Risk of Malnutrition in Adults Who Have Undergone Sleeve Gastrectomy: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Samples and Location
2.3. Data Collection and Instruments
2.4. Data Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Gastrointestinal Symptoms and Nutritional Supplement Intake
3.3. Postoperative Change in BMI and Its Key Predictors
3.4. Postoperative Change in PNI and Its Key Predictors
3.5. Postoperative Change in Hemoglobin and Its Key Predictors
4. Discussion
4.1. Postoperative Change in BMI and Its Key Predictors
4.2. Postoperative Change in PNI and Its Key Predictors
4.3. Postoperative Change in Hemoglobin and Its Key Predictors
4.4. Gastrointestinal Symptoms and Nutritional Supplement Intake
4.5. Study Limitations
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight, Fact Sheet no 311. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 March 2023).
- Taiwan Health Promotion Administration. National Nutrition and Health Survey (2017–2022). 2022. Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=4567 (accessed on 15 March 2023). (In Chinese)
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, D.J.; Murphy-Després, A.; Alméras, N.; Lemieux, I.; Larose, E.; Després, J.P. Overweight, obesity, and CVD risk: A focus on visceral/ectopic fat. Curr. Atheroscler. Rep. 2022, 24, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Taiwan National Health Insurance Administration. Considerations for the National Health Insurance Medical Expenses Review (Announcement of Revisions in the Past Year). 2020. Available online: https://www.nhi.gov.tw/Content_List.aspx?n=1A7099384DD1EE74&topn=5FE8C9FEAE863B46 (accessed on 15 March 2023). (In Chinese)
- American Society for Metabolic and Bariatric Surgery. Bariatric Surgery Procedures 2021. Available online: https://asmbs.org/patients/bariatric-surgery-procedures (accessed on 15 March 2023).
- Aldenbäck, E.; Johansson, H.-E. Anthropometric measurements and correlations to glucometabolic and cardiovascular risk in obese patients undergoing gastric bypass surgery. J. Obes. 2021, 2021, 6647328. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.L.; Ioniţa-Radu, F.; Jinga, M.; Gavrilă, A.I.; Săvulescu, F.A.; Fierbinţeanu-Braticevici, C. Laparoscopic sleeve gastrectomy and GI reflux. Rom. J. Intern. Med. 2018, 56, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, X.; Lu, X.; Mattar, S.; Kassab, G. Mechanisms of weight loss after sleeve gastrectomy and adjustable gastric banding: Far more than just restriction. Obesity 2019, 27, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, T.; Elgarf, S.; Elshora, A.; Moussa, G.; Swelam, A. Five-year outcomes of laparoscopic sleeve gastrectomy in the treatment of morbid obesity: A retrospective analysis. Egypt. J. Surg. 2020, 39, 814–821. [Google Scholar] [CrossRef]
- Taiwan Society for Metabolic and Bariatric Surgery. Satisitics of Bariatric Surgery in Taiwan. 2022. Available online: https://www.tsmbs.org.tw (accessed on 15 March 2023). (In Chinese).
- Ahmed, E.; Anas, M. Comparison between antral resection in laparoscopic sleeve gastrectomy and classical laparoscopic sleeve gastrectomy. Egypt. J. Surg. 2019, 38, 570–574. [Google Scholar] [CrossRef]
- Sioka, E.; Tzovaras, G.; Perivoliotis, K.; Bakalis, V.; Zachari, E.; Magouliotis, D.; Tassiopoulou, V.; Potamianos, S.; Kapsoritakis, A.; Poultsidi, A.; et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol. Res. Pract. 2018, 2018, 4135813. [Google Scholar] [CrossRef]
- Guerreiro, V.; Neves, J.S.; Salazar, D.; Ferreira, M.J.; Oliveira, S.C.; Souteiro, P.; Pedro, J.; Magalhães, D.; Varela, A.; Belo, S.; et al. Long-term weight loss and metabolic syndrome remission after bariatric surgery: The effect of sex, age, metabolic parameters and surgical technique. A 4-year follow-up study. Obes. Facts 2019, 12, 639–652. [Google Scholar] [CrossRef]
- Sewefy, A.M.; Saleh, A. The outcomes of single anastomosis sleeve jejunal bypass as a treatment for morbid obesity (two-year follow-up). Surg. Endosc. 2021, 35, 5698–5704. [Google Scholar] [CrossRef]
- Bertoni, L.; Valentini, R.; Zattarin, A.; Belligoli, A.; Bettini, S.; Vettor, R.; Foletto, M.; Spinella, P.; Busetto, L.; Devlieger, R. Assessment of protein intake in the first three months after sleeve gastrectomy in patients with severe obesity. Nutrients 2021, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Gangadharan, K.; Pitchumoni, C.S. Malnutrition in obesity before and after bariatric surgery. Disease-a-Month 2020, 66, 100866. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-F.; Soong, T.-C.; Hsu, Y.-R. Nutrition demand and care of patients undergoing bariatric surgery. J. Nurs. 2021, 68, 21–25. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, X.; Li, J.; Liu, Z.; Liu, W.; Zhang, J.; Zhou, Z. Anaemia and related nutritional deficiencies in Chinese patients with obesity, 12 months following laparoscopic sleeve gastrectomy. Diabetes Metab. Syndr. Obes. 2021, 14, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Brewczyński, A.; Jabłońska, B.; Pawlicki, K. Associations between nutritional parameters and clinicopathologic factors in patients with gastric cancer: A comprehensive study. Nutr. Cancer 2017, 69, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Cong, K.; Chunwei, G. Exploration of three different nutritional scores in predicting postoperative complications after pancreaticoduodenectomy. Nutr. Hosp. 2022, 39, 101–110. [Google Scholar] [PubMed]
- Oh, S.E.; Choi, M.-G.; Seo, J.-M.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S. Prognostic significance of perioperative nutritional parameters in patients with gastric cancer. Clin. Nutr. 2019, 38, 870–876. [Google Scholar] [CrossRef]
- Omarov, T.; Samadov, E.; Coskun, A.K.; Unlu, A. Comparison of weight loss in sleeve gastrectomy patients with and without antrectomy: A prospective randomized study. Obes. Surg. 2020, 30, 446–450. [Google Scholar] [CrossRef]
- Masood, A.; Alsheddi, L.; Alfayadh, L.; Bukhari, B.; Elawad, R.; Alfadda, A.A. Dietary and lifestyle factors serve as predictors of successful weight loss maintenance postbariatric surgery. J. Obes. 2019, 2019, 7295978. [Google Scholar] [CrossRef]
- Enani, G.; Bilgic, E.; Lebedeva, E.; Delisle, M.; Vergis, A.; Hardy, K. The incidence of iron deficiency anemia post-Roux-en-Y gastric bypass and sleeve gastrectomy: A systematic review. Surg. Endosc. 2020, 34, 3002–3010. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, N.; Gesquiere, I.; Matthys, C. The relevance of dietary protein after bariatric surgery: What do we know? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Suh, H.; Karantanis, W.; Jia, S.; Yang, Y.; Loi, K.W.K. Evaluation of micronutrient status post laparoscopic sleeve gastrectomy: An Australian perspective. Obes. Surg. 2021, 31, 1099–1104. [Google Scholar] [CrossRef]
- Camaschella, C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Fu, R.; Chen, P.; Du, N.; Chen, S.; Mao, D.; Chen, B.; Mao, F.; Khadaroo, P.A.; Jin, Q. In terms of nutrition, the most suitable method for bariatric surgery: Laparoscopic sleeve gastrectomy or Roux-en-Y gastric bypass? A systematic review and meta-analysis. Obes. Surg. 2020, 30, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Felinska, E.; Billeter, A.; Nickel, F.; Contin, P.; Berlth, F.; Chand, B.; Grimminger, P.; Mikami, D.; Schoppmann, S.F.; Muller-Stich, B. Do we understand the pathophysiology of GERD after sleeve gastrectomy? Ann. N. Y. Acad. Sci. 2020, 1482, 26–35. [Google Scholar] [CrossRef]
- Taiwan Health Promotion Administration. Evidence-Based Guidelines on Adult Obesity Prevention and Management. 2023. Available online: https://www.hpa.gov.tw/Pages/EBook.aspx?nodeid=1788 (accessed on 15 March 2023). (In Chinese)
| Variables | n | % | |
|---|---|---|---|
| Sex | |||
| Male | 52 | 43.3 | |
| Female | 68 | 56.7 | |
| Education level | |||
| Middle school and below | 10 | 8.3 | |
| High school | 37 | 30.8 | |
| College and above | 73 | 60.9 | |
| Diagnosis | |||
| Morbid obesity | 120 | 100 | |
| Sleep apnea | 56 | 46.7 | |
| Fatty liver | 73 | 60.8 | |
| Polycystic ovary syndrome | 9 | 7.5 | |
| Mean | SD | Range | |
| Age | 36.5 | 8.4 | 20–55 |
| Charlson Comorbidity Index | 1.6 | 1.0 | 0–4 |
| Number of outpatient visits | 6.0 | 1.4 | 3–10 |
| Number of nutritional consultations | 3.6 | 0.8 | 3–6 |
| Variable | T0 | T1 | T2 | T3 | Chi-Square |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Abdominal bloating | - | 117 (97.5) | 102 (85) | 36 (30) | |
| Reflux symptoms | - | 97 (80.8) | 86 (71.7) | 33 (27.5) | |
| Nausea | - | 87 (72.5) | 46 (38.3) | 6 (5.0) | |
| Vomit | - | 13 (10.8) | 10 (8.3) | 3 (2.5) | |
| Constipation | - | 3 (2.5) | 3 (2.5) | 2 (1.7) | |
| Diarrhea | - | 0 | 1 (0.8) | 1 (0.8) | |
| Multivitamin intake | - | 5 (4.2) | 25 (20.8) | 35 (29.2) | |
| Iron supplement intake | - | 0 (0) | 1 (0.8) | 5 (4.2) | |
| Protein powder intake | - | 2 (1.7) | 5 (4.2) | 4 (3.3) | |
| Level of obesity | - | 294.4 *** | |||
| Underweight | 0 | 0 | 0 | 1 (0.8) | |
| Healthy weight | 0 | 0 | 13 (10.8) | 23 (19.2) | |
| Overweight | 0 | 3 (2.5) | 18 (15.0) | 35 (29.2) | |
| Pre-obese | 0 | 13 (10.8) | 28 (23.3) | 33 (27.5) | |
| Obese class I | 15 (12.5) | 37 (30.8) | 45 (37.5) | 21 (17.5) | |
| Obese class II | 43 (35.8) | 38 (31.7) | 11 (9.2) | 4 (3.3) | |
| Obese class III | 62 (51.7) | 29 (24.2) | 5 (4.2) | 3 (2.5) | |
| Nutritional risk | 21.7 ** | ||||
| Normal | 116 (96.7) | 112 (93.3) | 108 (90) | 96 (80) | |
| Moderate | 3 (2.5) | 6 (5.0) | 11 (9.2) | 20 (16.7) | |
| Severe | 1 (0.8) | 2 (1.7) | 1 (0.8) | 4 (3.3) | |
| Anemia status | 12.8 * | ||||
| Nonanemic | 109 (90.8) | 107 (89.2) | 101 (84.2) | 91 (75.8) | |
| Anemic | 11 (9.2) | 13 (10.8) | 19 (15.8) | 29 (24.2) |
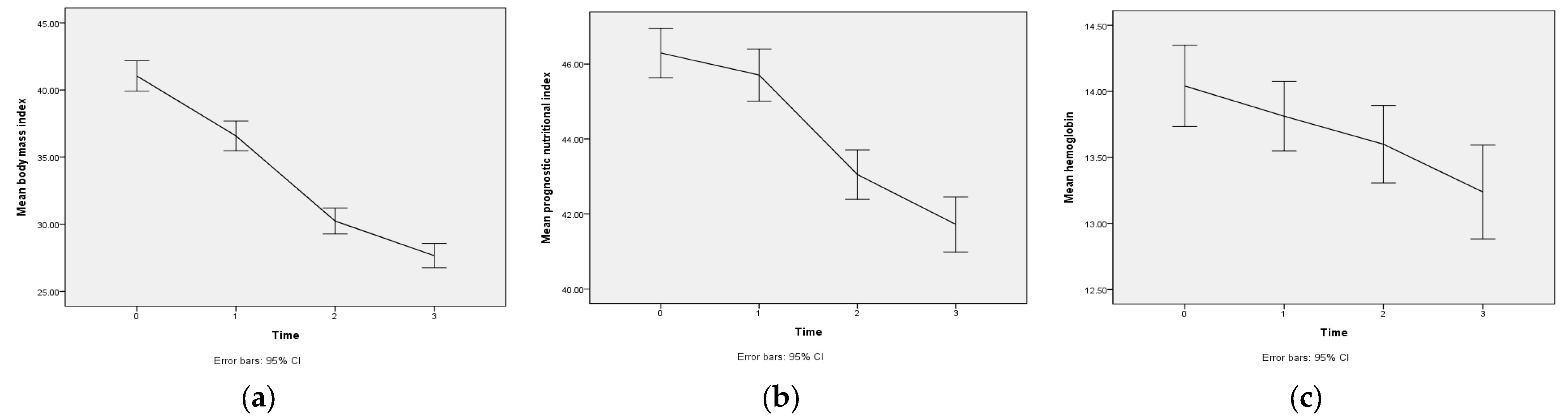
| Variable | Time | Mean | SD | Range | GEE | |
|---|---|---|---|---|---|---|
| B | 95%CI | |||||
| Body mass index | T3 | 27.7 | 5.0 | 18.0–49.6 | −13.5 *** | −14.1~−12.9 |
| T2 | 30.2 | 5.3 | 21.10–53.5 | −10.9 *** | −11.5~−10.3 | |
| T1 | 36.6 | 6.1 | 25.0–57.6 | −4.6 *** | −5.1~−4.0 | |
| T0 | 41.0 | 6.2 | 32.4–65.7 | 0 | ||
| PNI | T3 | 41.7 | 4.1 | 30.1–50.2 | −4.6 *** | −5.5~−3.7 |
| T2 | 43.1 | 3.6 | 32.1–52.2 | −3.2 *** | −4.0~−2.5 | |
| T1 | 45.7 | 3.8 | 30.2–53.1 | −0.6 * | −1.1~−0.1 | |
| T0 | 46.3 | 3.6 | 34.0–54.2 | 0 | ||
| Hemoglobin | T3 | 13.2 | 2.0 | 6.2–16.8 | −0.8 *** | −1.1~−0.5 |
| T2 | 13.6 | 1.6 | 8.6–17.7 | −0.4 *** | −0.7~−0.2 | |
| T1 | 13.8 | 1.5 | 9.3–16.9 | −0.2 | −0.5~−0.0 | |
| T0 | 14.0 | 1.7 | 9.0–17.4 | 0 | ||
| Independent Variable | Body Mass Index | Prognostic Nutritional Index | Hemoglobin | |||
|---|---|---|---|---|---|---|
| Dependent Variable | B | SE | B | SE | B | SE |
| Intercept | 44.1 *** | 2.6 | 48.1 *** | 1.3 | 14.7 *** | 0.5 |
| T3 vs. T0 | −11.0 *** | 0.9 | −3.3 ** | 1.1 | −1.2 *** | 0.3 |
| T2 vs. T0 | −8.2 *** | 0.9 | −1.5 | 1.2 | −0.8 ** | 0.3 |
| T1 vs. T0 | −1.7 | 0.9 | 1.4 | 1.1 | −0.6 | 0.3 |
| Age | −0.1 | 0.1 | −0.0 | 0.0 | 0.0 | 0.0 |
| Sex (female vs. male) | −0.6 | 1.0 | −0.8 | 0.5 | −1.7 *** | 0.2 |
| Charlson Comorbidity Index | 1.0 | 0.6 | 0.2 | 0.3 | 0.1 | 0.1 |
| Outpatient visits | −0.2 * | 0.1 | −0.1 | 0.1 | 0.0 | 0.0 |
| Nutritional consultations | −0.3 | 0.2 | −0.2 | 0.2 | 0.1 | 0.1 |
| Bloating (yes vs. no) | −0.7 | 0.5 | −0.5 | 0.5 | −0.2 | 0.2 |
| Reflux symptoms (yes vs. no) | 0.1 | 0.3 | −0.7 | 0.5 | 0.0 | 0.2 |
| Nausea (yes vs. no) | 0.1 | 0.4 | −0.2 | 0.4 | 0.1 | 0.1 |
| Vomit (yes vs. no) | 0.4 | 0.5 | −0.3 | 0.8 | −0.2 | 0.3 |
| Constipation (yes vs. no) | −0.3 | 0.6 | 1.6 * | 0.8 | −0.2 | 0.4 |
| Diarrhea (yes vs. no) | 1.7 ** | 0.5 | 3.0 *** | 0.6 | −1.5 *** | 0.2 |
| Multivitamin (yes vs. no) | 0.5 | 0.5 | 0.7 | 0.6 | −0.0 | 0.2 |
| Iron supplement (yes vs. no) | 1.7 | 0.9 | −0.3 | 1.3 | −1.8 * | 0.8 |
| Protein powder (yes vs. no) | 1.3 | 1.4 | 1.1 | 0.7 | 0.1 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.-C.; Wang, T.-J.; Liu, C.-Y.; Liu, T.-P.; Liang, S.-Y.; Chang, K.-S. Risk of Malnutrition in Adults Who Have Undergone Sleeve Gastrectomy: A Retrospective Study. Nutrients 2023, 15, 3858. https://doi.org/10.3390/nu15173858
Liao W-C, Wang T-J, Liu C-Y, Liu T-P, Liang S-Y, Chang K-S. Risk of Malnutrition in Adults Who Have Undergone Sleeve Gastrectomy: A Retrospective Study. Nutrients. 2023; 15(17):3858. https://doi.org/10.3390/nu15173858
Chicago/Turabian StyleLiao, Wan-Chun, Tsae-Jyy Wang, Chieh-Yu Liu, Tsang-Pai Liu, Shu-Yuan Liang, and Ko-Shih Chang. 2023. "Risk of Malnutrition in Adults Who Have Undergone Sleeve Gastrectomy: A Retrospective Study" Nutrients 15, no. 17: 3858. https://doi.org/10.3390/nu15173858
APA StyleLiao, W.-C., Wang, T.-J., Liu, C.-Y., Liu, T.-P., Liang, S.-Y., & Chang, K.-S. (2023). Risk of Malnutrition in Adults Who Have Undergone Sleeve Gastrectomy: A Retrospective Study. Nutrients, 15(17), 3858. https://doi.org/10.3390/nu15173858





