Disruption of Vitamin D Signaling Impairs Adaptation of Cerebrocortical Microcirculation to Carotid Artery Occlusion in Hyperandrogenic Female Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
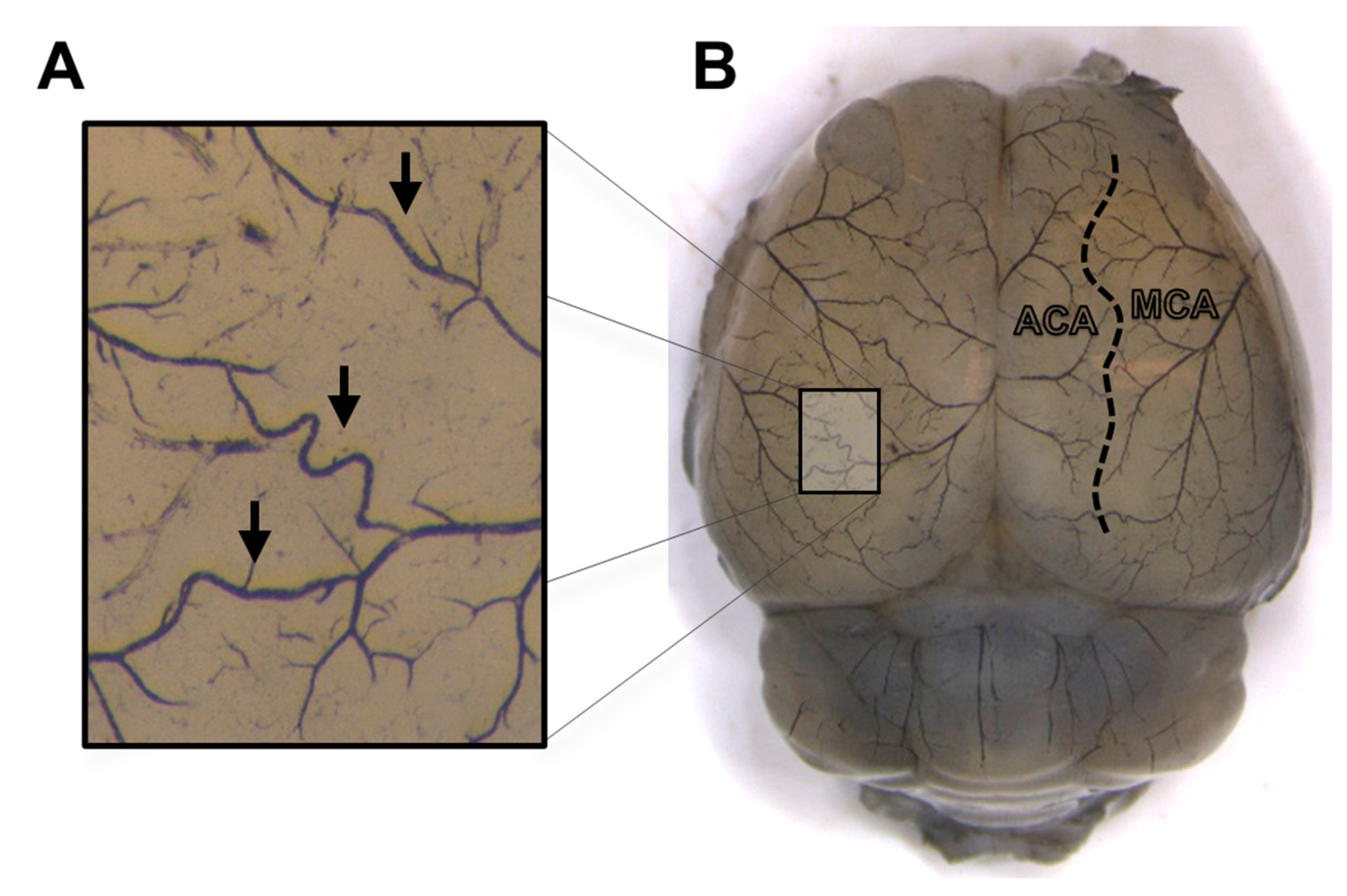
2.2. Morphological Analysis of Leptomeningeal Collaterals
2.3. Ovariectomy and Testosterone Treatment
2.4. Vaginal Cytology
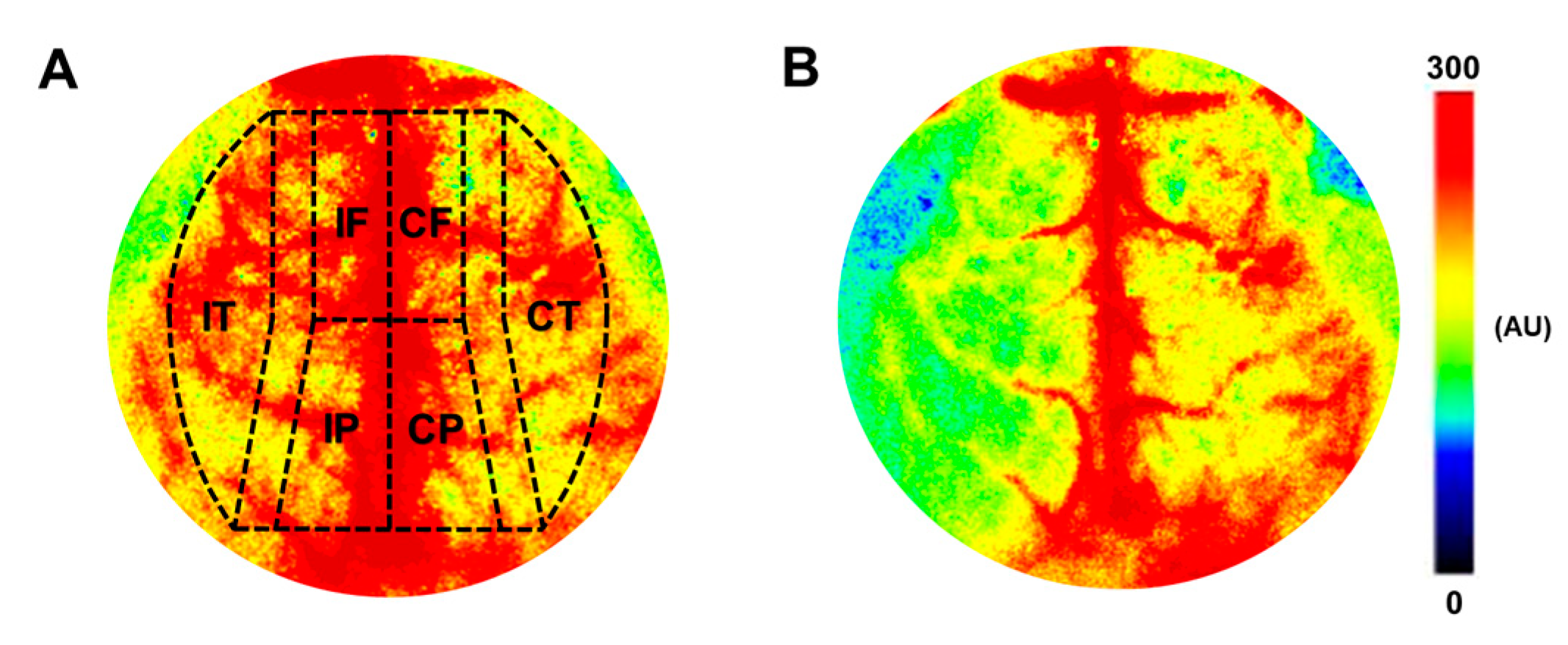
2.5. Determination of Cerebrocortical Blood Flow Changes after Carotid Artery Occlusion
2.5.1. Surgical Procedures
2.5.2. Measurement of Cerebrocortical Blood Flow, Arterial Blood Pressure, and Blood Gas Parameters
2.6. Measurement of Testosterone Levels
2.7. Statistical Analysis
3. Results
3.1. Anatomical and Physiological Parameters
3.1.1. Anatomical Traits
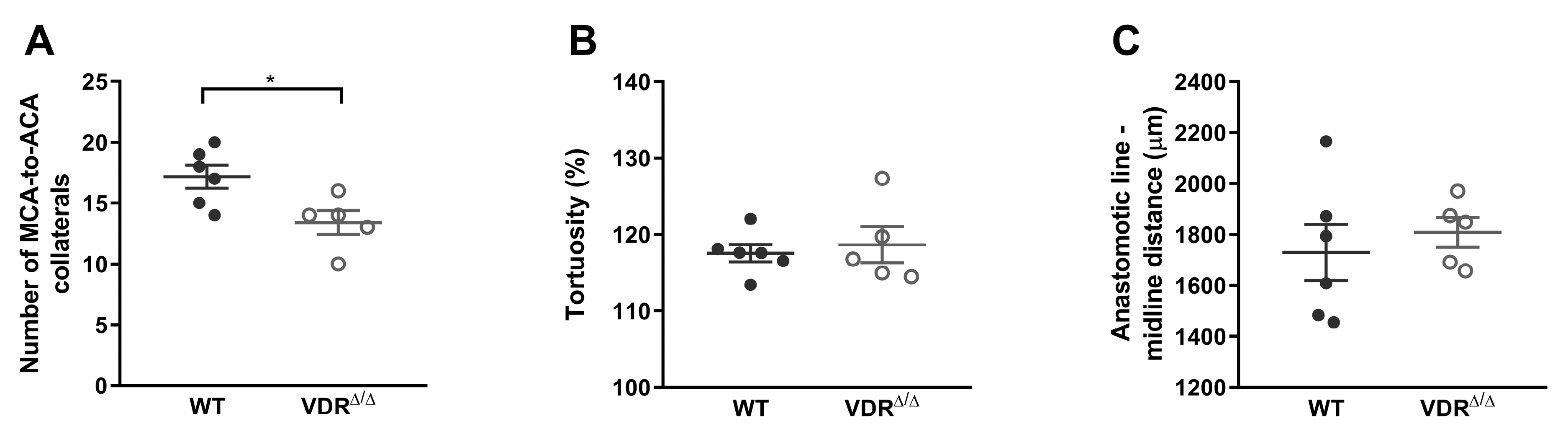
3.1.2. Morphology of Leptomeningeal Collaterals in Intact Females
3.1.3. Validation of Testosterone Treatment and Ovariectomy
3.1.4. In Vivo Blood Pressure Measurement
3.1.5. Analysis of Blood Gas, Acid–Base Parameters, and Plasma Ion Concentrations
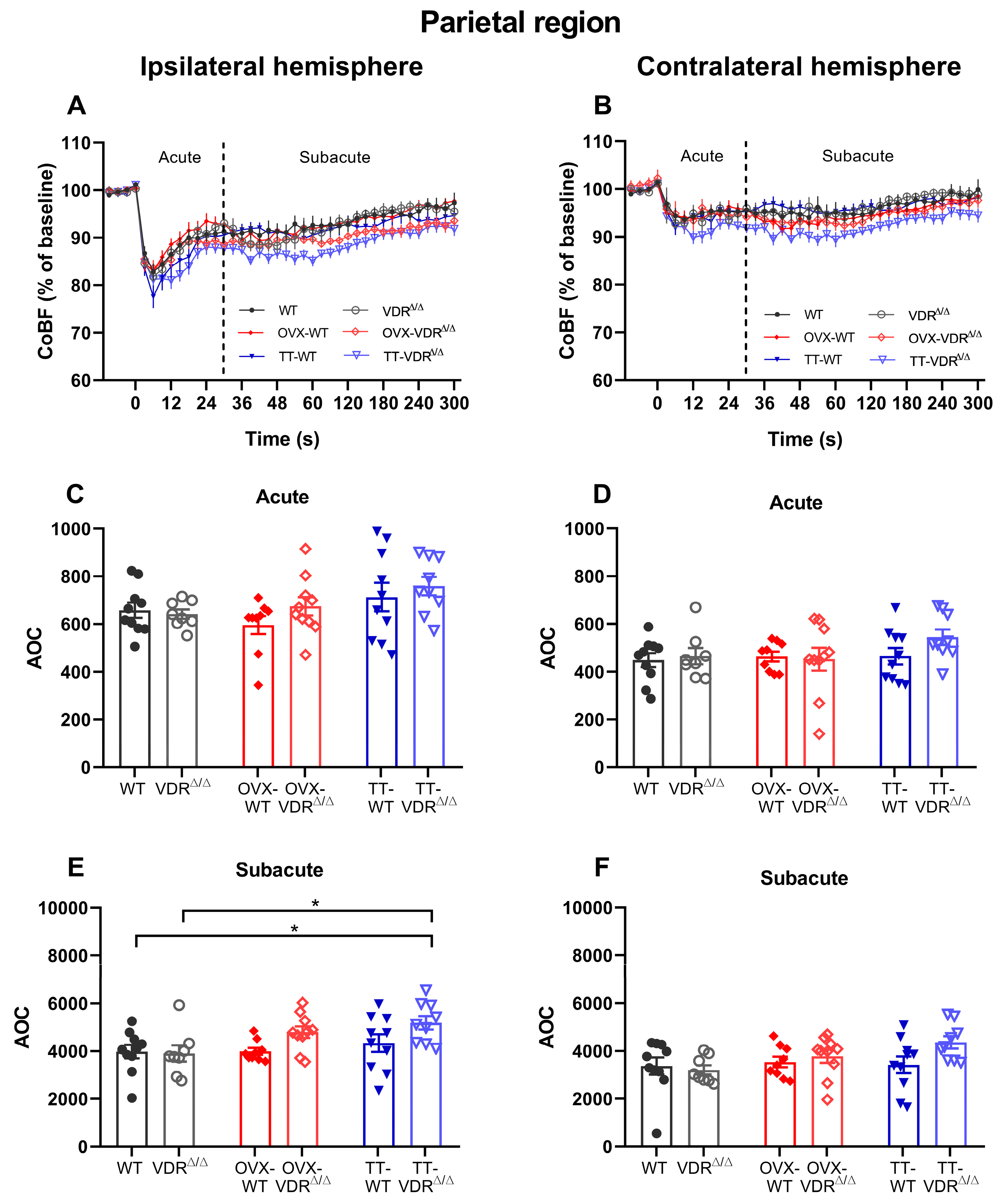
3.2. Regional Cerebrocortical Blood Flow Changes after Carotid Artery Occlusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D Deficiency and the Risk of Cerebrovascular Disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Crumpler, R.F.; Thomas, K.N.; Mazique, J.N.; Roman, R.J.; Fan, F. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol. Int. 2022; online ahead of print. [Google Scholar]
- Ungvari, Z.; Toth, P.; Tarantini, S.; Prodan, C.I.; Sorond, F.; Merkely, B.; Csiszar, A. Hypertension-induced cognitive impairment: From pathophysiology to public health. Nat. Rev. Nephrol. 2021, 17, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Gosalia, J.; Montgomery, P.S.; Zhang, S.; Pomilla, W.A.; Wang, M.; Liang, M.; Csiszar, A.; Ungvari, Z.; Yabluchanskiy, A.; Proctor, D.N.; et al. Increased pulse wave velocity is related to impaired working memory and executive function in older adults with metabolic syndrome. Geroscience 2022, 44, 2831–2844. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, S.; Csiszar, A.; Ungvari, Z. Midlife Obesity Impairs Neurovascular Coupling Responses. Obesity 2021, 29, 17. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Kiss, T.; Tarantini, S.; Nyul-Toth, A.; Ahire, C.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Tabak, A.; Institoris, A.; et al. Obesity-induced cognitive impairment in older adults: A microvascular perspective. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H740–H761. [Google Scholar] [CrossRef]
- Toth, L.; Czigler, A.; Hegedus, E.; Komaromy, H.; Amrein, K.; Czeiter, E.; Yabluchanskiy, A.; Koller, A.; Orsi, G.; Perlaki, G.; et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022, 44, 2771–2783. [Google Scholar] [CrossRef]
- Tarantini, S.; Nyul-Toth, A.; Yabluchanskiy, A.; Csipo, T.; Mukli, P.; Balasubramanian, P.; Ungvari, A.; Toth, P.; Benyo, Z.; Sonntag, W.E.; et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience 2021, 43, 2387–2394. [Google Scholar] [CrossRef]
- Tarantini, S.; Balasubramanian, P.; Yabluchanskiy, A.; Ashpole, N.M.; Logan, S.; Kiss, T.; Ungvari, A.; Nyul-Toth, A.; Schwartzman, M.L.; Benyo, Z.; et al. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: Implications for brain aging. Geroscience 2021, 43, 901–911. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Cunningham, J.T.; Sumien, N. Estrogen receptor involvement in vascular cognitive impairment and vascular dementia pathogenesis and treatment. Geroscience 2020, 43, 159–166. [Google Scholar] [CrossRef]
- Gardner, A.W.; Montgomery, P.S.; Wang, M.; Shen, B.; Casanegra, A.I.; Silva-Palacios, F.; Ungvari, Z.; Yabluchanskiy, A.; Csiszar, A.; Waldstein, S.R. Cognitive decrement in older adults with symptomatic peripheral artery disease. Geroscience 2021, 43, 2455–2465. [Google Scholar] [CrossRef]
- Istvan, L.; Czako, C.; Elo, A.; Mihaly, Z.; Sotonyi, P.; Varga, A.; Ungvari, Z.; Csiszar, A.; Yabluchanskiy, A.; Conley, S.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Liu, Y.; Fan, L.; Booz, G.W.; Roman, R.J.; Chen, Z.; Fan, F. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience 2020, 42, 547–561. [Google Scholar] [CrossRef]
- Brundel, M.; Reijmer, Y.D.; van Veluw, S.J.; Kuijf, H.J.; Luijten, P.R.; Kappelle, L.J.; Biessels, G.J. Cerebral microvascular lesions on high-resolution 7-Tesla MRI in patients with type 2 diabetes. Diabetes 2014, 63, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Brundel, M.; van den Berg, E.; Reijmer, Y.D.; de Bresser, J.; Kappelle, L.J.; Biessels, G.J. Cerebral haemodynamics, cognition and brain volumes in patients with type 2 diabetes. J. Diabetes Complicat. 2012, 26, 205–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Bresser, J.; Tiehuis, A.M.; van den Berg, E.; Reijmer, Y.D.; Jongen, C.; Kappelle, L.J.; Mali, W.P.; Viergever, M.A.; Biessels, G.J. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010, 33, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Cotch, M.F.; Sigurdsson, S.; Garcia, M.; Klein, R.; Jonasson, F.; Klein, B.E.; Eiriksdottir, G.; Harris, T.B.; van Buchem, M.A.; et al. Retinal and cerebral microvascular signs and diabetes: The age, gene/environment susceptibility-Reykjavik study. Diabetes 2008, 57, 1645–1650. [Google Scholar] [CrossRef]
- Cosentino, N.; Campodonico, J.; Milazzo, V.; De Metrio, M.; Brambilla, M.; Camera, M.; Marenzi, G. Vitamin D and Cardiovascular Disease: Current Evidence and Future Perspectives. Nutrients 2021, 13, 3603. [Google Scholar] [CrossRef]
- Gomez-Cabrero, D.; Walter, S.; Abugessaisa, I.; Miñambres-Herraiz, R.; Palomares, L.B.; Butcher, L.; Erusalimsky, J.D.; Garcia-Garcia, F.J.; Carnicero, J.; Hardman, T.C.; et al. A robust machine learning framework to identify signatures for frailty: A nested case-control study in four aging European cohorts. Geroscience 2021, 43, 1317–1329. [Google Scholar] [CrossRef]
- Huggins, B.; Farris, M. Vitamin D(3) promotes longevity in Caenorhabditis elegans. Geroscience 2023, 45, 345–358. [Google Scholar] [CrossRef]
- Vetter, V.M.; Sommerer, Y.; Kalies, C.H.; Spira, D.; Bertram, L.; Demuth, I. Vitamin D supplementation is associated with slower epigenetic aging. Geroscience 2022, 44, 1847–1859. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef] [PubMed]
- AlQuaiz, A.M.; Kazi, A.; Fouda, M.; Alyousefi, N. Age and gender differences in the prevalence and correlates of vitamin D deficiency. Arch. Osteoporos. 2018, 13, 49. [Google Scholar] [CrossRef]
- Boettger, S.F.; Angersbach, B.; Klimek, C.N.; Wanderley, A.L.M.; Shaibekov, A.; Sieske, L.; Wang, B.; Zuchowski, M.; Wirth, R.; Pourhassan, M. Prevalence and predictors of vitamin D-deficiency in frail older hospitalized patients. BMC Geriatr. 2018, 18, 219. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef]
- Hoseinzadeh-Chahkandak, F.; Zeinali, T.; Salmani, F.; Moodi, M.; Sharifi, F.; Rahimlou, M.; Ansarifar, E. Prevalence of vitamin D deficiency and its association with metabolic syndrome among the elderly population of Birjand, Iran. J. Diabetes Metab. Disord. 2022, 21, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Tomaschitz, A.; Drechsler, C.; Zittermann, A.; Dekker, J.M.; März, W. Vitamin D supplementation: A promising approach for the prevention and treatment of strokes. Curr. Drug Targets 2011, 12, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Peterfi, A.; Meszaros, A.; Szarvas, Z.; Penzes, M.; Fekete, M.; Feher, A.; Lehoczki, A.; Csipo, T.; Fazekas-Pongor, V. Comorbidities and increased mortality of COVID-19 among the elderly: A systematic review. Physiol. Int. 2022; online ahead of print. [Google Scholar]
- Gadó, K.; Kovács, A.K.; Domján, G.; Nagy, Z.Z.; Bednárik, G.D. COVID-19 and the elderly. Physiol. Int. 2022; online ahead of print. [Google Scholar]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Dosa, N.; Lehoczki, A.; Tarantini, S.; Varga, J.T. COVID-19 infection in patients with chronic obstructive pulmonary disease: From pathophysiology to therapy. Mini-review. Physiol. Int. 2022; online ahead of print. [Google Scholar]
- Quarleri, J.; Delpino, M.V. SARS-CoV-2 interacts with renin-angiotensin system: Impact on the central nervous system in elderly patients. Geroscience 2022, 44, 547–565. [Google Scholar] [CrossRef]
- Batra, A.; Clark, J.R.; Kang, A.K.; Ali, S.; Patel, T.R.; Shlobin, N.A.; Hoffman, S.C.; Lim, P.H.; Orban, Z.S.; Visvabharathy, L.; et al. Persistent viral RNA shedding of SARS-CoV-2 is associated with delirium incidence and six-month mortality in hospitalized COVID-19 patients. Geroscience 2022, 44, 1241–1254. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Tanaka, T.; Moore, A.Z.; Bandinelli, S.; Lakatta, E.G.; Ferrucci, L. Age-associated difference in circulating ACE2, the gateway for SARS-COV-2, in humans: Results from the InCHIANTI study. Geroscience 2021, 43, 619–627. [Google Scholar] [CrossRef]
- Nikolich-Zugich, J.; Knox, K.S.; Rios, C.T.; Natt, B.; Bhattacharya, D.; Fain, M.J. SARS-CoV-2 and COVID-19 in older adults: What we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience 2020, 42, 505–514. [Google Scholar] [CrossRef]
- Mercola, J.; Grant, W.B.; Wagner, C.L. Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity. Nutrients 2020, 12, 3361. [Google Scholar] [CrossRef]
- Pál, É.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Mezei, Z.; Lippai, N.; Magyar, A.; Heinzlmann, A.; Karvaly, G.; Monos, E.; et al. Vitamin D deficiency causes inward hypertrophic remodeling and alters vascular reactivity of rat cerebral arterioles. PLoS ONE 2018, 13, e0192480. [Google Scholar] [CrossRef]
- Pál, É.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Lippai, N.; Horváth, E.M.; Magyar, A.; Horváth, E.; Monos, E.; Nádasy, G.L.; et al. Gender, hyperandrogenism and vitamin D deficiency related functional and morphological alterations of rat cerebral arteries. PLoS ONE 2019, 14, e0216951. [Google Scholar] [CrossRef]
- Hadjadj, L.; Pál, É.; Monori-Kiss, A.; Sziva, R.E.; Korsós-Novák, Á.; Mária Horváth, E.; Benkő, R.; Magyar, A.; Magyar, P.; Benyó, Z.; et al. Vitamin D deficiency and androgen excess result eutrophic remodeling and reduced myogenic adaptation in small cerebral arterioles in female rats. Gynecol. Endocrinol. 2019, 35, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Powell, J.T. Vitamin D and cardiovascular disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Annweiler, C.; Duval, G.; Karras, S.; Tirabassi, G.; Salvio, G.; Balercia, G.; Kimball, S.; Kotsa, K.; Mascitelli, L.; et al. Vitamin D and cardiovascular disease: From atherosclerosis to myocardial infarction and stroke. Int. J. Cardiol. 2017, 230, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Marginean, O. Links between Vitamin D Deficiency and Cardiovascular Diseases. Biomed. Res. Int. 2015, 2015, 109275. [Google Scholar] [CrossRef] [PubMed]
- Pál, É.; Hricisák, L.; Lékai, Á.; Nagy, D.; Fülöp, Á.; Erben, R.G.; Várbíró, S.; Sándor, P.; Benyó, Z. Ablation of Vitamin D Signaling Compromises Cerebrovascular Adaptation to Carotid Artery Occlusion in Mice. Cells 2020, 9, 1457. [Google Scholar] [CrossRef]
- Guennoun, R.; Zhu, X.; Fréchou, M.; Gaignard, P.; Slama, A.; Liere, P.; Schumacher, M. Steroids in Stroke with Special Reference to Progesterone. Cell Mol. Neurobiol. 2019, 39, 551–568. [Google Scholar] [CrossRef]
- Stewart, C.E.; Sohrabji, F. Gonadal hormones and stroke risk: PCOS as a case study. Front. Neuroendocrinol. 2020, 58, 100853. [Google Scholar] [CrossRef]
- Thomson, R.L.; Spedding, S.; Buckley, J.D. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin. Endocrinol. 2012, 77, 343–350. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Chedraui, P.; Pilz, S. Vitamin D supplementation after the menopause. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820931291. [Google Scholar] [CrossRef] [PubMed]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Zuloaga, K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2019, 1710, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Al Mheid, I.; Quyyumi, A.A. Vitamin D and Cardiovascular Disease: Controversy Unresolved. J. Am. Coll. Cardiol. 2017, 70, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Duckles, S.P.; Pelligrino, D.A. Influence of sex steroid hormones on cerebrovascular function. J. Appl. Physiol. 2006, 101, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.N.; Duckles, S.P.; Gonzales, R.J. Local oestrogenic/androgenic balance in the cerebral vasculature. Acta Physiol. 2011, 203, 181–186. [Google Scholar] [CrossRef]
- Razmara, A.; Krause, D.N.; Duckles, S.P. Testosterone augments endotoxin-mediated cerebrovascular inflammation in male rats. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1843–H1850. [Google Scholar] [CrossRef]
- Erben, R.G.; Soegiarto, D.W.; Weber, K.; Zeitz, U.; Lieberherr, M.; Gniadecki, R.; Möller, G.; Adamski, J.; Balling, R. Deletion of deoxyribonucleic acid binding domain of the vitamin D receptor abrogates genomic nongenomic functions of vitamin D. Mol. Endocrinol. 2002, 16, 1524–1537. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef]
- Ng, K.Y.; Yong, J.; Chakraborty, T.R. Estrous cycle in ob/ob and ovariectomized female mice and its relation with estrogen and leptin. Physiol. Behav. 2010, 99, 125–130. [Google Scholar] [CrossRef]
- Polycarpou, A.; Hricisák, L.; Iring, A.; Safar, D.; Ruisanchez, É.; Horváth, B.; Sándor, P.; Benyó, Z. Adaptation of the cerebrocortical circulation to carotid artery occlusion involves blood flow redistribution between cortical regions and is independent of eNOS. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H972–H980. [Google Scholar] [CrossRef]
- Karvaly, G.; Kovács, K.; Mészáros, K.; Kocsis, I.; Patócs, A.; Vásárhelyi, B. The comprehensive characterization of adrenocortical steroidogenesis using two-dimensional ultra-performance liquid chromatography—Electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 153, 274–283. [Google Scholar] [CrossRef]
- Winship, I.R. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation 2015, 22, 228–236. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Pál, É.; Ungvári, Z.; Benyó, Z.; Várbíró, S. Role of Vitamin D Deficiency in the Pathogenesis of Cardiovascular and Cerebrovascular Diseases. Nutrients 2023, 15, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tremble, S.M.; Cipolla, M.J. Implications for understanding ischemic stroke as a sexually dimorphic disease: The role of pial collateral circulations. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1703–H1712. [Google Scholar] [CrossRef]
- Macut, D.; Antić, I.B.; Bjekić-Macut, J. Cardiovascular risk factors and events in women with androgen excess. J. Endocrinol. Invest. 2015, 38, 295–301. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Mallya, S.M.; Corrado, K.R.; Saria, E.A.; Yuan, F.F.; Tran, H.Q.; Saucier, K.; Atti, E.; Tetradis, S.; Arnold, A. Modeling vitamin D insufficiency and moderate deficiency in adult mice via dietary cholecalciferol restriction. Endocr. Res. 2016, 41, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Pirro, A.E.; Amling, M.; Delling, G.; Baron, R.; Bronson, R.; Demay, M.B. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc. Natl. Acad. Sci. USA 1997, 94, 9831–9835. [Google Scholar] [CrossRef] [PubMed]
- Malloy, P.J.; Pike, J.W.; Feldman, D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr. Rev. 1999, 20, 156–188. [Google Scholar] [PubMed]
- Hubscher, C.H.; Brooks, D.L.; Johnson, J.R. A quantitative method for assessing stages of the rat estrous cycle. Biotech. Histochem. 2005, 80, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.E.; Vandenput, L.; Tivesten, Å.; Norlén, A.K.; Lagerquist, M.K.; Windahl, S.H.; Börjesson, A.E.; Farman, H.H.; Poutanen, M.; Benrick, A.; et al. Measurement of a Comprehensive Sex Steroid Profile in Rodent Serum by High-Sensitive Gas Chromatography-Tandem Mass Spectrometry. Endocrinology 2015, 156, 2492–2502. [Google Scholar] [CrossRef]
- Clark, R.V.; Wald, J.A.; Swerdloff, R.S.; Wang, C.; Wu, F.C.W.; Bowers, L.D.; Matsumoto, A.M. Large divergence in testosterone concentrations between men and women: Frame of reference for elite athletes in sex-specific competition in sports, a narrative review. Clin. Endocrinol. 2019, 90, 15–22. [Google Scholar] [CrossRef]
- Borges, C.C.; Bringhenti, I.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Vitamin D deficiency aggravates the liver metabolism and inflammation in ovariectomized mice. Biomed. Pharmacother. 2018, 107, 878–888. [Google Scholar] [CrossRef]
- Hadjadj, L.; Várbíró, S.; Horváth, E.M.; Monori-Kiss, A.; Pál, É.; Karvaly, G.B.; Heinzlmann, A.; Magyar, A.; Szabó, I.; Sziva, R.E.; et al. Insulin resistance in an animal model of polycystic ovary disease is aggravated by vitamin D deficiency: Vascular consequences. Diab. Vasc. Dis. Res. 2018, 15, 294–301. [Google Scholar] [CrossRef]
- Alrabadi, N.; Al-Rabadi, G.J.; Maraqa, R.; Sarayrah, H.; Alzoubi, K.H.; Alqudah, M.; Al-U’datt D, G. Androgen effect on body weight and behaviour of male and female rats: Novel insight on the clinical value. Andrologia 2020, 52, e13730. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Akishita, M.; Kim, S.; Ako, J.; Hashimoto, M.; Iijima, K.; Ohike, Y.; Watanabe, T.; Sudoh, N.; Toba, K.; et al. Estrogen receptor beta is involved in the anorectic action of estrogen. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1103–1109. [Google Scholar] [CrossRef]
- Joksimovic Jovic, J.; Sretenovic, J.; Jovic, N.; Rudic, J.; Zivkovic, V.; Srejovic, I.; Mihajlovic, K.; Draginic, N.; Andjic, M.; Milinkovic, M.; et al. Cardiovascular Properties of the Androgen-Induced PCOS Model in Rats: The Role of Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 8862878. [Google Scholar] [CrossRef]
- Brozici, M.; van der Zwan, A.; Hillen, B. Anatomy and functionality of leptomeningeal anastomoses: A review. Stroke 2003, 34, 2750–2762. [Google Scholar] [CrossRef] [PubMed]
- Cuccione, E.; Padovano, G.; Versace, A.; Ferrarese, C.; Beretta, S. Cerebral collateral circulation in experimental ischemic stroke. Exp. Transl. Stroke Med. 2016, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Prabhakar, P.; Sealock, R.; Faber, J.E. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J. Cereb. Blood Flow Metab. 2010, 30, 923–934. [Google Scholar] [CrossRef]
- Zhang, H.; Chalothorn, D.; Faber, J.E. Collateral Vessels Have Unique Endothelial and Smooth Muscle Cell Phenotypes. Int. J. Mol. Sci. 2019, 20, 3608. [Google Scholar] [CrossRef] [PubMed]
- Faber, J.E.; Moore, S.M.; Lucitti, J.L.; Aghajanian, A.; Zhang, H. Sex Differences in the Cerebral Collateral Circulation. Transl. Stroke Res. 2017, 8, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Losordo, D.W.; Isner, J.M. Estrogen and angiogenesis: A review. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Hong, Y.; Weng, C.; Tan, C.; Imperato-McGinley, J.; Zhu, Y.S. Androgen stimulates endothelial cell proliferation via an androgen receptor/VEGF/cyclin A-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1210–H1221. [Google Scholar] [CrossRef]
- Goldie, L.C.; Nix, M.K.; Hirschi, K.K. Embryonic vasculogenesis and hematopoietic specification. Organogenesis 2008, 4, 257–263. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in regulation of physiological angiogenesis. Am. J. Physiol. Cell Physiol. 2001, 280, C1358–C1366. [Google Scholar] [CrossRef]
- Lucitti, J.L.; Mackey, J.K.; Morrison, J.C.; Haigh, J.J.; Adams, R.H.; Faber, J.E. Formation of the collateral circulation is regulated by vascular endothelial growth factor-A and a disintegrin and metalloprotease family members 10 and 17. Circ. Res. 2012, 111, 1539–1550. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, B.; Gu, Y.; Groome, L.J.; Sun, J.; Wang, Y. Activation of vitamin D receptor promotes VEGF and CuZn-SOD expression in endothelial cells. J. Steroid Biochem. Mol. Biol. 2014, 140, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Chopra, S.; Rohit, M.K.; Banerjee, D.; Chakraborti, A. Vitamin D regulates the production of vascular endothelial growth factor: A triggering cause in the pathogenesis of rheumatic heart disease? Med. Hypotheses 2016, 95, 62–66. [Google Scholar] [CrossRef]
- Plate, K.H. Mechanisms of angiogenesis in the brain. J. Neuropathol. Exp. Neurol. 1999, 58, 313–320. [Google Scholar] [CrossRef]
- Kim, D.H.; Meza, C.A.; Clarke, H.; Kim, J.S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Guo, Y.J.; Wang, K.Y.; Chen, L.M.; Jiang, P. Neuroprotective effects of vitamin D and 17ß-estradiol against ovariectomy-induced neuroinflammation and depressive-like state: Role of the AMPK/NF-κB pathway. Int. Immunopharmacol. 2020, 86, 106734. [Google Scholar] [CrossRef]
- Li, J.; Padwa, B.L.; Zhou, S.; Mullokandova, J.; LeBoff, M.S.; Glowacki, J. Synergistic effect of 1α,25-dihydroxyvitamin D(3) and 17β-estradiol on osteoblast differentiation of pediatric MSCs. J. Steroid Biochem. Mol. Biol. 2018, 177, 103–108. [Google Scholar] [CrossRef]
- Geary, G.G.; Krause, D.N.; Duckles, S.P. Estrogen reduces myogenic tone through a nitric oxide-dependent mechanism in rat cerebral arteries. Am. J. Physiol. 1998, 275, H292–H300. [Google Scholar] [CrossRef]
- Ospina, J.A.; Duckles, S.P.; Krause, D.N. 17beta-estradiol decreases vascular tone in cerebral arteries by shifting COX-dependent vasoconstriction to vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H241–H250. [Google Scholar] [CrossRef]
- Carswell, H.V.; Macrae, I.M.; Farr, T.D. Complexities of oestrogen in stroke. Clin. Sci. 2009, 118, 375–389. [Google Scholar] [CrossRef]
- Abi-Ghanem, C.; Robison, L.S.; Zuloaga, K.L. Androgens’ effects on cerebrovascular function in health and disease. Biol. Sex Differ. 2020, 11, 35. [Google Scholar] [CrossRef]
- Connelly, P.J.; Marie Freel, E.; Perry, C.; Ewan, J.; Touyz, R.M.; Currie, G.; Delles, C. Gender-Affirming Hormone Therapy, Vascular Health and Cardiovascular Disease in Transgender Adults. Hypertension 2019, 74, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017, 5, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Morgante, G.; Darino, I.; Spanò, A.; Luisi, S.; Luddi, A.; Piomboni, P.; Governini, L.; De Leo, V. PCOS Physiopathology and Vitamin D Deficiency: Biological Insights and Perspectives for Treatment. J. Clin. Med. 2022, 11, 4509. [Google Scholar] [CrossRef] [PubMed]
- Menichini, D.; Facchinetti, F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: A review. Gynecol. Endocrinol. 2020, 36, 1–5. [Google Scholar] [CrossRef] [PubMed]



| Experimental Group | WT | VDR∆/∆ | OVX-WT | OVX-VDR∆/∆ | TT-WT | TT-VDR∆/∆ |
|---|---|---|---|---|---|---|
| Vitamin D Signaling | + | − | + | − | + | − |
| Ovariectomy | − | − | + | + | − | − |
| Testosterone Treatment | − | − | − | − | + | + |
| Parameter | WT | VDR∆/∆ | OVX-WT | OVX-VDR∆/∆ | TT-WT | TT-VDR∆/∆ |
|---|---|---|---|---|---|---|
| Body Weight (g) | 22.10 ± 0.18 | 20.88 ± 0.44 | 23.56 ± 0.42 | 21.82 ± 0.30 | 25.34 ± 1.40 *,**,†,# | 22.22 ± 0.82 |
| Heart Weight (g) | 0.13 (0.12–0.14) | 0.12 (0.12–0.14) | 0.13 (0.12–0.13) | 0.12 (0.11–0.12) | 0.13 (0.12–0.14) | 0.12 (0.11–0.12) |
| Tibial Length (cm) | 1.66 ± 0.03 | 1.59 ± 0.04 **,† | 1.76 ± 0.03 | 1.67 ± 0.03 | 1.75 ± 0.03 | 1.68 ± 0.03 |
| Brain Weight (g) | 0.442 ± 0.005 | 0.438 ± 0.003 | 0.441 ± 0.008 | 0.442 ± 0.009 | 0.451 ± 0.006 | 0.440 ± 0.006 |
| Heart Weight/Body Weight (%) | 0.58 ± 0.02 | 0.60 ± 0.03 | 0.53 ± 0.02 | 0.53 ± 0.01 | 0.55 ± 0.05 | 0.58 ± 0.03 |
| MABP (mmHg) | WT | VDR∆/∆ | OVX-WT | OVX-VDR∆/∆ | TT-WT | TT-VDR∆/∆ |
|---|---|---|---|---|---|---|
| Baseline | 79.38 ± 3.11 | 76.12 ± 2.30 | 75.72 ± 3.34 | 70.54 ± 2.23 | 75.91 ± 2.07 | 75.72 ± 2.70 |
| 0–60 s | 83.17 ± 3.36 | 80.38 ± 2.72 | 81.21 ± 3.36 | 74.91 ± 1.63 | 83.04 ± 1.95 | 82.51 ± 2.90 |
| 61–120 s | 82.72 ± 3.62 | 79.02 ± 2.18 | 80.53 ± 3.00 | 74.94 ± 2.34 | 83.34 ± 2.11 | 80.62 ± 3.28 |
| 121–180 s | 83.08 ± 3.55 | 77.11 ± 2.58 | 79.16 ± 2.98 | 71.59 ± 2.18 | 81.97 ± 2.21 | 78.49 ± 2.80 |
| 181–240 s | 83.09 ± 3.53 | 76.87 ± 2.56 | 78.88 ± 3.11 | 71.28 ± 2.20 | 82.03 ± 2.28 | 78.72 ± 3.18 |
| 241–300 s | 83.72 ± 3.56 | 76.11 ± 2.53 | 78.37 ± 3.04 | 71.57 ± 2.10 | 81.60 ± 2.07 | 79.21 ± 3.50 |
| Parameter | WT | VDR∆/∆ | OVX-WT | OVX-VDR∆/∆ | TT-WT | TT-VDR∆/∆ |
|---|---|---|---|---|---|---|
| pH | 7.29 ± 0.02 | 7.27 ± 0.01 | 7.30 ± 0.01 | 7.28 ± 0.02 | 7.26 ± 0.02 | 7.27 ± 0.02 |
| pCO2 (mmHg) | 40.35 (35.30–47.03) | 38.20 (35.28–47.38) | 45.00 (36.65–46.90) | 35.60 (31.43–45.93) | 48.85 (31.70–50.85) | 43.60 (38.60–45.90) |
| pO2 (mmHg) | 96.50 ± 3.65 | 93.00 ± 4.56 | 94.22 ± 2.41 | 99.00 ± 4.81 | 93.4 ± 3.35 | 98.44 ± 3.36 |
| Hematocrit (%) | 42.10 ± 1.14 | 41.0 ± 1.09 | 39.89 ± 0.98 | 39.70 ± 0.60 | 39.30 ± 0.63 | 38.44 ± 0.58 |
| cNa⁺ (mmol/L) | 155.60 ± 1.19 | 156.10 ± 1.04 | 157.90 ± 1.20 | 157.90 ± 1.20 | 156.50 ± 0.82 | 154.80 ± 0.76 |
| cK⁺ (mmol/L) | 4.33 ± 0.13 | 4.35 ± 0.12 | 4.47 ± 0.06 | 4.29 ± 0.14 | 4.27 ± 0.10 | 4.34 ± 0.15 |
| cCa2⁺ (mmol/L) | 1.28 ± 0.02 | 1.22 ± 0.02 | 1.27 ± 0.03 | 1.25 ± 0.02 | 1.29 ± 0.01 | 1.27 ± 0.02 |
| cCl¯ (mmol/L) | 116.20 ± 1.87 | 115.80 ± 0.94 | 116.00 ± 1.23 | 115.40 ± 1.51 | 115.20 ± 0.92 | 115.10 ± 0.63 |
| cHCO3¯ (mmol/L) | 18.83 ± 0.82 | 17.90 ± 0.76 | 19.09 ± 0.96 | 18.22 ± 0.73 | 19.07 ± 0.28 | 18.84 ± 0.72 |
| O2 saturation (%) | 97.28 ± 0.51 | 96.20 ± 0.68 | 97.33 ± 0.34 | 97.25 ± 0.50 | 96.04 ± 0.62 | 97.17 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, D.; Hricisák, L.; Walford, G.P.; Lékai, Á.; Karácsony, G.; Várbíró, S.; Ungvári, Z.; Benyó, Z.; Pál, É. Disruption of Vitamin D Signaling Impairs Adaptation of Cerebrocortical Microcirculation to Carotid Artery Occlusion in Hyperandrogenic Female Mice. Nutrients 2023, 15, 3869. https://doi.org/10.3390/nu15183869
Nagy D, Hricisák L, Walford GP, Lékai Á, Karácsony G, Várbíró S, Ungvári Z, Benyó Z, Pál É. Disruption of Vitamin D Signaling Impairs Adaptation of Cerebrocortical Microcirculation to Carotid Artery Occlusion in Hyperandrogenic Female Mice. Nutrients. 2023; 15(18):3869. https://doi.org/10.3390/nu15183869
Chicago/Turabian StyleNagy, Dorina, László Hricisák, Guillaume Peter Walford, Ágnes Lékai, Gábor Karácsony, Szabolcs Várbíró, Zoltán Ungvári, Zoltán Benyó, and Éva Pál. 2023. "Disruption of Vitamin D Signaling Impairs Adaptation of Cerebrocortical Microcirculation to Carotid Artery Occlusion in Hyperandrogenic Female Mice" Nutrients 15, no. 18: 3869. https://doi.org/10.3390/nu15183869
APA StyleNagy, D., Hricisák, L., Walford, G. P., Lékai, Á., Karácsony, G., Várbíró, S., Ungvári, Z., Benyó, Z., & Pál, É. (2023). Disruption of Vitamin D Signaling Impairs Adaptation of Cerebrocortical Microcirculation to Carotid Artery Occlusion in Hyperandrogenic Female Mice. Nutrients, 15(18), 3869. https://doi.org/10.3390/nu15183869







