The Effects of 12 Weeks Colostrum Milk Supplementation on the Expression Levels of Pro-Inflammatory Mediators and Metabolic Changes among Older Adults: Findings from the Biomarkers and Untargeted Metabolomic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Informed Consent
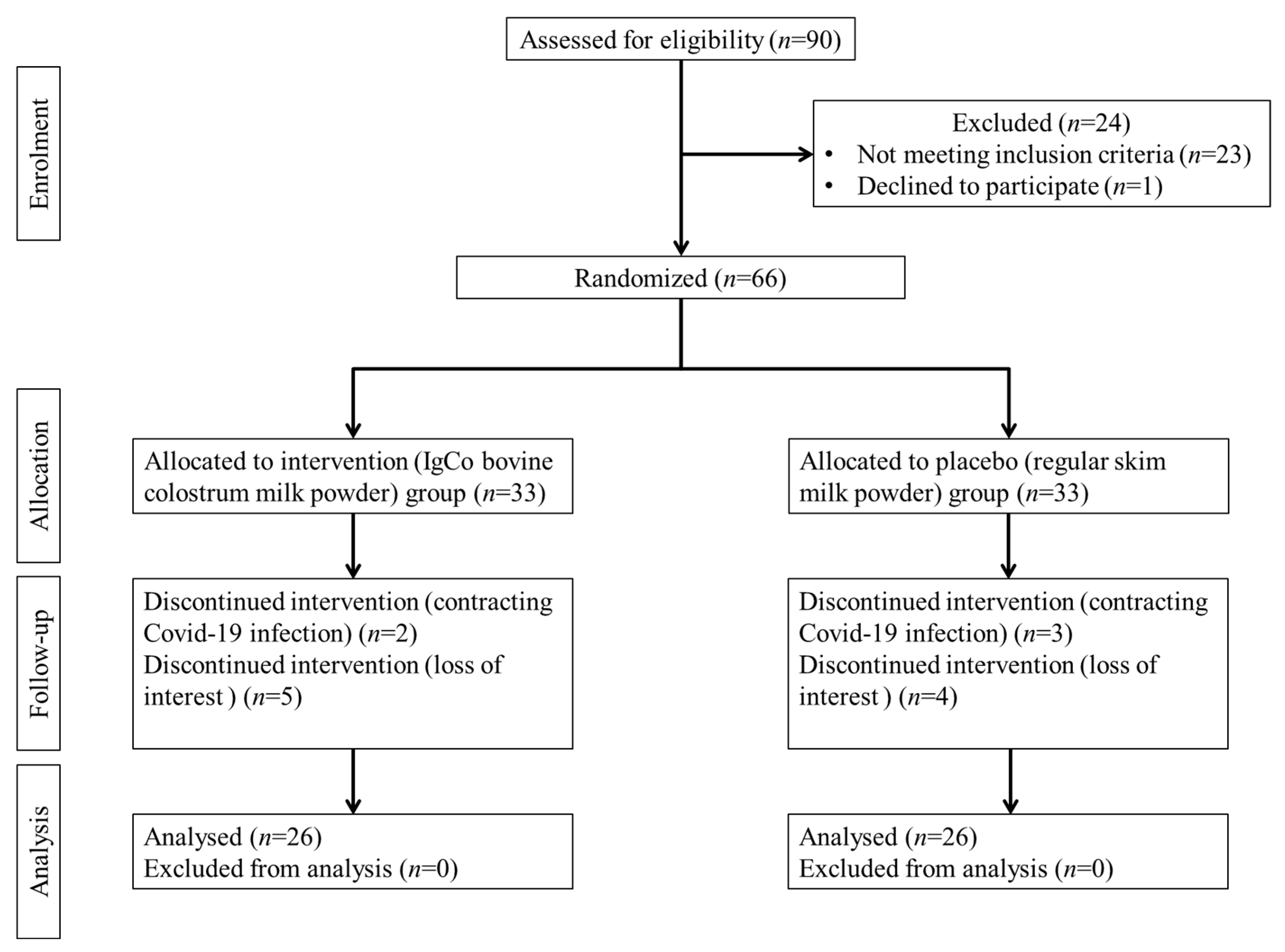
2.2. Study Design and Participants
2.3. Investigational Product
2.4. Data Collection
2.5. Blood Samples Collection and Clinical Laboratory Testing
2.6. Biomarkers Detection
2.7. Untargeted Metabolomics Analysis
2.7.1. Samples Preparation
2.7.2. UPLC-MS Analysis
2.7.3. Metabolite Ion Peak Extraction and Metabolite Identification
2.7.4. Bioinformatics Analysis
2.8. Statistical Analysis
3. Results
3.1. The Baseline Attributes of Participants
3.2. The Effects of 12 Weeks Intervention on the Expression of Biomarkers
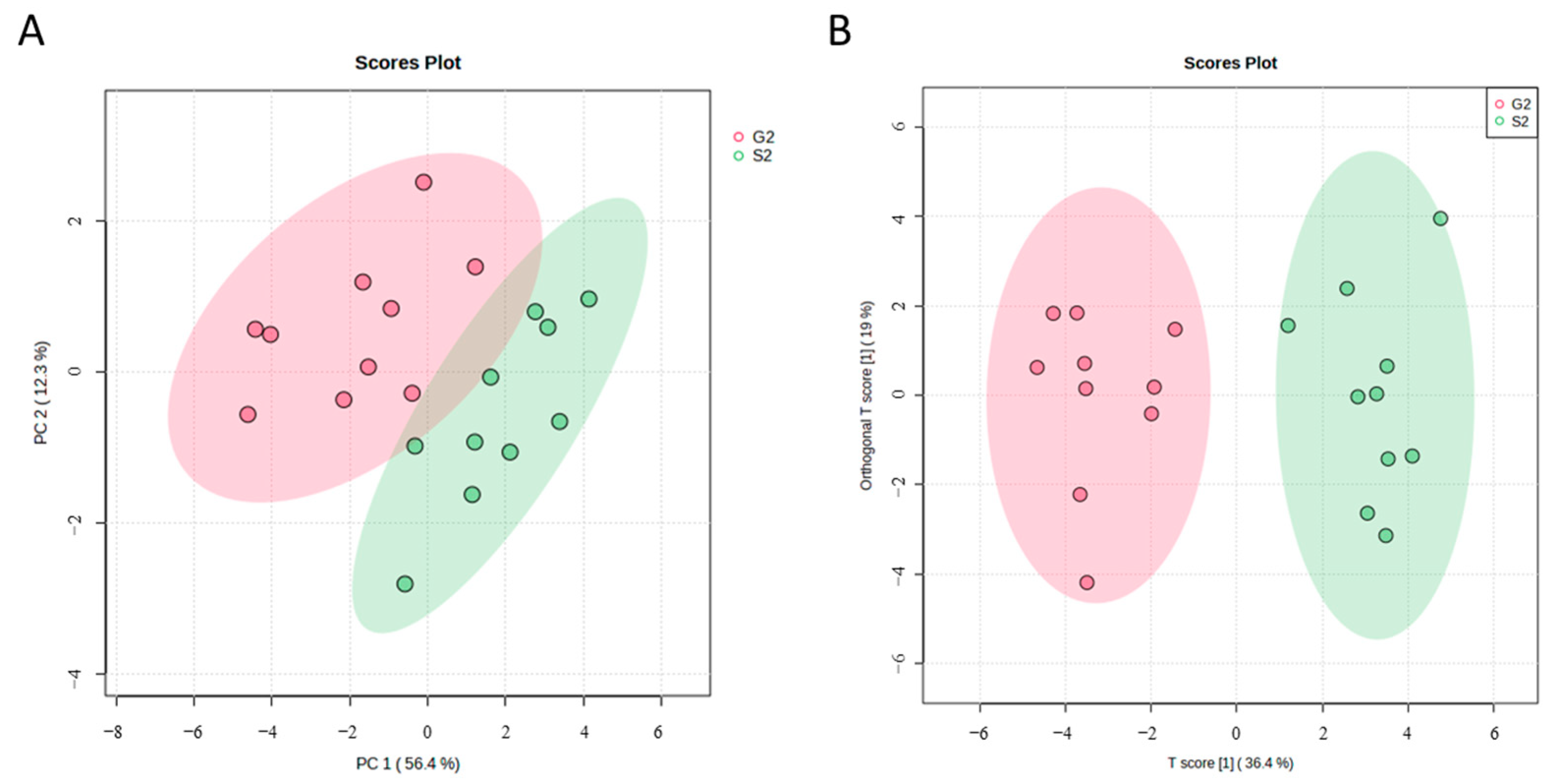
3.3. Metabolic Profiles of Bovine Colostrum Supplemented Group and Placebo Group
3.4. Detection and Identification of Metabolic Markers
3.5. Characterization and Functional Analysis of Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Ageing 2020 Highlights: Living Arrangements of Older Persons (ST/ESA/SER.A/451); United Nations Publication: New York, NY, USA, 2020; ISBN 978-92-1-148347-5. [Google Scholar]
- Fulmer, T.; Reuben, D.B.; Auerbach, J.; Fick, D.M.; Galambos, C.; Johnson, K.S. Actualizing Better Health and Health Care For Older Adults. Health Aff. 2021, 40, 219–225. [Google Scholar] [CrossRef]
- Valls Martínez, M.; Santos-Jaén, J.M.; Amin, F.U.; Martín-Cervantes, P.A. Pensions, Ageing and Social Security Research: Literature Review and Global Trends. Mathematics 2021, 9, 3258. [Google Scholar] [CrossRef]
- Chung, H.Y.; Kim, D.H.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Huston, P. A Sedentary and Unhealthy Lifestyle Fuels Chronic Disease Progression by Changing Interstitial Cell Behaviour: A Network Analysis. Front. Physiol. 2022, 13, 904107. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Główka, N.; Woźniewicz, M. Potential Use of Colostrum Bovinum Supplementation in Athletes—A Review. Acta Sci. Pol. Technol. Aliment. 2019, 18, 115–123. [Google Scholar] [CrossRef]
- Przybylska, J.; Albera, E.; Kankofer, M. Antioxidants in Bovine Colostrum. Reprod. Domest. Anim. 2007, 42, 402–409. [Google Scholar] [CrossRef]
- Albera, E.; Kankofer, M. Antioxidants in Colostrum and Milk of Sows and Cows. Reprod. Domest. Anim. 2009, 44, 606–611. [Google Scholar] [CrossRef]
- Kwon, O.Y.; Lee, J.S.; Choi, H.S.; Hong, H.P.; Jang, K.H.; Paek, J.H.; Ah Kan, S.; Ko, Y.G. Antioxidant and Anticytokine Effects of Bovine Colostrum in Intestinal Ischemia/Reperfusion Injured Rat Model. Food Sci. Biotechnol. 2010, 19, 1295–1301. [Google Scholar] [CrossRef]
- Appukutty, M.; Radhakrishnan, A.K.; Ramasamy, K.; Ramasamy, R.; Abdul Majeed, A.B.; Noor, M.I.; Safii, N.S.; Koon, P.B.; Chinna, K.; Haleagrahara, N. Colostrum Supplementation Protects against Exercise—Induced Oxidative Stress in Skeletal Muscle in Mice. BMC Res. Notes 2012, 5, 649. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Ooi, T.C.; Singh, D.K.A.; Shahar, S.; Rajab, N.F.; Vanoh, D.; Sharif, R.; Tan, M.P. Incidence and Multidimensional Predictors of Occasional and Recurrent Falls among Malaysian Community-dwelling Older Persons. BMC Geriatr. 2021, 21, 154. [Google Scholar] [CrossRef]
- Ooi, T.C.; Ishak, W.S.; Sharif, R.; Shahar, S.; Rajab, N.F.; Singh, D.K.A.; Mukari, S.Z.M.S. Multidimensional Risk Factors of Age-Related Hearing Loss among Malaysian Community-Dwelling Older Adults. Clin. Interv. Aging 2021, 16, 2033–2046. [Google Scholar] [CrossRef]
- Ooi, T.C.; Singh, D.K.A.; Shahar, S.; Sharif, R.; Rivan, N.F.M.; Meramat, A.; Rajab, N.F. Higher Lead and Lower Calcium Levels Are Associated with Increased Risk of Mortality in Malaysian Older Population: Findings from the LRGS-TUA Longitudinal Study. Int. J. Environ. Res. Public Health 2022, 19, 6955. [Google Scholar] [CrossRef]
- Ooi, T.C.; Singh, D.K.A.; Shahar, S.; Rajab, N.F.; Sharif, R. Higher Levels of Lead and Aluminium Are Associated with Increased Risk of Falls among Community-Dwelling Older Adults: An 18-Month Follow-up Study. Geriatr. Gerontol. Int. 2021, 21, 1026–1032. [Google Scholar] [CrossRef]
- Ooi, T.C.; Meramat, A.; Rajab, N.F.; Shahar, S.; Ismail, I.S.; Azam, A.A.; Sharif, R. Intermittent Fasting Enhanced the Cognitive Function in Older Adults with Mild Cognitive Impairment by Inducing Biochemical and Metabolic Changes: A 3-Year Progressive Study. Nutrients 2020, 12, 2644. [Google Scholar] [CrossRef]
- Gomes, R.D.S.; Anaya, K.; Galdino, A.B.S.; Oliveira, J.P.F.; Gama, M.A.S.; Medeiros, C.A.C.X.; Gavioli, E.C.; Porto, A.L.F.; Rangel, A.H.N. Bovine Colostrum: A Source of Bioactive Compounds for Prevention and Treatment of Gastrointestinal Disorders. NFS J. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.; Son, D.O.; Satsu, H.; Takano, Y.; Kawakami, H.; Totsuka, M.; Shimizu, M. Inhibitory Effect of Lactoperoxidase on the Secretion of Pro-inflammatory Cytokine Interleukin-8 in Human Intestinal Epithelial Caco-2 Cells. Int. Dairy J. 2008, 18, 932–938. [Google Scholar] [CrossRef]
- Yamakaze, J.; Lu, Z. Deletion of the Lactoperoxidase Gene Causes Multisystem Inflammation and Tumors in Mice. Sci. Rep. 2021, 11, 12429. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Rasheed, N.; Alghasham, A.; Rasheed, Z. Lactoferrin from Camelus Dromedarius Inhibits Nuclear Transcription Factor-kappa B Activation, Cyclooxygenase-2 Expression and Prostaglandin E2 Production in Stimulated Human Chondrocytes. Pharmacogn. Res. 2016, 8, 135–141. [Google Scholar] [CrossRef]
- Gasser, M.; Lissner, R.; Nawalaniec, K.; Hsiao, L.-L.; Waaga-Gasser, A.M. KMP01D Demonstrates Beneficial Anti-Inflammatory Effects on Immune Cells: An Ex Vivo Preclinical Study of Patients with Colorectal Cancer. Front. Immunol. 2020, 11, 684. [Google Scholar] [CrossRef]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine Colostrum Modulates Cytokine Production in Human Peripheral Blood Mononuclear Cells Stimulated with Lipopolysaccharide and Phytohemagglutinin. J. Interferon Cytokine Res. 2008, 29, 37–44. [Google Scholar] [CrossRef]
- Watson, H. Biological Membranes. Essays Biochem. 2015, 59, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Pietzner, M.; Kaul, A.; Henning, A.-K.; Kastenmüller, G.; Artati, A.; Lerch, M.M.; Adamski, J.; Nauck, M.; Friedrich, N. Comprehensive Metabolic Profiling of Chronic Low-Grade Inflammation among Generally Healthy Individuals. BMC Med. 2017, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.A.; He, S.; Jones, D.P.; Sun, Y.V.; Ramirez-Zea, M.; Stein, A.D. Metabolomic Profiling Demonstrates Postprandial Changes in Fatty Acids and Glycerophospholipids Are Associated with Fasting Inflammation in Guatemalan Adults. J. Nutr. 2021, 151, 2564–2573. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in Inflammation in the Blood and the Vessel. Front. Pharmacol. 2022, 13, 997403. [Google Scholar] [CrossRef] [PubMed]
- Lone, A.; Taskén, K. Pro-inflammatory and Immunoregulatory Roles of Eicosanoids in T Cells. Front. Immunol. 2013, 4, 130. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, Q.; Dong, Z.; Yan, Y.; Fu, Y.; Liu, X.; Zhao, B.; Duan, X. Phosphatidylcholine Ameliorates LPS-Induced Systemic Inflammation and Cognitive Impairments via Mediating the Gut–Brain Axis Balance. J. Agric. Food Chem. 2020, 68, 14884–14895. [Google Scholar] [CrossRef]
- Choudhary, V.; Uaratanawong, R.; Patel, R.R.; Patel, H.; Bao, W.; Hartney, B.; Cohen, E.; Chen, X.; Zhong, Q.; Isales, C.M.; et al. Phosphatidylglycerol Inhibits Toll-Like Receptor–Mediated Inflammation by Danger-Associated Molecular Patterns. J. Investig. Dermatol. 2019, 139, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic Acid: Physiological Roles and Potential Health Benefits—A Review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Mendes-Frias, A.; Santos-Lima, B.; Furtado, D.Z.S.; Ruperez, F.J.; Assunção, N.A.; Matias, M.J.; Gomes, V.; Gaifem, J.; Barbas, C.; Castro, A.G.; et al. Dysregulation of Glycerophospholipid Metabolism during Behçet’s Disease Contributes to a pro-Inflammatory Phenotype of Circulating Monocytes. J. Transl. Autoimmun. 2020, 3, 100056. [Google Scholar] [CrossRef] [PubMed]
- Sevastou, I.; Kaffe, E.; Mouratis, M.-A.; Aidinis, V. Lysoglycerophospholipids in Chronic Inflammatory Disorders: The PLA2/LPC and ATX/LPA Axes. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1831, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Bansal, P.; Gaur, S.N.; Arora, N. Lysophosphatidylcholine Plays Critical Role in Allergic Airway Disease Manifestation. Sci. Rep. 2016, 6, 27430. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, Y.; Mai, J.; Guo, G.; Meng, J.; Fang, X.; Chen, X.; Liu, C.; Zhong, S. Comprehensive Metabolic Profiling of Inflammation Indicated Key Roles of Glycerophospholipid and Arginine Metabolism in Coronary Artery Disease. Front. Immunol. 2022, 13, 829425. [Google Scholar] [CrossRef] [PubMed]
- Klein Geltink, R.I.; Pearce, E.L. The Importance of Methionine Metabolism. eLife 2019, 8, e47221. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. The Sulfur-Containing Amino Acids: An Overview. J. Nutr. 2006, 136, 1636S–1640S. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The Metabolism and Significance of Homocysteine in Nutrition and Health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef]
- Esse, R.; Barroso, M.; de Almeida, I.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef]
- Li, J.-J.; Li, Q.; Du, H.-P.; Wang, Y.-L.; You, S.-J.; Wang, F.; Xu, X.-S.; Cheng, J.; Cao, Y.-J.; Liu, C.-F.; et al. Homocysteine Triggers Inflammatory Responses in Macrophages through Inhibiting CSE-H2S Signaling via DNA Hypermethylation of CSE Promoter. Int. J. Mol. Sci. 2015, 16, 12560–12577. [Google Scholar] [CrossRef]
- Ji, J.; Xu, Y.; Zheng, M.; Luo, C.; Lei, H.; Qu, H.; Shu, D. Methionine Attenuates Lipopolysaccharide-Induced Inflammatory Responses via DNA Methylation in Macrophages. ACS Omega 2019, 4, 2331–2336. [Google Scholar] [CrossRef]
- Sharma, S.; Dixon, T.; Jung, S.; Graff, E.C.; Forney, L.A.; Gettys, T.W.; Wanders, D. Dietary Methionine Restriction Reduces Inflammation Independent of FGF21 Action. Obesity 2019, 27, 1305–1313. [Google Scholar] [CrossRef]

| Parameters | n (%) or Mean ± SD | |||
|---|---|---|---|---|
| Total 52 (100.0) | Bovine Colostrum Milk 26 (50.0) | Placebo 26 (50.0) | p-Value | |
| Age | 61.71 ± 7.14 | 60.46 ± 7.07 | 62.96 ± 7.13 | 0.210 |
| Sex | ||||
| Male | 23 (44.2) | 11 (42.3) | 12 (46.2) | 0.780 |
| Female | 29 (55.8) | 15 (57.7) | 14 (53.8) | |
| Marital status | ||||
| Single/Divorced | 2 (3.8) | 0 (0.0) | 2 (7.7) | 0.149 |
| Married | 50 (96.2) | 26 (100.0) | 24 (92.3) | |
| Smoking status | 2 (3.8) | 1 (3.8) | 1 (3.8) | 1.000 |
| BMI (kg/m2) | 27.19 ± 4.61 | 26.55 ± 5.16 | 27.84 ± 3.99 | 0.318 |
| Waist circumference (cm) | 90.32 ± 10.20 | 88.03 ± 11.15 | 92.81 ± 8.60 | 0.098 |
| Hip circumference (cm) | 102.60 ± 8.80 | 100.49 ± 8.62 | 104.89 ± 8.58 | 0.077 |
| Systolic pressure (mmHg) | 133.78 ± 16.13 | 130.65 ± 13.94 | 136.90 ± 17.79 | 0.165 |
| Diastolic pressure (mmHg) | 82.61 ± 9.79 | 81.37 ± 10.14 | 83.85 ± 9.45 | 0.366 |
| Medical history | ||||
| Diabetes | 11 (21.2) | 4 (15.4) | 7 (26.9) | 0.308 |
| Hypercholesterolemia | 17 (32.7) | 9 (34.6) | 8 (30.8) | 0.768 |
| Hypertension | 15 (28.8) | 7 (26.9) | 8 (30.8) | 0.760 |
| Bovine Colostrum Milk (n = 26) | Placebo (n = 26) | Group Effect | Time Effect | Group × Time Effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p | Partial Eta Squared | Power | p | Partial Eta Squared | Power | p | Partial Eta Squared | Power | |||
| Vitamin D (µmol/L) | |||||||||||
| Baseline | 105.74 ± 53.61 | 142.22 ± 60.79 | 0.012 * | 0.125 | 0.729 | 0.265 | 0.026 | 0.197 | 0.119 | 0.050 | 0.343 |
| Post | 60.64 ± 24.05 ### | 70.28 ± 50.52 ### | |||||||||
| MDA (µmol/L) | |||||||||||
| Baseline | 0.74 ± 0.40 | 1.14 ± 0.91 | 0.047 * | 0.080 | 0.515 | 0.586 | 0.006 | 0.084 | 0.490 | 0.010 | 0.105 |
| Post | 1.11 ± 0.48 ## | 1.31 ± 0.75 | |||||||||
| SOD activity (U/mL) | |||||||||||
| Baseline | 122.23 ± 12.42 | 122.88 ± 25.13 | 0.306 | 0.022 | 0.173 | 0.002 | 0.175 | 0.879 | 0.942 | 0.000 | 0.051 |
| Post | 113.12 ± 11.36 ## | 117.04 ± 20.47 | |||||||||
| 8-OHdG (ng/mL) | |||||||||||
| Baseline | 37.16 ± 36.43 | 30.03 ± 15.18 | 0.842 | 0.001 | 0.054 | 0.163 | 0.040 | 0.285 | 0.196 | 0.035 | 0.251 |
| Post | 42.76 ± 38.95 | 45.83 ± 44.50 | |||||||||
| CRP (ng/mL) | |||||||||||
| Baseline | 5.03 ± 3.26 | 4.70 ± 1.91 | 0.640 | 0.005 | 0.075 | 0.657 | 0.004 | 0.072 | 0.015 * | 0.117 | 0.694 |
| Post | 3.34 ± 2.00 ## | 4.55 ± 2.16 | |||||||||
| IL-6 (pg/mL) | |||||||||||
| Baseline | 3.32 ± 1.19 | 3.45 ± 1.43 | 0.082 | 0.062 | 0.413 | 0.156 | 0.041 | 0.292 | 0.018 * | 0.111 | 0.671 |
| Post | 2.67 ± 1.13 # | 3.64 ± 1.49 | |||||||||
| TNF-α (pg/mL) | |||||||||||
| Baseline | 91.88 ± 56.80 | 80.26 ± 46.85 | 0.898 | 0.000 | 0.052 | 0.717 | 0.003 | 0.065 | 0.008 ** | 0.138 | 0.774 |
| Post | 51.31 ± 44.07 ### | 64.51 ± 37.03 # | |||||||||
| Telomerase (ng/mL) | |||||||||||
| Baseline | 456.29 ± 179.56 | 465.68 ± 193.06 | 0.915 | 0.000 | 0.051 | 0.070 | 0.067 | 0.443 | 0.819 | 0.001 | 0.056 |
| Post | 495.59 ± 185.98 | 500.60 ± 229.12 | |||||||||
| Metabolite | Fold Change | State | p-Value | VIP |
|---|---|---|---|---|
| Propionic acid | 18.634 | Up | 0.001 | 5.251 |
| Isovaleric acid | 9.308 | Up | 0.003 | 4.594 |
| n-butyl acetate | 31.712 | Up | 0.004 | 4.464 |
| Ethyl acetate | 43.492 | Up | 0.016 | 4.306 |
| Gamma-glutamylglutamic acid | 10.617 | Up | 0.010 | 3.876 |
| Linoleamide | 2.749 | Up | 0.000 | 3.250 |
| 4,4’-diapolycopenedial | 2.678 | Up | 0.000 | 3.012 |
| Oleamide | 2.247 | Up | 0.000 | 2.882 |
| 2,4,12-octadecatrienoic acid isobutylamide | 2.563 | Up | 0.000 | 2.702 |
| Leukotriene E3 | 2.268 | Up | 0.000 | 2.511 |
| Gamma-linolenic acid | 1.937 | Up | 0.001 | 2.240 |
| N-oleoylethanolamine | 2.049 | Up | 0.017 | 2.121 |
| Etiocholanolone | 0.467 | Down | 0.037 | 1.934 |
| Glycerophosphocholine | 1.832 | Up | 0.002 | 1.913 |
| Octadecanamide | 1.733 | Up | 0.000 | 1.911 |
| N,N-dimethylsphingosine | 1.747 | Up | 0.007 | 1.807 |
| N-arachidonoyl dopamine | 1.755 | Up | 0.004 | 1.775 |
| Leukotriene C5 | 1.668 | Up | 0.013 | 1.759 |
| 3-oxododecanoic acid | 0.689 | Down | 0.036 | 1.700 |
| PE(P-18:0/18:2(9Z,12Z)) | 0.480 | Down | 0.018 | 1.605 |
| PE(O-18:1(1Z)/20:4(5Z,8Z,11Z,14Z)) | 0.629 | Down | 0.014 | 1.552 |
| But-2-enoic acid | 0.563 | Down | 0.046 | 1.479 |
| 3-hydroxybutyric acid | 0.694 | Down | 0.035 | 1.462 |
| PE(P-18:0/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 0.652 | Down | 0.003 | 1.407 |
| LysoPC(18:3(9Z,12Z,15Z)) | 1.319 | Up | 0.004 | 1.405 |
| LysoPE(18:0/0:0) | 1.350 | Up | 0.006 | 1.368 |
| Ubiquinone-4 | 0.725 | Down | 0.023 | 1.266 |
| PC(o-18:1(9Z)/16:0) | 0.673 | Down | 0.005 | 1.212 |
| LysoPC(16:1(9Z)) | 1.383 | Up | 0.040 | 1.161 |
| PE(P-16:0/20:3(8Z,11Z,14Z)) | 0.663 | Down | 0.028 | 1.141 |
| PE(P-18:1(9Z)/22:6(4Z,7Z,10Z,13Z,16Z,19Z)) | 0.713 | Down | 0.039 | 1.111 |
| Indolepyruvate | 1.500 | Up | 0.038 | 1.071 |
| Docosanamide | 1.396 | Up | 0.002 | 1.055 |
| Pathway Name | Hits | Raw p | –log(p) | Holm Adjust | FDR | Impact |
|---|---|---|---|---|---|---|
| Glycerophospholipid metabolism | 3 | 0.0004 | 3.4391 | 0.0029 | 0.0016 | 0.1700 |
| Steroid hormone biosynthesis | 1 | 0.0024 | 2.6193 | 0.0120 | 0.0038 | 0.0052 |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 1 | 2.52 × 10−5 | 4.5993 | 0.0002 | 0.0002 | 0.0040 |
| Synthesis and degradation of ketone bodies | 1 | 0.0007 | 3.1494 | 0.0050 | 0.0016 | 0.0000 |
| Butanoate metabolism | 1 | 0.0007 | 3.1494 | 0.0050 | 0.0016 | 0.0000 |
| Propanoate metabolism | 1 | 0.0025 | 2.6011 | 0.0120 | 0.0038 | 0.0000 |
| Biosynthesis of unsaturated fatty acids | 1 | 0.0806 | 1.0936 | 0.2418 | 0.1037 | 0.0000 |
| Ether lipid metabolism | 1 | 0.2411 | 0.6177 | 0.4823 | 0.2713 | 0.0000 |
| Tryptophan metabolism | 1 | 0.5021 | 0.2992 | 0.5021 | 0.5021 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, T.C.; Ahmad, A.; Rajab, N.F.; Sharif, R. The Effects of 12 Weeks Colostrum Milk Supplementation on the Expression Levels of Pro-Inflammatory Mediators and Metabolic Changes among Older Adults: Findings from the Biomarkers and Untargeted Metabolomic Analysis. Nutrients 2023, 15, 3184. https://doi.org/10.3390/nu15143184
Ooi TC, Ahmad A, Rajab NF, Sharif R. The Effects of 12 Weeks Colostrum Milk Supplementation on the Expression Levels of Pro-Inflammatory Mediators and Metabolic Changes among Older Adults: Findings from the Biomarkers and Untargeted Metabolomic Analysis. Nutrients. 2023; 15(14):3184. https://doi.org/10.3390/nu15143184
Chicago/Turabian StyleOoi, Theng Choon, Azizan Ahmad, Nor Fadilah Rajab, and Razinah Sharif. 2023. "The Effects of 12 Weeks Colostrum Milk Supplementation on the Expression Levels of Pro-Inflammatory Mediators and Metabolic Changes among Older Adults: Findings from the Biomarkers and Untargeted Metabolomic Analysis" Nutrients 15, no. 14: 3184. https://doi.org/10.3390/nu15143184
APA StyleOoi, T. C., Ahmad, A., Rajab, N. F., & Sharif, R. (2023). The Effects of 12 Weeks Colostrum Milk Supplementation on the Expression Levels of Pro-Inflammatory Mediators and Metabolic Changes among Older Adults: Findings from the Biomarkers and Untargeted Metabolomic Analysis. Nutrients, 15(14), 3184. https://doi.org/10.3390/nu15143184







