Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Medicine Samples
2.2. Extraction Procedure
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Flavonoid Content
2.4.1. Procedure 1
2.4.2. Procedure 2
2.5. Determination of Monomeric Anthocyanin Content (MAC)
2.6. Determination of Oxygen Radical Absorbance Capacity (ORAC)
2.7. Determination of DPPH-Free Radical Scavenging Capacity
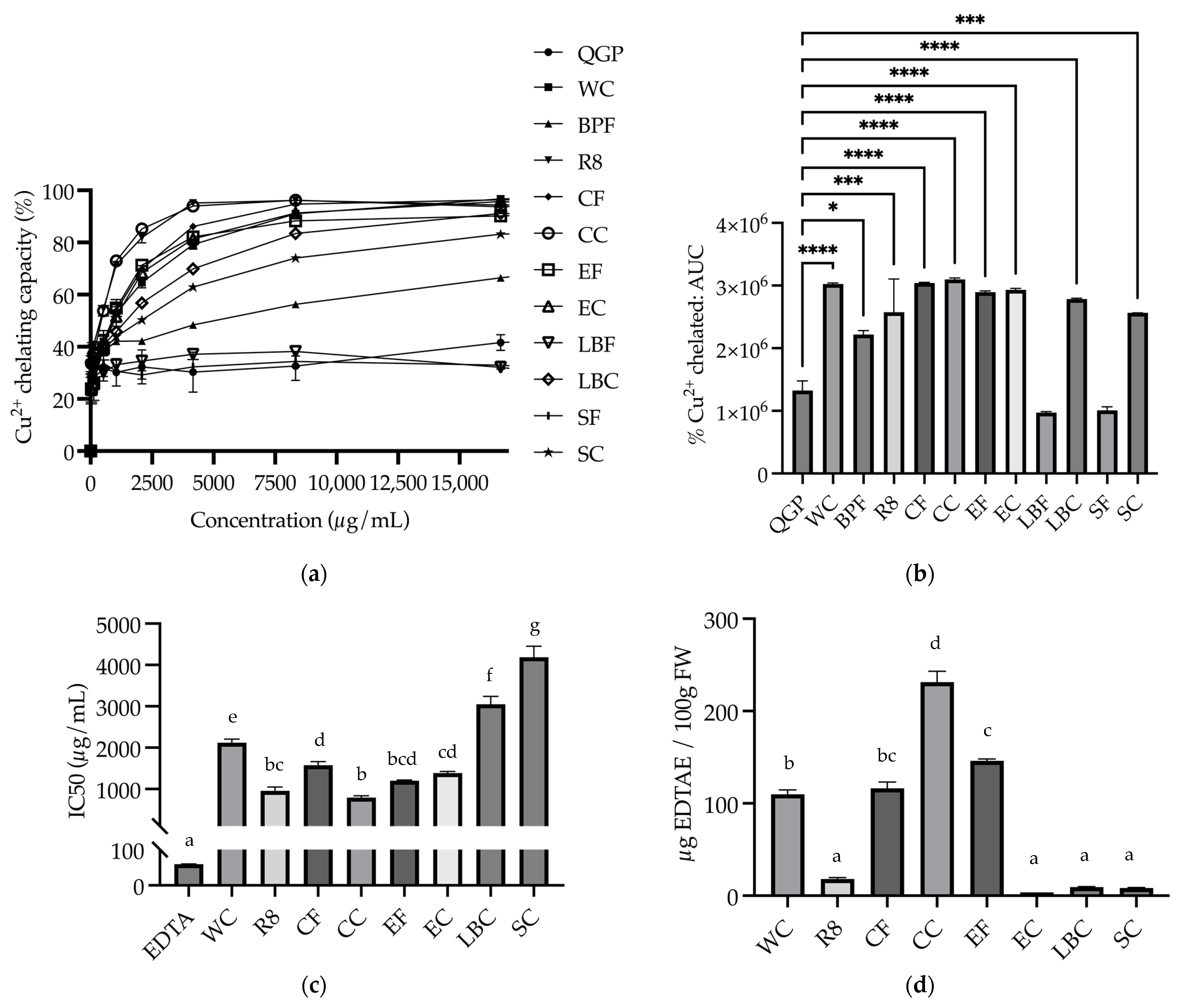
2.8. Determination of Cu2+ Chelating Capacity
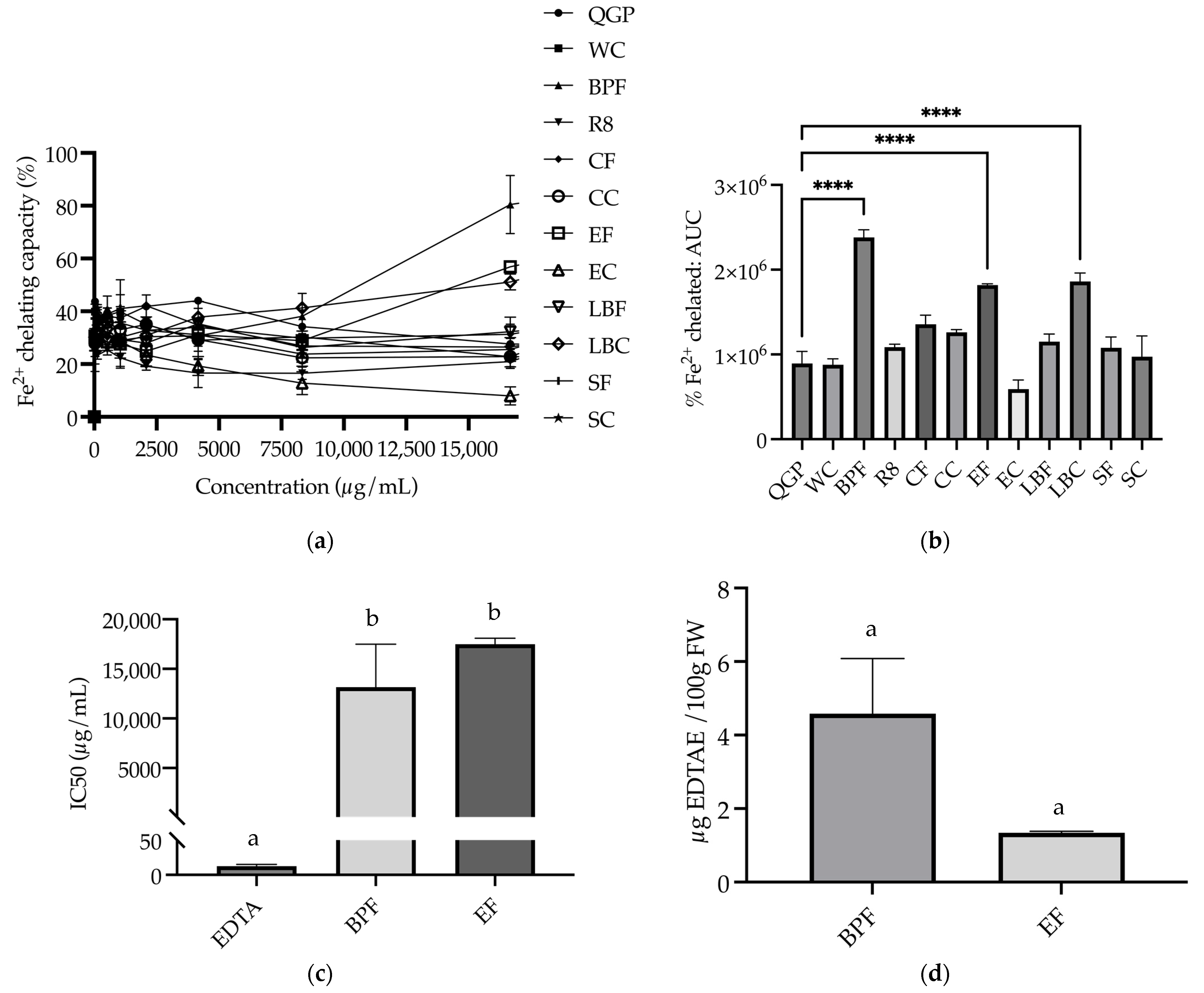
2.9. Determination of Fe2+ Chelating Capacity
2.10. Analysis of In Vitro Effects of Samples on H2O2-Induced Oxidative Stress Using SH-SY5Y Cells
2.10.1. Cell Culture and Growth
2.10.2. Dose Response of Samples on Cell Viability
2.10.3. Dose Response of Oxidative Stressor on Cell Viability
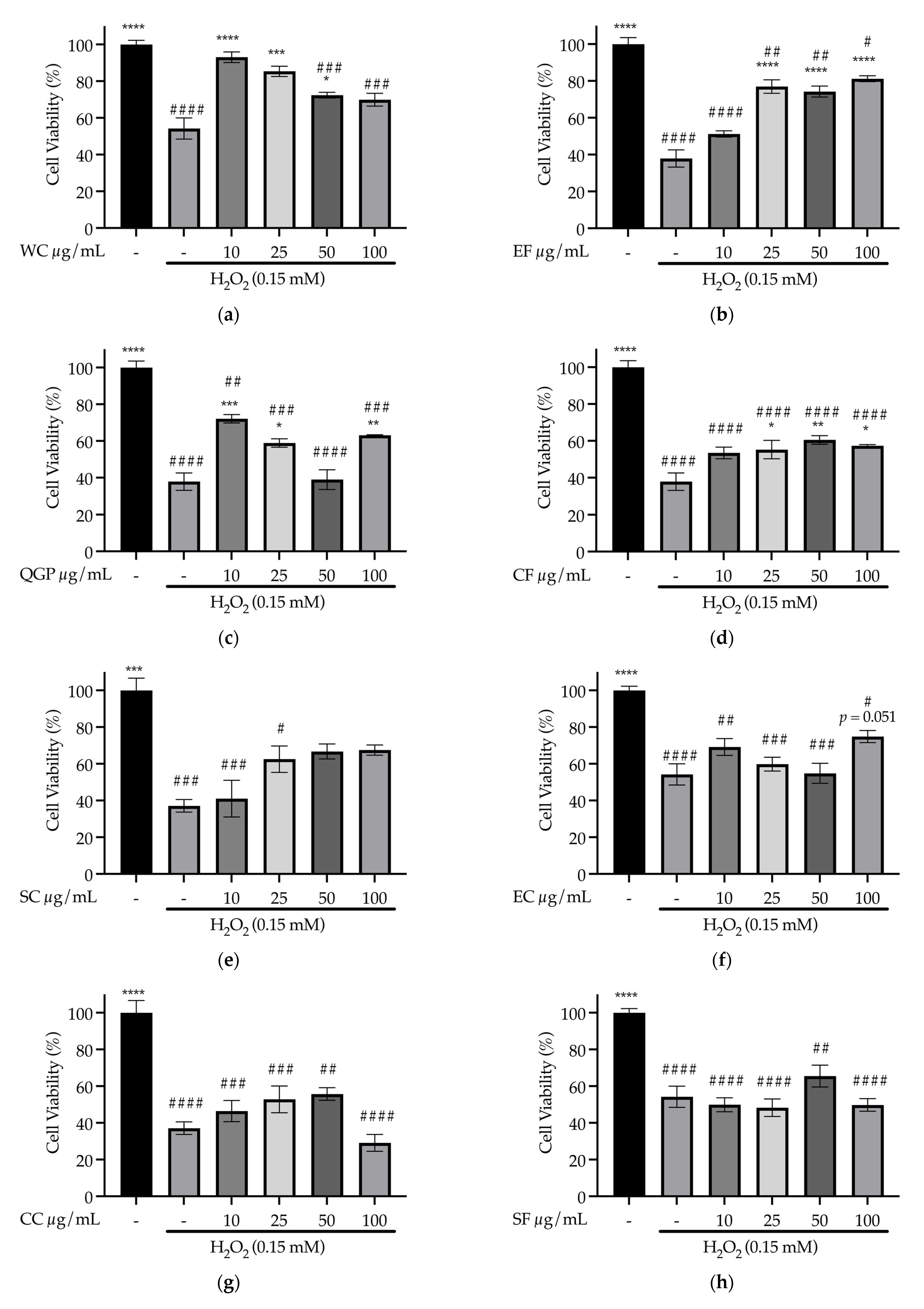
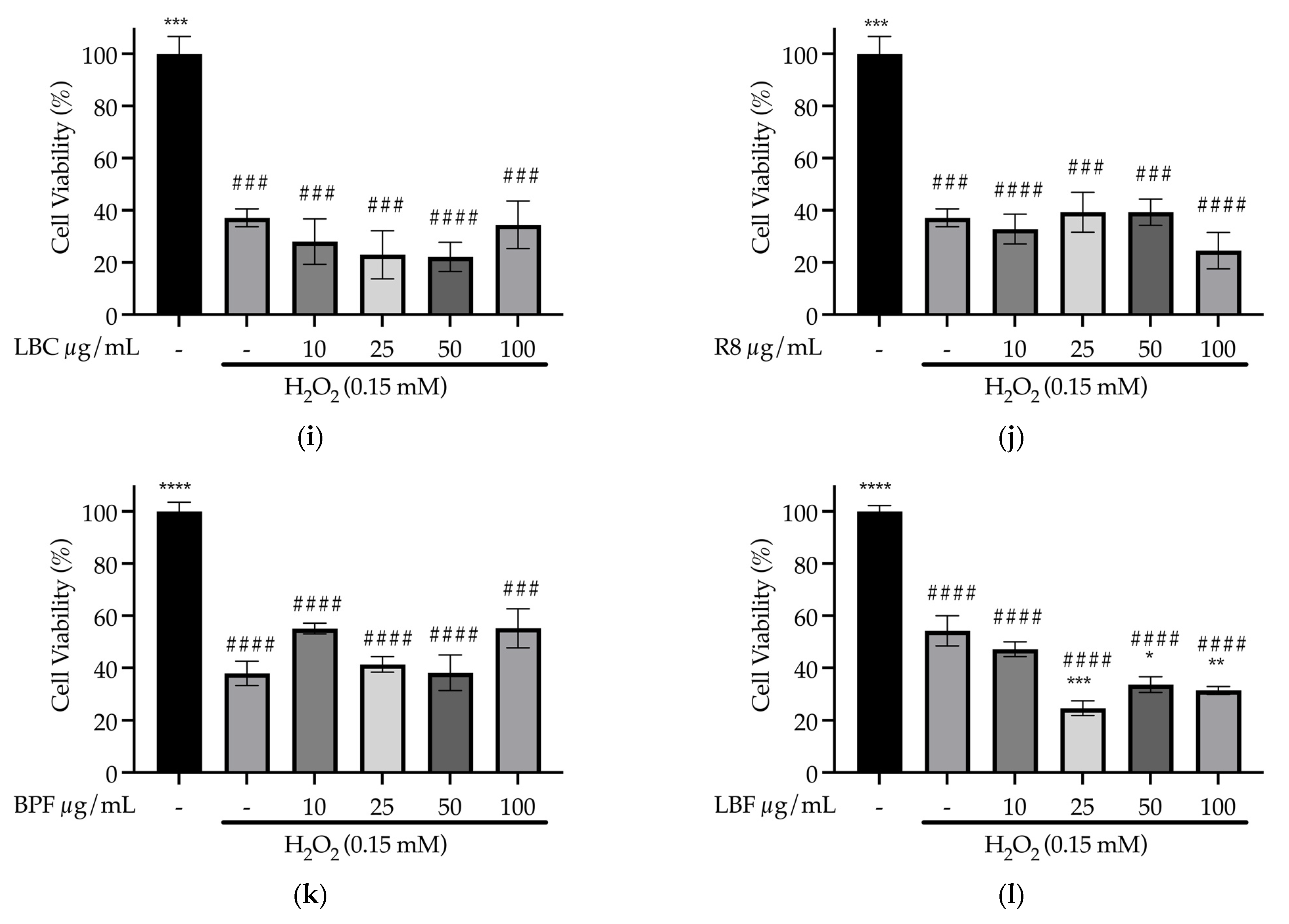
2.10.4. Oxidative Stress Prevention Paradigm and Cell Viability MTT Assay
2.10.5. Oxidative Stress Treatment Paradigm and Cell Viability MTT Assay
2.11. Statistical Analysis
3. Results
3.1. Total Phenolic, Flavonoid and Monomeric Anthocyanin Content
3.1.1. Total Phenolic Content
3.1.2. Flavonoids
3.1.3. Monomeric Anthocyanin Content
3.2. Antioxidant Capacity
3.2.1. ORAC
3.2.2. DPPH
3.3. Metal Chelating Capacity
3.3.1. Cu2+ Chelating
3.3.2. Fe2+ Chelating
3.4. Cell Culture Experiments to Determine Effects of Samples on Oxidative Stress in SH-SY5Y Cells (In Vitro)
3.4.1. Dose Response of Samples on SH-SY5Y Cell Viability in the Absence of Stressor
3.4.2. Dose Response of Oxidative Stressor on SH-SY5Y Cell Viability
3.4.3. Effect of Samples on Oxidative Stress Prevention in SH-SY5Y Cells (In Vitro)
3.4.4. Effect of Samples on Treatment of Oxidative Stress in SH-SY5Y Cells (In Vitro)
3.4.5. Correlations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, Mitochondria and Oxidative Stress: Cross-Talk and Redox Signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Melo, A.; Monteiro, L.; Lima, R.M.F.; de Oliveira, D.M.; de Cerqueira, M.D.; El-Bachá, R.S. Oxidative Stress in Neurodegenerative Diseases: Mechanisms and Therapeutic Perspectives. Oxidative Med. Cell. Longev. 2011, 2011, 467180. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of Oxidative Stress in Depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Nantachai, G.; Vasupanrajit, A.; Tunvirachaisakul, C.; Solmi, M.; Maes, M. Oxidative Stress and Antioxidant Defenses in Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 79, 101639. [Google Scholar] [CrossRef]
- Berr, C.; Balansard, B.; Arnaud, J.; Roussel, A.-M.; Alpérovitch, A.; Group, E.S. Cognitive Decline Is Associated with Systemic Oxidative Stress: The EVA Study. J. Am. Geriatr. Soc. 2000, 48, 1285–1291. [Google Scholar] [CrossRef]
- Hajjar, I.; Hayek, S.S.; Goldstein, F.C.; Martin, G.; Jones, D.P.; Quyyumi, A. Oxidative Stress Predicts Cognitive Decline with Aging in Healthy Adults: An Observational Study. J. Neuroinflamm. 2018, 15, 17. [Google Scholar] [CrossRef]
- Jorgensen, A.; Baago, I.B.; Rygner, Z.; Jorgensen, M.B.; Andersen, P.K.; Kessing, L.V.; Poulsen, H.E. Association of Oxidative Stress–Induced Nucleic Acid Damage with Psychiatric Disorders in Adults: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2022, 79, 920–931. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston–Green, K.; et al. Food Anthocyanins Decrease Concentrations of TNF-α in Older Adults with Mild Cognitive Impairment: A Randomized, Controlled, Double Blind Clinical Trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef]
- Lorzadeh, E.; Heidary, Z.; Mohammadi, M.; Nadjarzadeh, A.; Ramezani-Jolfaie, N.; Salehi-Abargouei, A. Does Pomegranate Consumption Improve Oxidative Stress? A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Clin. Nutr. ESPEN 2022, 47, 117–127. [Google Scholar] [CrossRef]
- Munteanu, I.G. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, 13394. [Google Scholar] [CrossRef]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res. 2019, 10, 1567–1574. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Castellano, S.; Currenti, W.; Galvano, F.; Ferri, R.; Grosso, G. Association Between Dietary Flavonoids Intake and Cognitive Function in an Italian Cohort. Biomolecules 2020, 10, 1300. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Yuan, C.; Ascherio, A.; Rosner, B.; Willett, W.; Blacker, D. Long-Term Dietary Flavonoid Intake and Subjective Cognitive Decline in US Men and Women. Neurology 2021, 97, 1041–1056. [Google Scholar] [CrossRef]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative Effect of Kaempferol, a Flavonoid, on Oxidative Stress in Streptozotocin-Induced Diabetic Rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Ekeanyanwu, R.C.; Njoku, O.U. Flavonoid-Rich Fraction of the MonoMonodora Tenuifolia Seed Extract Attenuates Behavioural Alterations and Oxidative Damage in Forced-Swim Stressed Rats. Chin. J. Nat. Med. 2015, 13, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E. Flavonoids and Brain Health: Multiple Effects Underpinned by Common Mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The Effects of Flavonoids on Cardiovascular Health: A Review of Human Intervention Trials and Implications for Cerebrovascular Function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P.E. The Mechanisms of Action of Flavonoids in the Brain: Direct versus Indirect Effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef]
- Medina dos Santos, N.; Berilli Batista, P.; Batista, Â.G.; Maróstica Júnior, M.R. Current Evidence on Cognitive Improvement and Neuroprotection Promoted by Anthocyanins. Curr. Opin. Food Sci. 2019, 26, 71–78. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; El-Shazly, M.; Fahmy, N.M.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Mesnage, R.; Fernandes, F.; Abhayaratne, N.; Xu, Y.; Zhang, Z.; Bell, L.; Williams, C.; Rodriguez-Mateos, A. Wild Blueberry (Poly)Phenols Can Improve Vascular Function and Cognitive Performance in Healthy Older Individuals: A Double-Blind Randomized Controlled Trial. Am. J. Clin. Nutr. 2023, 117, 1306–1319. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Ghosh, D.; McGhie, T.K.; Zhang, J.; Adaim, A.; Skinner, M. Effects of Anthocyanins and Other Phenolics of Boysenberry and Blackcurrant as Inhibitors of Oxidative Stress and Damage to Cellular DNA in SH-SY5Y and HL-60 Cells. J. Sci. Food Agric. 2006, 86, 678–686. [Google Scholar] [CrossRef]
- Vargas, C.G.; da Silva Junior, J.D.; Rabelo, T.K.; Moreira, J.C.F.; Gelain, D.P.; Rodrigues, E.; Augusti, P.R.; de Oliveira Rios, A.; Flôres, S.H. Bioactive Compounds and Protective Effect of Red and Black Rice Brans Extracts in Human Neuron-like Cells (SH-SY5Y). Food Res. Int. 2018, 113, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fanning, K.J.; Topp, B.; Russell, D.; Stanley, R.; Netzel, M. Japanese Plums (Prunus Salicina Lindl.) and Phytochemicals—Breeding, Horticultural Practice, Postharvest Storage, Processing and Bioactivity. J. Sci. Food Agric. 2014, 94, 2137–2147. [Google Scholar] [CrossRef]
- Netzel, M.; Fanning, K.; Netzel, G.; Zabaras, D.; Karagianis, G.; Treloar, T.; Russell, D.; Stanley, R. Urinary Excretion of Antioxidants in Healthy Humans Following Queen Garnet Plum Juice Ingestion: A New Plum Variety Rich in Antioxidant Compounds. J. Food Biochem. 2012, 36, 159–170. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Kundur, A.R.; Fanning, K.; Netzel, M.; Stanley, R.; Singh, I. Consumption of Anthocyanin-Rich Queen Garnet Plum Juice Reduces Platelet Activation Related Thrombogenesis in Healthy Volunteers. J. Funct. Foods 2015, 12, 11–22. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A Systematic Review on Black Pepper (Piper Nigrum L.): From Folk Uses to Pharmacological Applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- Chonpathompikunlert, P.; Wattanathorn, J.; Muchimapura, S. Piperine, the Main Alkaloid of Thai Black Pepper, Protects against Neurodegeneration and Cognitive Impairment in Animal Model of Cognitive Deficit like Condition of Alzheimer’s Disease. Food Chem. Toxicol. 2010, 48, 798–802. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, A.; Sachan, N.; Chandra, P. Anxiolytic and Antidepressant-like Effects of Essential Oil from the Fruits of Piper Nigrum Linn. (Black Pepper) in Mice: Involvement of Serotonergic but Not GABAergic Transmission System. Heliyon 2021, 7, e06884. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Kuete, V.; Mihasan, M. Methanolic Extract of Piper Nigrum Fruits Improves Memory Impairment by Decreasing Brain Oxidative Stress in Amyloid Beta(1–42) Rat Model of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2014, 34, 437–449. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and Antidepressant Profile of the Methanolic Extract of Piper Nigrum Fruits in Beta-Amyloid (1–42) Rat Model of Alzheimer’s Disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xian, Y.-F.; Ip, S.-P.; Che, C.-T. Involvement of Serotonergic System in the Antidepressant-like Effect of Piperine. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Dodd, F.L.; Robertson, B.C.; Okello, E.J.; Reay, J.L.; Scholey, A.B.; Haskell, C.F. Monoterpenoid Extract of Sage (Salvia lavandulaefolia) with Cholinesterase Inhibiting Properties Improves Cognitive Performance and Mood in Healthy Adults. J. Psychopharmacol. 2011, 25, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Tildesley, N.T.J.; Kennedy, D.O.; Perry, E.K.; Ballard, C.G.; Wesnes, K.A.; Scholey, A.B. Positive Modulation of Mood and Cognitive Performance Following Administration of Acute Doses of Salvia Lavandulaefolia Essential Oil to Healthy Young Volunteers. Physiol. Behav. 2005, 83, 699–709. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Pace, S.; Haskell, C.; Okello, E.J.; Milne, A.; Scholey, A.B. Effects of Cholinesterase Inhibiting Sage (Salvia officinalis) on Mood, Anxiety and Performance on a Psychological Stressor Battery. Neuropsychopharmacology 2006, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; García-Beltrán, Y.; Mora, S.; Díaz-Véliz, G.; Viana, G.S.B.; Tortoriello, J.; Ramírez, G. Antidepressant and Anxiolytic Effects of Hydroalcoholic Extract from Salvia Elegans. J. Ethnopharmacol. 2006, 107, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Sajjadi, S.E.; Jafarian, A.; Vaseghi, G. Anxiolytic Effects of Salvia Reuterana Boiss. on the Elevated plus-Maze Model of Anxiety in Mice. J. Ethnopharmacol. 2005, 101, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Neekhra, S.; Awasthi, H.; Prasad Singh, D. Beneficial Effects of Sambucus Nigra in Chronic Stress-Induced Neurobehavioral and Biochemical Perturbation in Rodents. Pharmacogn. J. 2021, 13, 155–161. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Cui, J.; Simonyi, A.; Engel, V.A.; Chen, S.; Fritsche, K.L.; Thomas, A.L.; Applequist, W.L.; Folk, W.R.; Lubahn, D.B.; et al. Dietary Sutherlandia and Elderberry Mitigate Cerebral Ischemia-Induced Neuronal Damage and Attenuate P47phox and Phospho-ERK1/2 Expression in Microglial Cells. ASN Neuro 2014, 6, 1759091414554946. [Google Scholar] [CrossRef]
- Moghaddam, M.H.; Bayat, A.-H.; Eskandari, N.; Abdollahifar, M.; Fotouhi, F.; Forouzannia, A.; Rafiei, R.; Hatari, S.; Seraj, A.; Shahidi, A.M.E.J.; et al. Elderberry Diet Ameliorates Motor Function and Prevents Oxidative Stress-Induced Cell Death in Rat Models of Huntington Disease. Brain Res. 2021, 1762, 147444. [Google Scholar] [CrossRef]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-khodaei, M.; Sadigh-Eteghad, S. Melissa Officinalis L. Hydro-Alcoholic Extract Inhibits Anxiety and Depression through Prevention of Central Oxidative Stress and Apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Leite, F.B.; Lucena, G.M.; Barros, M.; Silveira, D.; Silva, M.V.; Ferreira, V.M. Anxiolytic and Antidepressant-like Effects of Melissa Officinalis (Lemon Balm) Extract in Rats: Influence of Administration and Gender. Indian J. Pharmacol. 2012, 44, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Soodi, M.; Naghdi, N.; Hajimehdipoor, H.; Choopani, S.; Sahraei, E. Memory-Improving Activity of Melissa Officinalis Extract in Naïve and Scopolamine-Treated Rats. Res. Pharm. Sci. 2014, 9, 107–114. [Google Scholar] [PubMed]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The Effects of Melissa Officinalis Supplementation on Depression, Anxiety, Stress, and Sleep Disorder in Patients with Chronic Stable Angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Heydari, N.; Dehghani, M.; Emamghoreishi, M.; Akbarzadeh, M. Effect of Melissa Officinalis Capsule on the Mental Health of Female Adolescents with Premenstrual Syndrome: A Clinical Trial Study. Int. J. Adolesc. Med. Health 2019, 31, 20170015. [Google Scholar] [CrossRef]
- Galal, A.; Abdellatief, S. Neuropharmacological Studies on Syzygium Aromaticum (Clove) Essential Oil. Int. J. Pharma Sci. 2015, 5, 1013–1018. [Google Scholar]
- Mehta, A.K.; Halder, S.; Khanna, N.; Tandon, O.P.; Sharma, K.K. The Effect of the Essential Oil of Eugenia Caryophyllata in Animal Models of Depression and Locomotor Activity. Nutr. Neurosci. 2013, 16, 233–238. [Google Scholar] [CrossRef]
- Halder, S.; Mehta, A.; Kar, R.; Mustafa, M.; Mediratta, P.; Sharma, K. Clove Oil Reverses Learning and Memory Deficits in Scopolamine-Treated Mice. Planta Medica 2010, 77, 830–834. [Google Scholar] [CrossRef]
- Mammen, R.R.; Natinga Mulakal, J.; Mohanan, R.; Maliakel, B.; Illathu Madhavamenon, K. Clove Bud Polyphenols Alleviate Alterations in Inflammation and Oxidative Stress Markers Associated with Binge Drinking: A Randomized Double-Blinded Placebo-Controlled Crossover Study. J. Med. Food 2018, 21, 1188–1196. [Google Scholar] [CrossRef]
- Li, X.; Zhao, T.; Gu, J.; Wang, Z.; Lin, J.; Wang, R.; Duan, T.; Li, Z.; Dong, R.; Wang, W.; et al. Intake of Flavonoids from Astragalus Membranaceus Ameliorated Brain Impairment in Diabetic Mice via Modulating Brain-Gut Axis. Chin. Med. 2022, 17, 22. [Google Scholar] [CrossRef]
- Bhaswant, M.; Brown, L.; Mathai, M.L. Queen Garnet Plum Juice and Raspberry Cordial in Mildly Hypertensive Obese or Overweight Subjects: A Randomized, Double-Blind Study. J. Funct. Foods 2019, 56, 119–126. [Google Scholar] [CrossRef]
- Chandra Singh, M.; Probst, Y.; Price, W.E.; Kelso, C. Relative Comparisons of Extraction Methods and Solvent Composition for Australian Blueberry Anthocyanins. J. Food Compos. Anal. 2022, 105, 104232. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian Fruits—A Novel Source of Antioxidants for Food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Musci, M.; Yao, S. Optimization and Validation of Folin–Ciocalteu Method for the Determination of Total Polyphenol Content of Pu-Erh Tea. Int. J. Food Sci. Nutr. 2017, 68, 913–918. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Ortiz, R.; Antilén, M.; Speisky, H.; Aliaga, M.E.; López-Alarcón, C.; Baugh, S. Application of a Microplate-Based ORAC-Pyrogallol Red Assay for the Estimation of Antioxidant Capacity: First Action 2012.03. J. AOAC Int. 2012, 95, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I.; Vizmanos, J.L. A Simple and a Reliable Method to Quantify Antioxidant Activity In Vivo. Antioxidants 2019, 8, 142. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Bouriche, H.; Meziti, H.; Senator, A.; Arnhold, J. Anti-Inflammatory, Free Radical-Scavenging, and Metal-Chelating Activities of Malva Parviflora. Pharm. Biol. 2011, 49, 942–946. [Google Scholar] [CrossRef]
- Gülçin, I.; Alwasel, S. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Santos, J.S.; Alvarenga Brizola, V.R.; Granato, D. High-Throughput Assay Comparison and Standardization for Metal Chelating Capacity Screening: A Proposal and Application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, P.; Chen, X.; Xie, Y.; Weston-Green, K.; Solowij, N.; Chew, Y.L.; Huang, X.-F. Cannabidiol Induces Autophagy and Improves Neuronal Health Associated with SIRT1 Mediated Longevity. Geroscience 2022, 44, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the Use of SH-SY5Y Neuroblastoma Cells in Neurobiology. In Neuronal Cell Culture: Methods and Protocols; Amini, S., White, M.K., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2013; pp. 9–21. ISBN 978-1-62703-640-5. [Google Scholar]
- Dhanalakshmi, C.; Manivasagam, T.; Nataraj, J.; Justin Thenmozhi, A.; Essa, M.M. Neurosupportive Role of Vanillin, a Natural Phenolic Compound, on Rotenone Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Evid.-Based Complement. Altern. Med. 2015, 2015, 626028. [Google Scholar] [CrossRef]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef]
- Hamdi, D.; Ouachikh, O.; Ouchchane, L.; Omara-Reda, H.; Messaoud, C. The Neuroprotective Effect of Clove Essential Oil against 6-OHDA-Induced Cell Death in SH-SY5Y and a Rat Model of Parkinson’s Disease. 2023. preprint. Available online: https://www.researchsquare.com (accessed on 14 August 2023).
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols Contents and Antioxidant Capacity of 68 Chinese Herbals Suitable for Medical or Food Uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Xu, X.; Li, F.; Zhang, X.; Li, P.; Zhang, X.; Wu, Z.; Li, D. In Vitro Synergistic Antioxidant Activity and Identification of Antioxidant Components from Astragalus Membranaceus and Paeonia Lactiflora. PLoS ONE 2014, 9, e96780. [Google Scholar] [CrossRef]
- Gouthamchandra, K.; Venkataramana, S.H.; Sathish, A.; Amritharaj; Basavegowda, L.H.; Puttaswamy, N.; Kodimule, S.P. Anti-Inflammatory and Neuroprotective Activity of Viphyllin a Standardized Extract of β-Caryophyllene from Black Pepper (Piper nigrum L.) and Its Associated Mechanisms in Mouse Macrophage Cells and Human Neuroblastoma SH-SY5Y Cells. 2021. preprint. Available online: https://www.biorxiv.org/content/10.1101/2021.12.16.472916v1 (accessed on 14 August 2023).
- Su, L.; Yin, J.-J.; Charles, D.; Zhou, K.; Moore, J.; Yu, L. Total Phenolic Contents, Chelating Capacities, and Radical-Scavenging Properties of Black Peppercorn, Nutmeg, Rosehip, Cinnamon and Oregano Leaf. Food Chem. 2007, 100, 990–997. [Google Scholar] [CrossRef]
- Walch, S.; Ngaba Tinzoh, L.; Zimmermann, B.; Stühlinger, W.; Lachenmeier, D. Antioxidant Capacity and Polyphenolic Composition as Quality Indicators for Aqueous Infusions of Salvia officinalis L. (Sage Tea). Front. Pharmacol. 2011, 2, 79–85. [Google Scholar] [CrossRef]
- Echeverry, C.; Arredondo, F.; Abin-Carriquiry, J.A.; Midiwo, J.O.; Ochieng, C.; Kerubo, L.; Dajas, F. Pretreatment with Natural Flavones and Neuronal Cell Survival after Oxidative Stress: A Structure−Activity Relationship Study. J. Agric. Food Chem. 2010, 58, 2111–2115. [Google Scholar] [CrossRef]
- Weston-Green, K. The United Chemicals of Cannabis: Beneficial Effects of Cannabis Phytochemicals on the Brain and Cognition. In Recent Advances in Cannabinoid Research; Costain, W.J., Laprairie, R.B., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-151-9. [Google Scholar]
- Weston-Green, K.; Clunas, H.; Jimenez Naranjo, C. A Review of the Potential Use of Pinene and Linalool as Terpene-Based Medicines for Brain Health: Discovering Novel Therapeutics in the Flavours and Fragrances of Cannabis. Front. Psychiatry 2021, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Hulme, A.J.; McArthur, J.R.; Maksour, S.; Miellet, S.; Ooi, L.; Adams, D.J.; Finol-Urdaneta, R.K.; Dottori, M. Molecular and Functional Characterization of Neurogenin-2 Induced Human Sensory Neurons. Front. Cell. Neurosci. 2020, 14, 600895. [Google Scholar] [CrossRef]
- Garcia, G.; Nanni, S.; Figueira, I.; Ivanov, I.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Pinto, P.; Silva, R.F.M.; Brites, D.; et al. Bioaccessible (Poly)Phenol Metabolites from Raspberry Protect Neural Cells from Oxidative Stress and Attenuate Microglia Activation. Food Chem. 2017, 215, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Georges, N.; Selomulya, C. Microencapsulation of Active Ingredients in Functional Foods: From Research Stage to Commercial Food Products. Trends Food Sci. Technol. 2018, 78, 167–179. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.-H.; Chung, S.-O. Effects of Temperature, Relative Humidity, and Carbon Dioxide Concentration on Growth and Glucosinolate Content of Kale Grown in a Plant Factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of Cannabinoids in Commercial Hemp Seed Oil and Decarboxylation Kinetics Studies of Cannabidiolic Acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef]
- Fanning, K.; Edwards, D.; Netzel, M.; Stanley, R.; Netzel, G.; Russell, D.; Topp, B. Increasing Anthocyanin Content in Queen Garnet Plum and Correlations with In-Field Measures. Acta Hortic. 2013, 97–104. [Google Scholar] [CrossRef]
- Kodagoda, G.; Hong, H.T.; O’Hare, T.J.; Sultanbawa, Y.; Topp, B.; Netzel, M.E. Effect of Storage on the Nutritional Quality of Queen Garnet Plum. Foods 2021, 10, 352. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Protective Properties of Salvia Lavandulifolia Vahl. Essential Oil against Oxidative Stress-Induced Neuronal Injury. Food Chem. Toxicol. 2015, 80, 154–162. [Google Scholar] [CrossRef]











| Sample ID | Name | Scientific Name | Form | Place of Origin |
|---|---|---|---|---|
| QGP | Queen Garnet Plum | Prunus salicina | Fresh | QLD, Australia |
| BPF | Black Peppercorn | Piper nigrum | Dried | India |
| CF | Clove | Syzygium aromaticum | Dried | Madagascar |
| EF | Elderberry | Sambucus nigra | Dried | Germany |
| LBF | Lemon Balm | Melissa officinalis | Fresh | Illawarra Region, NSW, Australia |
| SF | Sage | Salvia officinalis | Fresh | Illawarra Region, NSW, Australia |
| Sample ID | Name of Product | Active Ingredients | Form |
|---|---|---|---|
| WC | WelleCo Super Boosters Immune system support with Kakadu Plum, Welle Pty Ltd., Melbourne, VIC, Australia. | Astragalus (Astragalus membranaceus extract 500 mg) equiv. to dry root 1.4 g; Echinacea (Echinacea purpurea extract 200 mg) equiv. to dry root 400 mg; Acerola (Malpighia glabra extract 442 mg) equiv. to fresh fruit 4.4 g (standardized to contain vitamin C ((ascorbic acid)) 75.1 mg); Moringa (Moringa oleifera) leaf powder 300 mg; Olive (Olea europaea extract 111.1 mg) equiv to dry leaf 400 mg; Kakadu plum (Terminalia ferdinandiana) fruit flesh powder 412.5 mg (standardized to contain vitamin C (ascorbic acid) 41.3 mg); Total vitamin C 104.8 mg; Ginger (Zingiber officinale extract 375 mg) equiv. to dry rhizome 1.5 g. | Powder |
| R8 | Relax–Stress Relief, Regul8 Pty Ltd., Crows Nest, NSW, Australia. | Lemon balm (M. officinalis) extract dry concentrate 100 mg, derived from dry leaf and flower 1 g; Rehmannia glutinosa extract dry concentrate 100 mg, derived from dry root 1 g; passionflower (Passiflora incarnata) extract dry concentrate 37.5 mg, derived from dry herb top flowering 750 mg, rose root (Rhodiola rosea) extract dry concentrate 100 mg, derived from dry root 800 mg standardized to rosavin 3 mg; P. nigrum (BioPerine®, Sabinsa®, Sydney, NSW, Australia) 244 μg, derived from dry fruit 5 mg; mekabu seaweed (Undaria pinnatifida) extract concentrate 25 mg, derived from dry whole plant 300 mg, standardized to fucoidan 12.5 mg | Capsule |
| CC | Fresh Ground Cloves Herbal Supplement, Kroeger Herb® Products Co., Inc., Longmont, CO, USA. | Cloves (S. aromaticum) 450 mg | Capsule |
| EC | Sambucol Black Elderberry Cold & Flu, Pharmacare Laboratories Pty Ltd., Mona Vale, NSW, Australia. | Black elderberry (S. nigra) fruit juice dry equivalent to fresh juice 3.8 g | Capsule |
| LBC | Nature’s Sunshine Lemon Balm, Nature’s Sunshine Products of Australia Pty Ltd., Baulkham Hills, NSW, Australia. | Herbal extract standardized equiv. to dry lemon balm (M. officinalis) leaf 1.95 g equiv. to Rosmarinic acid 33 mg | Capsule |
| SC | Hilde Hemmes’ Herbals Sage 1000 capsule, Herbal Supplies Pty Ltd., St. Agnes, SA, Australia. | Extract equivalent to dried sage (S. officinalis) leaf 1 g | Capsule |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, N.; de Sousa Alves Neri, J.L.; Clunas, H.; Shi, J.; Parkes, E.; Dongol, A.; Wang, Z.; Jimenez Naranjo, C.; Yu, Y.; Huang, X.-F.; et al. Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects. Nutrients 2023, 15, 3912. https://doi.org/10.3390/nu15183912
May N, de Sousa Alves Neri JL, Clunas H, Shi J, Parkes E, Dongol A, Wang Z, Jimenez Naranjo C, Yu Y, Huang X-F, et al. Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects. Nutrients. 2023; 15(18):3912. https://doi.org/10.3390/nu15183912
Chicago/Turabian StyleMay, Naomi, Julianna Lys de Sousa Alves Neri, Helen Clunas, Jiahua Shi, Ella Parkes, Anjila Dongol, Zhizhen Wang, Carlos Jimenez Naranjo, Yinghua Yu, Xu-Feng Huang, and et al. 2023. "Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects" Nutrients 15, no. 18: 3912. https://doi.org/10.3390/nu15183912
APA StyleMay, N., de Sousa Alves Neri, J. L., Clunas, H., Shi, J., Parkes, E., Dongol, A., Wang, Z., Jimenez Naranjo, C., Yu, Y., Huang, X.-F., Charlton, K., & Weston-Green, K. (2023). Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects. Nutrients, 15(18), 3912. https://doi.org/10.3390/nu15183912










