Kotani, M.; Takeuchi, A.; Tabei, T.; Masamoto, Y.; Nakamura, H.; Takigawa, M.; Suemura, M.; Takeuchi, H.; et al. Vegetarian diet ameliorates symptoms of atopic dermatitis through reduction of the number of peripheral eosinophils and of PGE2 synthesis by monocytes. J. Physiol. Anthropol. Appl. Hum. Sci. 2001, 20, 353–361. [Google Scholar] [CrossRef]
| Year Location | Study Design | Subjects and Intervention | Results | |
|---|---|---|---|---|
| 2022 | RCT | Patients (n = 60) with | Improved allergic symptoms | [113] |
| Tokyo | eye/nose allergic symptoms | including eye itching, | ||
| Japan | Supplementation of | sneezing, nasal discharge, | ||
| 200 mg quercetin for 4 wks | sleep disorder | |||
| vs. the placebo food | ↓ Nasal discharge ecosipophil | |||
| Improved life quality | ||||
| 2022 | RCT | AR patients (n = 16) | ↑ Overall symptoms in | [51] |
| Chiang Mai | Treatment with10 mg cetirizine | 62.5% in shallot group | ||
| Thailand | for 4 wks plus oral supplement | 37.5% in placebo group | ||
| of 3 g shallot capsule vs. | ↓ Overall symptom score | |||
| the placebo capsule | ↓ Total ocular symptom score | |||
| 2022 | RCT | AR patients (n = 77) | Improved all symptoms | [114] |
| Tehran, | Treatment with 60 mg | except cough in both groups | ||
| Mashhad | Fexofenadine (FX) for 14 d. | MS better in nasal congestion, | ||
| Iran | vs. 15 g dried power of, | postnasal drip, and headache | ||
| Ma-al-Shaeer (MS), | ↓ Serum total IgE in both groups | |||
| a barley-based hot-water | ||||
| extracted formulation | ||||
| 2022 | RCT | Allergic women (n = 51) | ↓ Total nasal symptom score | [49] |
| Vienna, | Supplement for 6-month of | 42% improvement in treated | ||
| Austria | a lozenge called holoBLG (n = 25) | group vs. 13% in placebo group | ||
| containing β-lactoglobulin with | 45%, 31%, 40% improvement in | |||
| iron, polyphenol, retinoic acid, | combined symptom score in | |||
| zinc vs. placebo (n = 26) | holoBLG group in birch peak, | |||
| entire birch season, the entire | ||||
| grass pollen season | ||||
| ↑ Iron levels in circulating | ||||
| CD14+ monocytes | ||||
| ↑ Hematocrit values | ||||
| ↓ Red cell distribution width | ||||
| 2018 | RCT | Patients with AD (n = 65) | ↑ Serum vitamin D level | [115] |
| Mexico City | Standard treatment with | Inverse relationship between | ||
| Mexico | Vitamin D3 5000 IU/day | final serum vitamin D level | ||
| for 12 wks vs. no extra vitamin | and severity of AD | |||
| Serum vitamin D > 20 ng·/mL | ||||
| with standard therapy is sufficient to | ||||
| reduce AD severity | ||||
| 2019 | RCT | Asthma patients (n = 17) | Inulin decreased airway | [92] |
| Newcastle | Treated with 7 d inulin | eosinophils and HDAC9 | ||
| Australia | (6 g powder twice daily), | expression in sputum cells | ||
| inulin + probiotic, placebo | Inulin improved asthma | |||
| with a 2 wks run-in and | control in poorly controlled | |||
| 2 wks wash out periods | eosinophilic asthmatics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients 2023, 15, 3683. https://doi.org/10.3390/nu15173683
Zhang P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients. 2023; 15(17):3683. https://doi.org/10.3390/nu15173683
Chicago/Turabian StyleZhang, Ping. 2023. "The Role of Diet and Nutrition in Allergic Diseases" Nutrients 15, no. 17: 3683. https://doi.org/10.3390/nu15173683
APA StyleZhang, P. (2023). The Role of Diet and Nutrition in Allergic Diseases. Nutrients, 15(17), 3683. https://doi.org/10.3390/nu15173683






