Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Study Design
2.3. Diet Formula Adjustment Policy
2.4. Measurements
2.5. BIA Measurements
2.6. Statistics
3. Results
3.1. Patients Grouped by Intervention or Nutritional Risk
3.1.1. Baseline Characteristics
3.1.2. Primary Outcomes
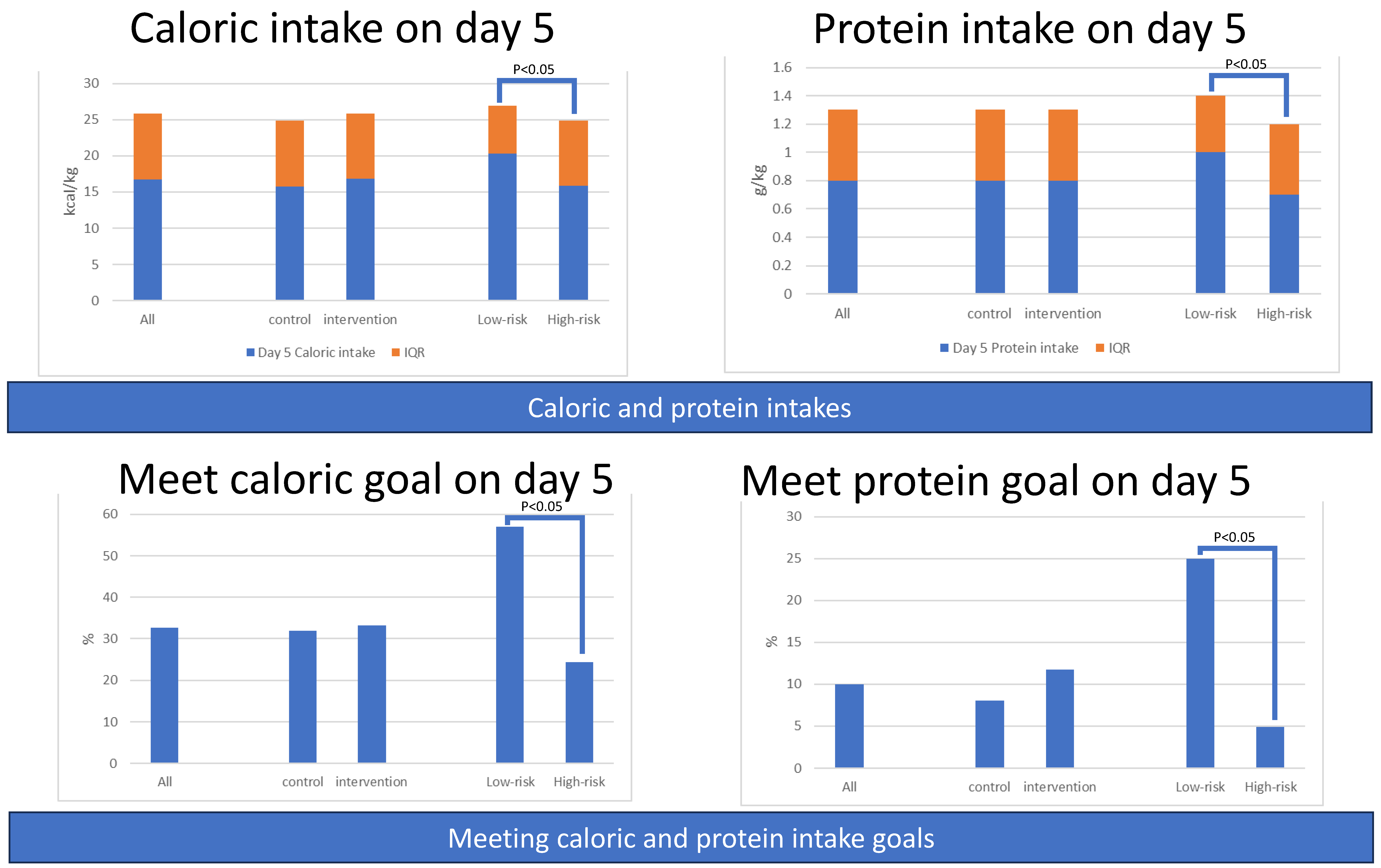
3.1.3. Secondary Outcomes: Amounts of Caloric and Protein Intake
3.1.4. Secondary Outcomes: Percentage of Patients Meeting Caloric or Protein Goals
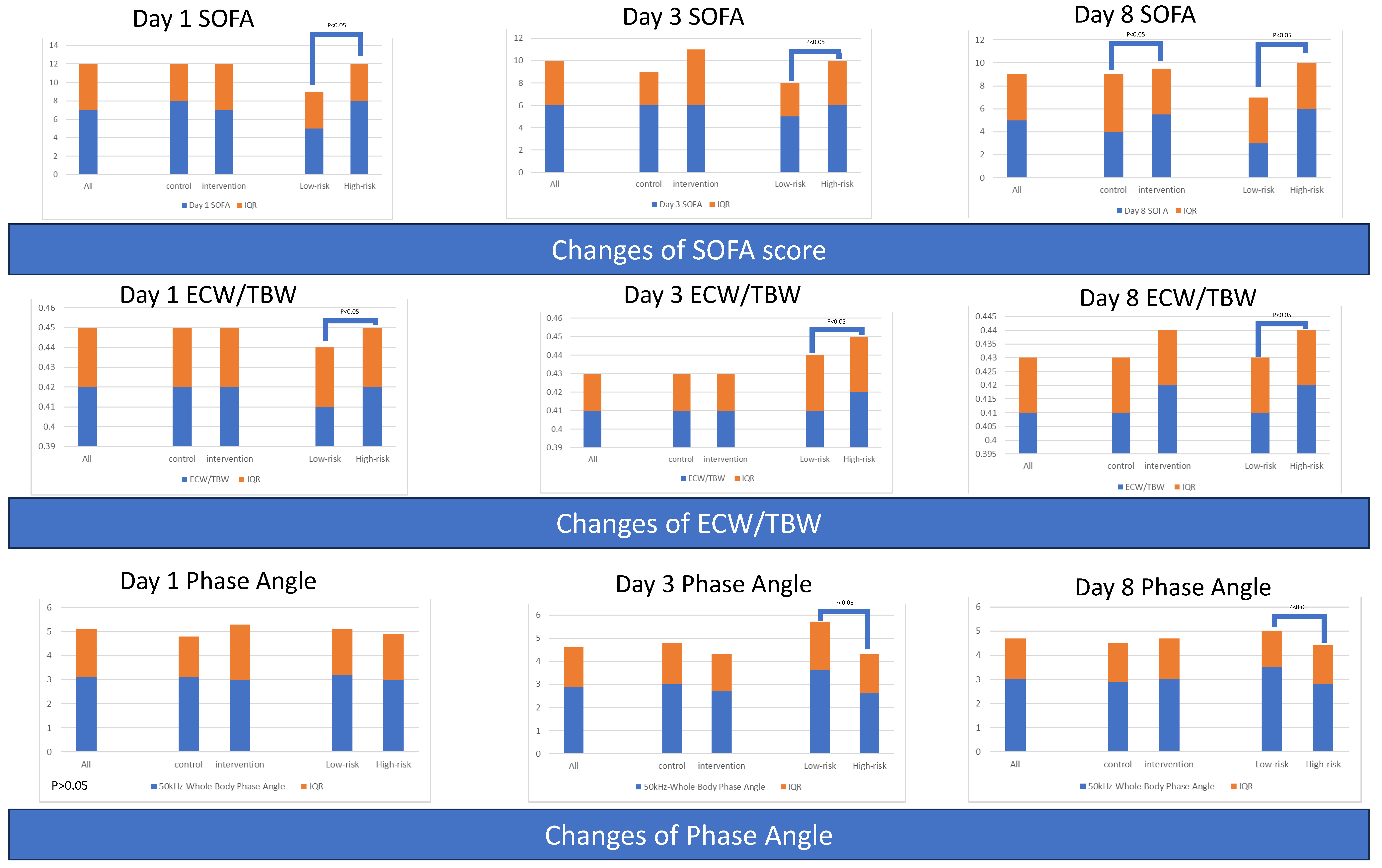
3.1.5. Serial Severity Score for All Patients and Grouped by Intervention or Risk
3.1.6. Serial Body Composition Variables for All Patients and Grouped by Intervention or Risk
3.2. Grouped by Intervention and Risk
3.2.1. Baseline Characteristics of Four Groups
3.2.2. Primary Outcomes: Length of Stay and Mortality among Four Groups
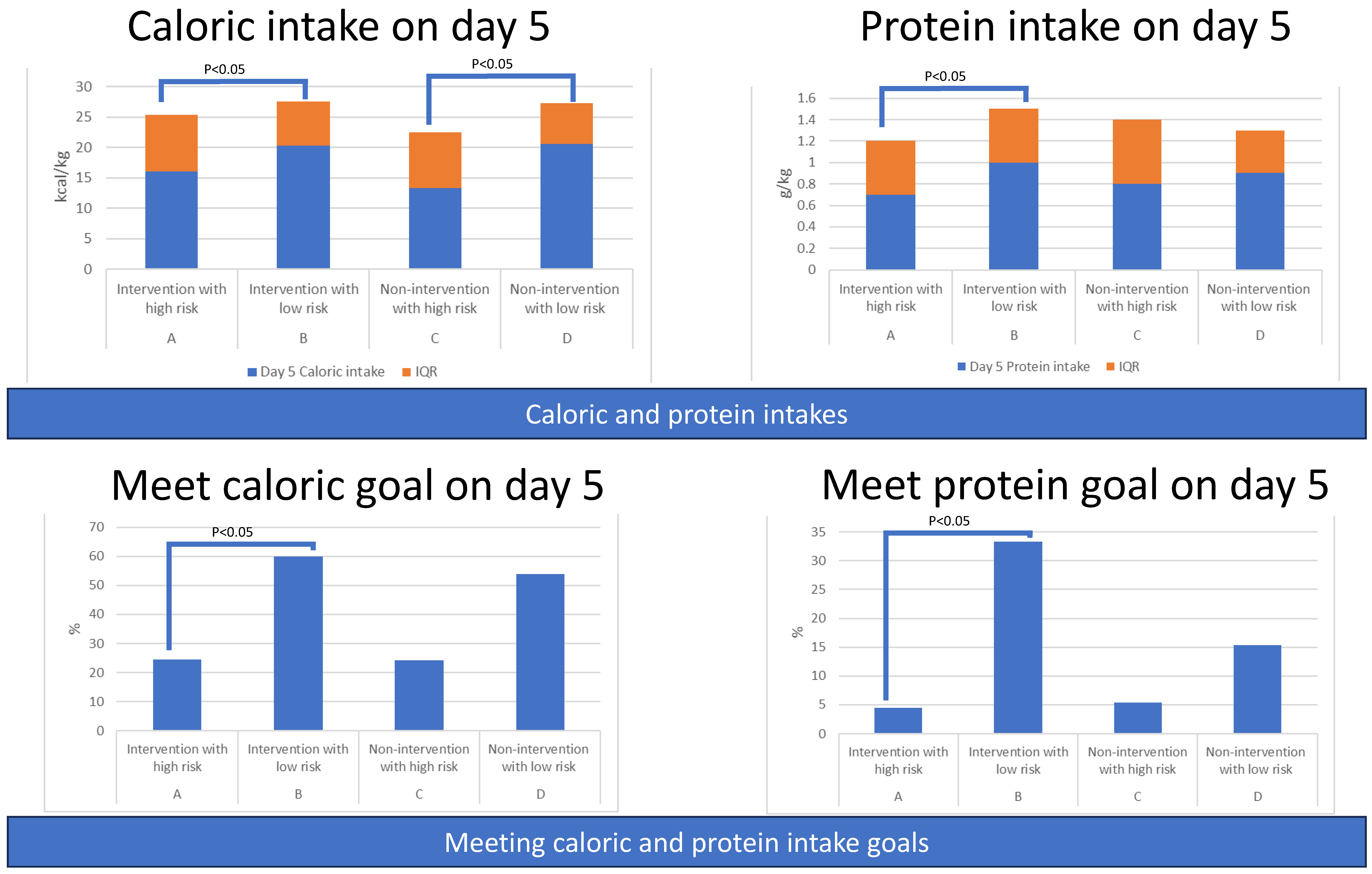
3.2.3. Secondary Outcomes: Amount of Caloric and Protein Intake among Four Groups
3.2.4. Secondary Outcomes: Percentage of Patients Meeting Caloric or Protein Goals among Four Groups
3.2.5. Serial Severity Scores among Four Groups
3.2.6. Serial Body Composition Variables among Four Groups
3.3. Items of Statistically Significant Differences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, A.; Ling, L.; Qin, H.; Arabi, Y.M.; Myatra, S.N.; Egi, M.; Kim, J.H.; Mat Nor, M.B.; Son, D.N.; Fang, W.F.; et al. Epidemiology, Management, and Outcomes of Sepsis in ICUs among Countries of Differing National Wealth across Asia. Am. J. Respir. Crit. Care Med. 2022, 206, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Manga, G.; Calin, G.A.; Manuc, M.; Droc, G.; Tudor, S. New Definitions of Sepsis and the Quest for Specific Biomarkers. Are the miRNAs the Answer? Chirurgia 2018, 113, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.F.; Douglas, I.S.; Chen, Y.M.; Lin, C.Y.; Kao, H.C.; Fang, Y.T.; Huang, C.H.; Chang, Y.T.; Huang, K.T.; Wang, Y.H.; et al. Development and validation of immune dysfunction score to predict 28-day mortality of sepsis patients. PLoS ONE 2017, 12, e0187088. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.F.; Chen, Y.M.; Lin, C.Y.; Huang, K.T.; Kao, H.C.; Fang, Y.T.; Huang, C.H.; Chang, Y.T.; Wang, Y.H.; Wang, C.C.; et al. Immune profiles and clinical outcomes between sepsis patients with or without active cancer requiring admission to intensive care units. PLoS ONE 2017, 12, e0179749. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.Y.; Chen, Y.M.; Wang, C.C.; Wang, Y.H.; Lin, C.Y.; Chang, Y.T.; Huang, K.T.; Lin, M.C.; Fang, W.F. Insufficient Nutrition and Mortality Risk in Septic Patients Admitted to ICU with a Focus on Immune Dysfunction. Nutrients 2019, 11, 367. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Caiaffa, W.T.; Correia, M.I. Hospital malnutrition: The Brazilian national survey (IBRANUTRI): A study of 4000 patients. Nutrition 2001, 17, 573–580. [Google Scholar] [CrossRef]
- Correia, M.I.; Campos, A.C.; Study, E.C. Prevalence of hospital malnutrition in Latin America: The multicenter ELAN study. Nutrition 2003, 19, 823–825. [Google Scholar] [CrossRef]
- Kamath, S.K.; Lawler, M.; Smith, A.E.; Kalat, T.; Olson, R. Hospital malnutrition: A 33-hospital screening study. J. Am. Diet. Assoc. 1986, 86, 203–206. [Google Scholar] [CrossRef]
- O’Flynn, J.; Peake, H.; Hickson, M.; Foster, D.; Frost, G. The prevalence of malnutrition in hospitals can be reduced: Results from three consecutive cross-sectional studies. Clin. Nutr. 2005, 24, 1078–1088. [Google Scholar] [CrossRef]
- Artinian, V.; Krayem, H.; DiGiovine, B. Effects of early enteral feeding on the outcome of critically ill mechanically ventilated medical patients. Chest 2006, 129, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Hooper, M.H. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Aldawood, A.S.; Haddad, S.H.; Al-Dorzi, H.M.; Tamim, H.M.; Jones, G.; Mehta, S.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N. Engl. J. Med. 2015, 372, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Y.M.; Aldawood, A.S.; Al-Dorzi, H.M.; Tamim, H.M.; Haddad, S.H.; Jones, G.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; Sadat, M.; et al. Permissive Underfeeding or Standard Enteral Feeding in High- and Low-Nutritional-Risk Critically Ill Adults. Post Hoc Analysis of the PermiT Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Paixão, E.M.S.; Gonzalez, M.C.; Nakano, E.Y.; Ito, M.K.; Pizato, N. Weight loss, phase angle, and survival in cancer patients undergoing radiotherapy: A prospective study with 10-year follow-up. Eur. J. Clin. Nutr. 2021, 75, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Karimi, E.; Vingrys, K.; Shirani, F. Is phase angle a valuable prognostic tool in cancer patients’ survival? A systematic review and meta-analysis of available literature. Clin. Nutr. 2021, 40, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Bear, D.E.; Berger, M.M.; De Waele, E.; Gunst, J.; McClave, S.A.; Prado, C.M.; Puthucheary, Z.; Ridley, E.J.; Van den Berghe, G.; et al. Personalized nutrition therapy in critical care: 10 expert recommendations. Crit. Care 2023, 27, 261. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Phua, J.; Koh, Y.; Du, B.; Faruq, M.O.; Nishimura, M.; Fang, W.F.; Gomersall, C.; Al Rahma, H.N.; Tamim, H.; et al. Structure, Organization, and Delivery of Critical Care in Asian ICUs. Crit. Care Med. 2016, 44, e940–e948. [Google Scholar] [CrossRef]
- See, K.C.; Zhao, M.Y.; Nakataki, E.; Chittawatanarat, K.; Fang, W.F.; Faruq, M.O.; Wahjuprajitno, B.; Arabi, Y.M.; Wong, W.T.; Divatia, J.V.; et al. Professional burnout among physicians and nurses in Asian intensive care units: A multinational survey. Intensive Care Med. 2018, 44, 2079–2090. [Google Scholar] [CrossRef]
- Tseng, C.C.; Tu, C.Y.; Chen, C.H.; Wang, Y.T.; Chen, W.C.; Fu, P.K.; Chen, C.M.; Lai, C.C.; Kuo, L.K.; Ku, S.C.; et al. Significance of the Modified NUTRIC Score for Predicting Clinical Outcomes in Patients with Severe Community-Acquired Pneumonia. Nutrients 2021, 14, 198. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Wang, Y.H.; Chen, Y.M.; Hung, K.Y.; Chang, Y.C.; Fang, Y.T.; Chang, Y.T.; Chen, H.C.; Huang, K.T.; Chang, H.C.; et al. Dynamic monitoring of kidney injury status over 3 days in the intensive care unit as a sepsis phenotype associated with hospital mortality and hyperinflammation. Biomed. J. 2021, 45, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Han, M.; Song, Z.; Kuang, L. Stress ulcer prophylaxis in critically ill adult patients with sepsis at risk of gastrointestinal bleeding: A retrospective cohort study. Intern. Med. J. 2023, 53, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Kyriazopoulou, E.; Tsangaris, I.; Routsi, C.; Vincent, J.L.; Giamarellos-Bourboulis, E.J. The early change of SOFA score as a prognostic marker of 28-day sepsis mortality: Analysis through a derivation and a validation cohort. Crit. Care 2019, 23, 387. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.C.; Tseng, C.H.; Chien, Y.C.; Sheu, C.C.; Tsai, M.J.; Fang, W.F.; Chen, Y.M.; Kao, K.C.; Hu, H.C.; Perng, W.C.; et al. Association of day 4 cumulative fluid balance with mortality in critically ill patients with influenza: A multicenter retrospective cohort study in Taiwan. PLoS ONE 2018, 13, e0190952. [Google Scholar] [CrossRef]
- Ko, S.J.; Cho, J.; Choi, S.M.; Park, Y.S.; Lee, C.H.; Lee, S.M.; Yoo, C.G.; Kim, Y.W.; Lee, J. Phase Angle and Frailty Are Important Prognostic Factors in Critically Ill Medical Patients: A Prospective Cohort Study. J. Nutr. Health Aging 2021, 25, 218–223. [Google Scholar] [CrossRef]
- Puthucheary, Z.A.; Rawal, J.; McPhail, M.; Connolly, B.; Ratnayake, G.; Chan, P.; Hopkinson, N.S.; Phadke, R.; Dew, T.; Sidhu, P.S.; et al. Acute skeletal muscle wasting in critical illness. JAMA 2013, 310, 1591–1600. [Google Scholar] [CrossRef]
- Casey, P.; Alasmar, M.; McLaughlin, J.; Ang, Y.; McPhee, J.; Heire, P.; Sultan, J. The current use of ultrasound to measure skeletal muscle and its ability to predict clinical outcomes: A systematic review. J. Cachexia Sarcopenia Muscle 2022, 13, 2298–2309. [Google Scholar] [CrossRef]
- Phua, J.; Faruq, M.O.; Kulkarni, A.P.; Redjeki, I.S.; Detleuxay, K.; Mendsaikhan, N.; Sann, K.K.; Shrestha, B.R.; Hashmi, M.; Palo, J.E.M.; et al. Critical Care Bed Capacity in Asian Countries and Regions. Crit. Care Med. 2020, 48, 654–662. [Google Scholar] [CrossRef]



| All | Control | Intervention | Low-Risk | High-Risk | |||
|---|---|---|---|---|---|---|---|
| N = 132 | N = 63 | N = 69 | p † | N = 39 | N = 93 | p † | |
| Demographic characteristics, mean (SD) or median (IQR) | |||||||
| Age (years) | 71.0 (14.7) | 69.2 (11.9) | 72.6 (12.0) | 0.101 | 63.7 (10.0) | 74.1 (11.5) | 0.000 |
| Gender(female), n (%) | 57 (5.50) | 28.0 (44.4) | 29.0 (42.0) | 0.780 | 14.0 (35.9) | 43.0 (46.2) | 0.327 |
| Body weight (kg) | 60.5 (7.0) | 60.9 (15.0) | 60.2 (14.5) | 0.763 | 61.2 (14.9) | 60.2 (14.7) | 0.720 |
| BMI (kg/m2) | 23.3 (44.40) | 23.2 (5.9) | 23.3 (5.2) | 0.932 | 22.9 (5.5) | 23.4 (5.6) | 0.619 |
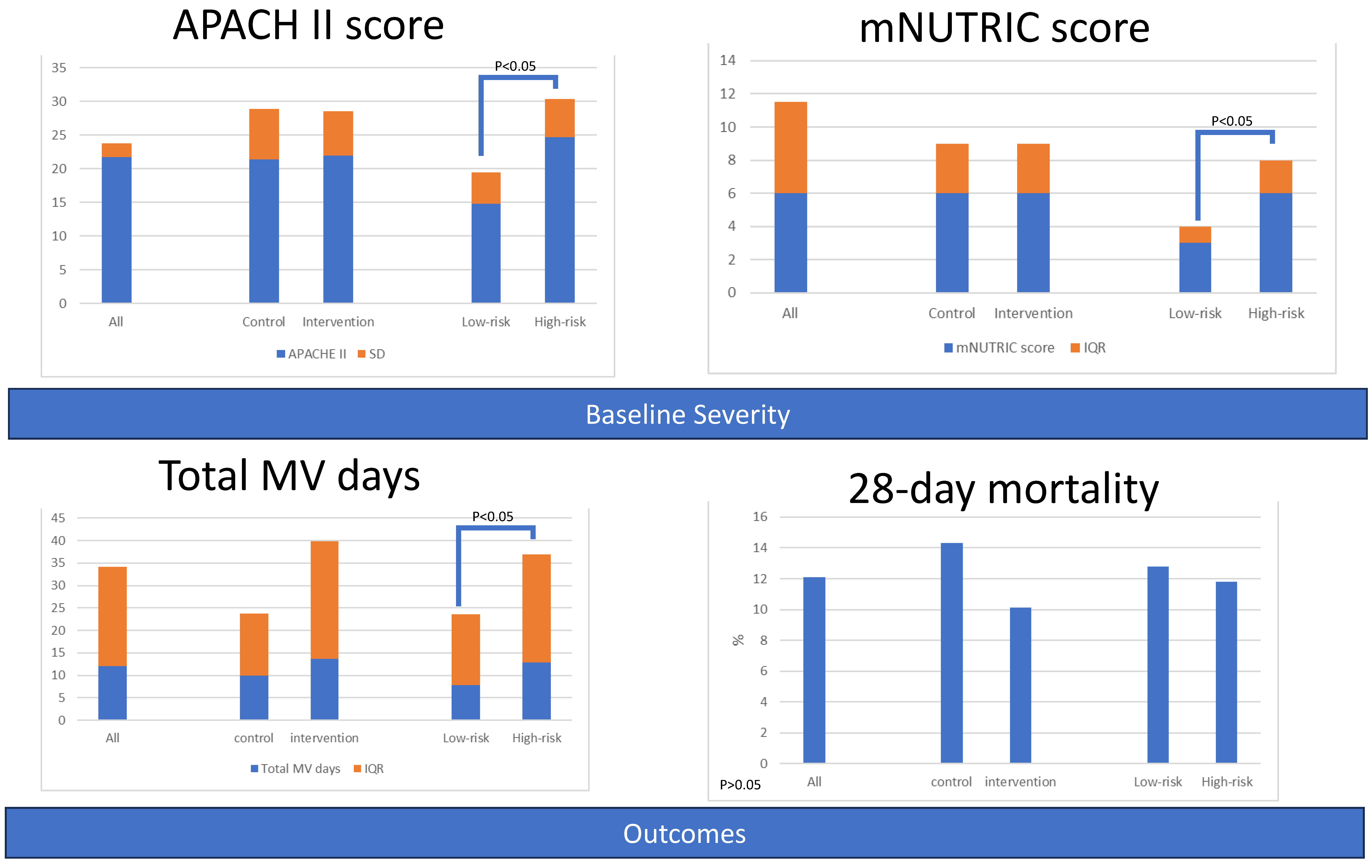
| APACHE II | 21.8 (2.0) | 21.4 (7.5) | 22.0 (6.6) | 0.636 | 14.8 (4.7) | 24.7 (5.7) | 0.000 |
| PSI score | 133.9 (3.0) | 133.4 (47.7) | 134.4 (41.6) | 0.902 | 108.3 (31.9) | 144.7 (44.7) | 0.000 |
| CURB-65 score | 2.0 (3.0) | 2.0 (1.0) | 2.0 (2.0) | 0.149 | 1.0 (1.0) | 2.0 (2.0) | 0.000 |
| Charlson comorbidity index | 5.0 (14.7) | 5.0 (4.0) | 5.0 (3.0) | 0.804 | 4.0 (2.0) | 6.0 (4.0) | 0.000 |
| mNUTRIC score | 6.0 (5.5) | 6 (3.0) | 6.0 (3.0) | 0.776 | 3.0 (1.0) | 6.0 (2.0) | 0.000 |
| Site of suspected infection, n (%) | |||||||
| Lung | 109 (82.6) | 53 (84.1) | 56 (81.2) | 0.655 | 27 (69.2) | 82 (88.2) | 0.009 |
| Intra-abdomen | 6 (4.5) | 2 (3.2) | 4 (5.8) | 0.472 | 1 (2.6) | 5 (5.4) | 0.481 |
| UTI | 93 (70.5) | 40. (63.5) | 53 (76.8) | 0.095 | 26 (66.7) | 67 (72.0) | 0.538 |
| Bacteremia | 71 (53.8) | 31 (49.2) | 40 (58.0) | 0.315 | 23 (59.0) | 48 (51.6) | 0.441 |
| Others | 5 (3.8) | 4 (6.3) | 1 (1.4) | 0.142 | 3 (7.7) | 2 (2.2) | 0.130 |
| Comorbidities, n (%) | |||||||
| Coronary artery disease, | 23 (17.4) | 11 (17.5) | 12 (17.4) | 0.992 | 4 (10.3) | 19 (20.4) | 0.161 |
| Hypertension | 76 (57.6) | 36 (57.1) | 40 (58.0) | 0.924 | 17 (43.6) | 59 (63.4) | 0.036 |
| COPD | 15 (11.4) | 9 (14.3) | 6 (8.7) | 0.314 | 3 (7.7) | 12 (12.9) | 0.391 |
| Cancer | 44 (33.3) | 26 (41.3) | 18 (26.1) | 0.066 | 13 (33.3) | 31 (33.3) | 1.000 |
| Liver cirrhosis | 6 (4.5) | 3 (4.8) | 3 (4.3) | 0.910 | 1 (2.6) | 5 (5.4) | 0.481 |
| Diabetes mellitus | 65 (49.2) | 29 (46.0) | 36 (52.2) | 0.482 | 13 (33.3) | 52 (55.9) | 0.018 |
| CKD | 43 (32.6) | 22 (34.9) | 21 (30.4) | 0.584 | 7 (17.9) | 36 (38.7) | 0.021 |
| All | Control | Intervention | Low-Risk | High-Risk | |||
|---|---|---|---|---|---|---|---|
| N = 132 | N = 63 | N = 69 | p † | N = 39 | N = 93 | p † | |
| Length of stay, median (IQR) | |||||||
| ICU Days | 9.0 (8.4) | 7.8 (10.1) | 10.0 (8.8) | 0.101 | 6.6 (9.6) | 10.0 (7.8) | 0.024 |
| Hospital days | 26.6 (31.0) | 24.4 (29.0) | 29.0 (33.5) | 0.390 | 21.6 (24.6) | 29.6 (30.4) | 0.176 |
| Total MV days | 12.0 (22.2) | 9.9 (13.8) | 13.7 (26.1) | 0.073 | 7.9 (15.7) | 12.9 (24.1) | 0.021 |
| mortality, n (%) | |||||||
| ICU Mortality, | 15 (11.4) | 7 (11.1) | 8 (11.6) | 0.931 | 4 (10.3) | 11 (11.8) | 0.832 |
| Hospital Mortality | 27 (20.5) | 13 (20.6) | 14 (20.3) | 0.961 | 7 (17.9) | 20 (21.5) | 0.693 |
| 7-day Mortality | 5 (3.8) | 4 (6.3) | 1 (1.4) | 0.142 | 1 (2.6) | 4 (4.3) | 0.652 |
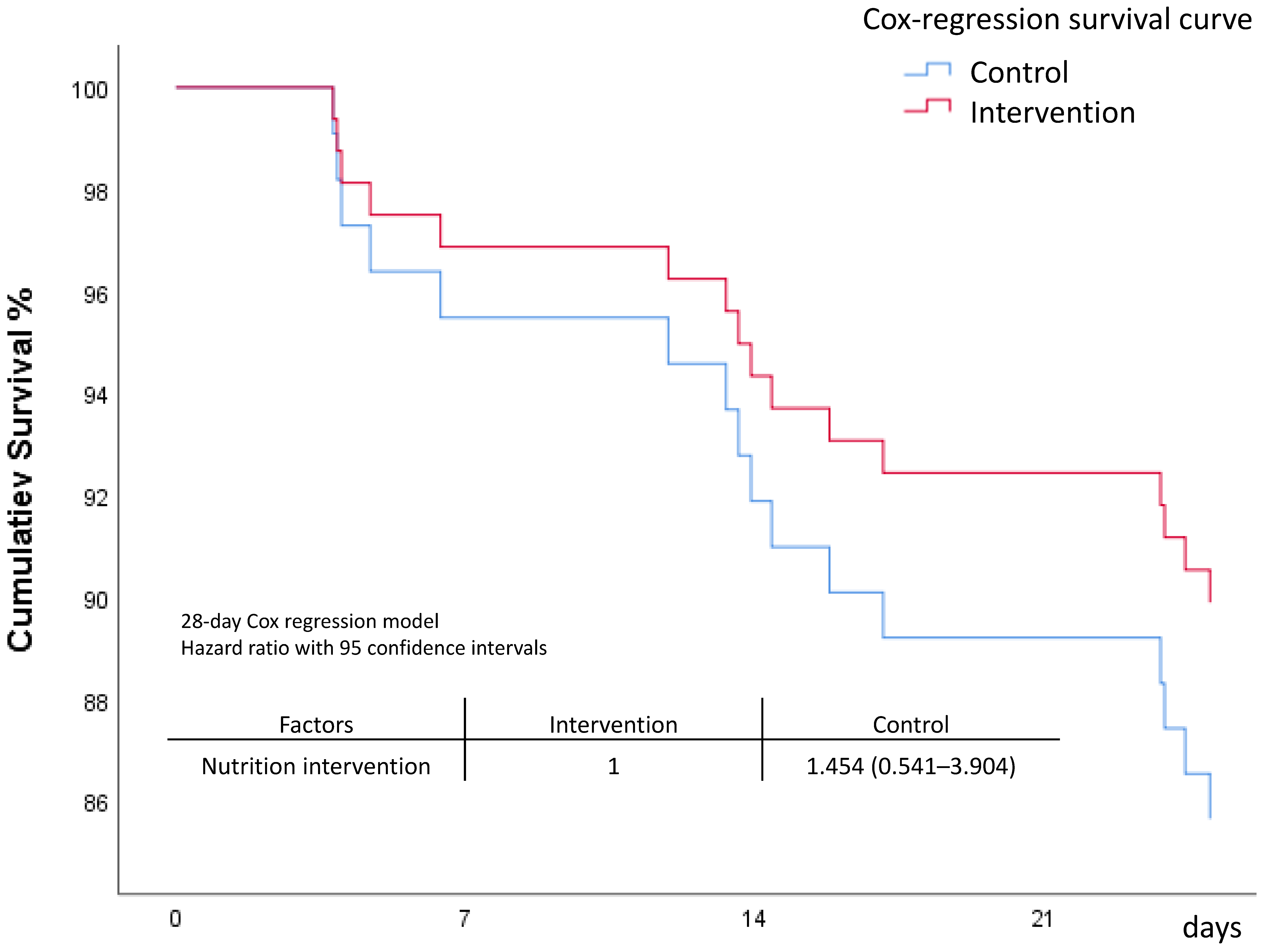
| 28-day Mortality | 16 (12.1) | 9 (14.3) | 7 (10.1) | 0.468 | 5 (12.8) | 11 (11.8) | 0.834 |
| All | Control | Intervention | Low-Risk | High-Risk | |||
|---|---|---|---|---|---|---|---|
| N = 132 | N = 63 | N = 69 | p † | N = 39 | N = 93 | p † | |
| Serial severity scores, median (IQR) | |||||||
| Day 1 SOFA | 7 (5) | 8 (4) | 7 (5) | 0.711 | 5 (4) | 8 (4) | 0.000 |
| Day 3 SOFA | 6 (4) | 6 (3) | 6 (5) | 0.474 | 5 (3) | 6 (4) | 0.007 |
| Day 8 SOFA | 5 (4) | 4 (5) | 5.5 (4) | 0.014 | 3 (4) | 6 (4) | 0.000 |
| Difference of severity score (day 3 value minus day 1 value), median (IQR) | |||||||
| ΔSOFA | −2 (3) | −2 (4) | −2.0 (4) | 0.057 | −1.5 (5) | −2 (3) | 0.030 |
| All | Control | Intervention | Low-Risk | High-Risk | |||
|---|---|---|---|---|---|---|---|
| N = 132 | N = 63 | N = 69 | p † | N = 39 | N = 93 | p † | |
| Day 1, median (IQR) | |||||||
| Total Body Water (kg) | 29.8 (8.7) | 30.9 (8.9) | 29.6 (8.7) | 0.099 | 30.2 (7.9) | 29.8 (8.2) | 0.826 |
| Intracellular Water (kg) | 17.8 (5.0) | 19.0 (5.3) | 17.6 (5.2) | 0.087 | 17.8 (4.9) | 17.9 (5.0) | 0.998 |
| Extracellular Water (kg) | 12.2 (3.8) | 12.7 (4.2) | 11.9 (3.6) | 0.107 | 12.3 (3.6) | 11.7 (4.0) | 0.673 |
| Body Fat Mass (kg) | 17.2 (3.6) | 15.6 (12.2) | 19.1 (14.7) | 0.089 | 16.6 (12.9) | 17.9 (14.2) | 0.965 |
| Soft Lean Mass (kg) | 38.4 (10.6) | 39.6 (11.4) | 37.6 (11.1) | 0.082 | 38.4 (10.1) | 38.4 (10.4) | 0.850 |
| Skeletal Muscle Mass (kg) | 21.3 (6.5) | 22.7 (7.0) | 21.0 (6.8) | 0.081 | 21.2 (6.4) | 21.3 (6.4) | 0.976 |
| ECW/TBW | 0.42 (0.03) | 0.42 (0.03) | 0.42 (0.03) | 0.516 | 0.41 (0.03) | 0.42 (0.03) | 0.042 |
| 50 kHz-Whole Body Phase Angle | 3.1 (2.0) | 3.1 (1.7) | 3.0 (2.3) | 0.720 | 3.2 (1.9) | 3.0 (1.9) | 0.075 |
| Skeletal Muscle Index (kg/m2) | 6.1 (2.2) | 6.4 (2.4) | 5.8 (1.9) | 0.286 | 5.7 (1.6) | 6.3 (2.2) | 0.228 |
| Day 3, median (IQR) | |||||||
| Total Body Water (kg) | 30.6 (9.2) | 30.4 (9.2) | 30.6 (8.4) | 0.852 | 31.0 (9.6) | 30.4 (8.8) | 0.677 |
| Intracellular Water (kg) | 17.9 (5.0) | 18.0 (5.7) | 17.8 (4.8) | 0.650 | 18.6 (5.7) | 17.7 (4.8) | 0.517 |
| Extracellular Water (kg) | 12.6 (3.5) | 12.2 (3.6) | 12.8 (3.4) | 0.777 | 13.0 (4.1) | 12.3 (3.3) | 0.919 |
| Body Fat Mass (kg) | 16.7 (14.5) | 15.2 (13.9) | 18.1 (14.2) | 0.265 | 16.9 (13.8) | 16.5 (14.7) | 0.721 |
| Soft Lean Mass (kg) | 38.9 (10.9) | 38.9 (11.9) | 38.8 (10.3) | 0.785 | 40.7 (11.9) | 38.7 (10.7) | 0.604 |
| Skeletal Muscle Mass (kg) | 21.3 (6.6) | 21.4 (7.4) | 21.2 (6.3) | 0.656 | 22.2 (7.3) | 21.1 (6.2) | 0.523 |
| ECW/TBW | 0.41 (0.02) | 0.41 (0.02) | 0.41 (0.02) | 0.505 | 0.41 (0.03) | 0.42 (0.03) | 0.006 |
| 50 kHz-Whole Body Phase Angle | 2.9 (1.7) | 3.0 (1.8) | 2.7 (1.6) | 0.185 | 3.6 (2.1) | 2.6(1.7) | 0.002 |
| Skeletal Muscle Index (kg/m2) | 6.1 (1.7) | 6.1 (1.9) | 6.0 (1.7) | 0.959 | 5.8 (1.9) | 6.1 (1.7) | 0.721 |
| Day 8, median (IQR) | |||||||
| Total Body Water (kg) | 29.7 (8.4) | 29.8 (8.8) | 29.7 (8.5) | 0.863 | 30.2 (10.8) | 29.0 (8.1) | 0.800 |
| Intracellular Water (kg) | 17.6 (4.9) | 17.9 (5.1) | 17.4 (4.6) | 0.628 | 17.7 (6.4) | 17.6 (4.8) | 0.718 |
| Extracellular Water (kg) | 12.1 (3.9) | 12.1 (3.7) | 12.4 (4.1) | 0.901 | 12.3 (4.7) | 12.2 (3.9) | 0.975 |
| Body Fat Mass (kg) | 19.5 (13.6) | 18.3 (14.4) | 20.6 (12.0) | 0.175 | 16.1 (13.9) | 20.6 (13.6) | 0.379 |
| Soft Lean Mass (kg) | 37.7 (11.0) | 37.9 (11.2) | 37.7 (10.8) | 0.828 | 38.5 (13.9) | 36.9 (10.3) | 0.797 |
| Skeletal Muscle Mass (kg) | 20.9 (6.4) | 21.3 (6.6) | 20.7 (6.0) | 0.646 | 21.1 (8.3) | 20.9 (6.2) | 0.712 |
| ECW/TBW | 0.41 (0.02) | 0.41 (0.02) | 0.42 (0.02) | 0.309 | 0.41 (0.02) | 0.42 (0.02) | 0.028 |
| 50 kHz-Whole Body Phase Angle | 3.0 (1.7) | 2.9 (1.6) | 3.0 (1.7) | 0.276 | 3.5 (1.5) | 2.8 (1.6) | 0.015 |
| Skeletal Muscle Index (kg/m2) | 5.9 (1.7) | 6.1 (1.8) | 5.8 (1.8) | 0.613 | 5.9 (2.0) | 5.9 (1.7) | 0.721 |
| Difference of body composition variable (day 3 value minus day 1 value), median (IQR) | |||||||
| Total Body Water (kg) | −0.50 (4.85) | −1.05 (4.23) | 0.10 (5.40) | 0.222 | −0.35 (3.88) | −0.50 (5.43) | 0.985 |
| Intracellular Water (kg) | −0.50 (2.90) | −0.65 (2.63) | −0.20 (3.00) | 0.428 | −0.45 (1.98) | −0.50 (3.10) | 0.730 |
| Extracellular Water (kg) | 0.00 (2.38) | −0.30 (2.20) | 0.30 (2.53) | 0.218 | −0.20 (1.78) | 0.05 (2.58) | 0.615 |
| Body Fat Mass (kg) | 1.50 (4.93) | 1.70 (4.43) | 1.00 (6.05) | 0.234 | −1.00 (4.38) | 1.85 (5.32) | 0.055 |
| Soft Lean Mass (kg) | −0.60 (6.13) | −1.30 (5.93) | −0.25 (6.55) | 0.231 | −0.55 (4.85) | −0.65 (6.65) | 0.897 |
| Skeletal Muscle Mass (kg) | −0.60 (3.80) | −0.80 (3.45) | −0.30 (4.00) | 0.405 | −0.55 (2.70) | −0.60 (4.10) | 0.706 |
| ECW/TBW | 0.00 (0.01) | 0.00 (0.01) | 0.00 (0.01) | 0.691 | 0.00 (0.01) | 0.00 (0.01) | 0.753 |
| 50 kHz-Whole Body Phase Angle | −0.01 (0.88) | −0.10 (0.60) | −0.10 (1.25) | 0.860 | 0.00 (0.88) | −0.20 (0.98) | 0.317 |
| Skeletal Muscle Index (kg/m2) | −0.01 (1.00) | −0.10 (0.90) | 0.20 (1.03) | 0.193 | 0.05 (0.60) | −0.10 (1.00) | 0.409 |
| Four Groups Category | A | B | C | D | |||
|---|---|---|---|---|---|---|---|
| Intervention with High Risk (n = 49) | Intervention with Low Risk (n = 20) | p † | Non-Intervention with High Risk (n = 44) | Non-Intervention with Low Risk (n = 19) | p † | p * | |
| Demographic characteristics, mean (SD) or median (IQR) | |||||||
| Age (years) | 76.1 (10.8) | 64.0 (10.5) | 0.000 | 71.9 (11.8) | 63.0 (9.7) | 0.023 | 0.000 |
| Gender(female), n (%) | 22 (44.9) | 7 (35.0) | 0.453 | 21 (47.7) | 7 (38.9) | 0.529 | 0.779 |
| Body weight (kg) | 58.1 (13.2) | 65.2 (16.4) | 0.406 | 62.6 (16.0) | 57.1 (12.1) | 1.000 | 0.150 |
| BMI (kg/m2) | 22.9 (4.8) | 24.4 (6.2) | 1.000 | 24.1 (6.4) | 21.4 (4.2) | 0.451 | 0.236 |
| APACHE II | 24.6 (5.2) | 15.8 (5.5) | 0.000 | 24.8 (6.3) | 13.8 (3.6) | 0.000 | 0.000 |
| PSI score | 144.7 (42.9) | 109.3 (25.0) | 0.000 | 144.8 (47.1) | 107.2 (38.5) | 0.008 | 0.000 |
| CURB-65 score | 2.0 (1.0) | 1.0 (2.0) | 0.000 | 2.0 (2.0) | 1.0 (1.0) | 0.008 | 0.000 |
| Charlson comorbidity index | 6.0 (3.0) | 4.0 (3.0) | 0.001 | 6.0 (4.0) | 4.0 (3.0) | 0.030 | 0.002 |
| mNUTRIC score | 6.0 (2.0) | 3.5 (1.0) | 0.000 | 6.0 (2.0) | 3.0 (1.0) | 0.000 | 0.000 |
| Site of suspected infection, n (%) | |||||||
| Lung | 43 (87.8) | 13 (65.0) | 0.029 | 39 (88.6) | 14 (73.7) | 0.139 | 0.062 |
| Intra-abdomen | 3 (6.1) | 1 (5.0) | 0.857 | 2 (4.5) | 0 (0.0) | 0.349 | 0.756 |
| UTI | 38 (77.6) | 15 (75.0) | 0.821 | 29 (65.9) | 11 (59.7) | 0.547 | 0.357 |
| Bacteremia | 26 (53.1) | 14 (70.0) | 0.199 | 22 (50.0) | 9 (47.4) | 0.849 | 0.445 |
| Others | 0 (0.0) | 1 (5.0) | 0.118 | 2 (4.5) | 2 (10.5) | 0.375 | 0.220 |
| Length of stay, median (IQR) | |||||||
| ICU Days | 10.7 (8.2) | 7.3 (10.7) | 0.224 | 9.0 (8.4) | 6.6 (4.7) | 0.065 | 0.054 |
| Hospital days | 29.4 (34.3) | 28.4 (33.9) | 0.526 | 30.8 (31.2) | 18.2 (14.5) | 0.245 | 0.437 |
| Total MV days | 14.0 (23.2) | 9.3 (31.8) | 0.098 | 10.8 (25.6) | 7.6 (14.9) | 0.178 | 0.036 |
| mortality, n (%) | |||||||
| ICU Mortality, | 6 (12.2) | 2 (10.0) | 0.793 | 5 (11.4) | 2 (11.1) | 0.977 | 0.995 |
| Hospital Mortality | 10 (20.4) | 4 (20.0) | 0.970 | 10 (22.7) | 3 (16.7) | 0.598 | 0.961 |
| 7-day Mortality | 1 (2.0) | 0 (0.0) | 0.523 | 3 (6.8) | 1 (5.6) | 0.855 | 0.489 |
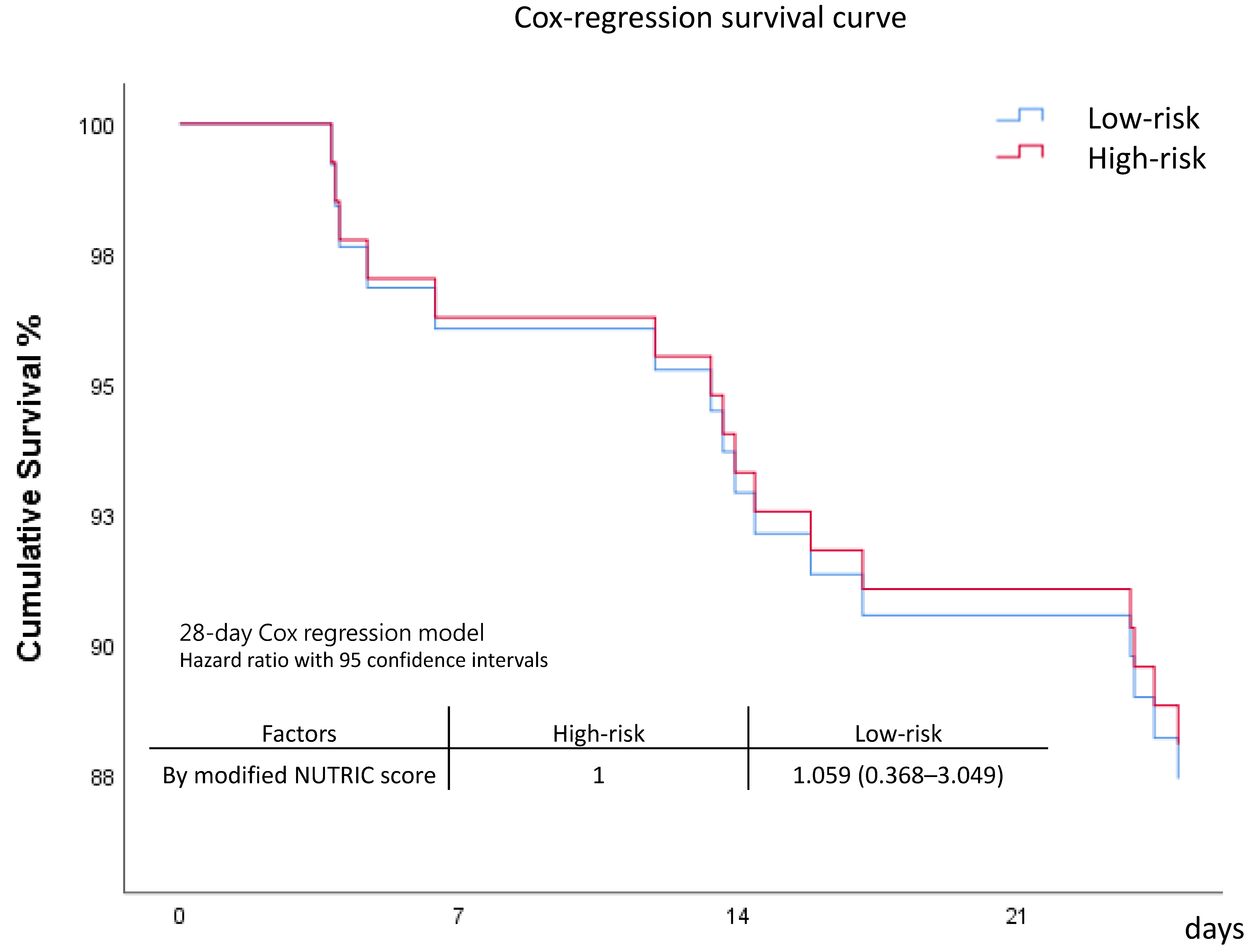
| 28-day Mortality | 5 (10.2) | 2 (10.0) | 0.980 | 6 (13.6) | 3 (16.7) | 0.760 | 0.876 |
| Intervention > Control | Intervention < Control | High-Risk > Low-Risk | Low-Risk > High-Risk | |
|---|---|---|---|---|
| baseline | Age, APACH II, PSI, CURB 65, Charlson comorbidity index, Modified NUTRIC score, Lung infection, HT, Diabetes mellitus, CKD | |||
| outcomes | ICU days Total MV days | Amount of caloric and protein intake (day 1, 2, 3, 4, 5, 6, 8; day 1, 2, 3, 4, 5, 6, 8) Percentage of meeting caloric and protein intake goals (day 1, 2, 3, 4, 5; day 4, 5, 6, 8) | ||
| Serial SOFA | Day 8 SOFA score | Day 1, 3, and 8 SOFA score | ΔSOFA | |
| Body composition variable | Day 1, 3, and 8 ECW/TBW | Day 3 and 8, 50 kHz-Whole Body Phase Angle |
| A > B | B > A | C > D | C < D | |
|---|---|---|---|---|
| baseline | Age, APACH II, PSI, CURB 65, Charlson comorbidity index, Modified NUTRIC score, Lung infection | Age, APACH II, PSI, CURB 65, Charlson comorbidity index, Modified NUTRIC score, Lung infection | Amount of caloric intake (day 5) | |
| outcomes | Amount of caloric and protein intake (day 1, 4, 5, 8; day 1, 4, 5, 6, 7, 8) Percentage of meeting caloric and protein intake goals (day 1, 3, 4, 5; day 3, 4, 5, 6, 8) | |||
| Serial SOFA | Day 1 and 8 SOFA score | Day 1, 3, and 8 SOFA score | ΔSOFA | |
| Body composition variable | ΔBody Fat Mass | Day 3 ECW/TBW | Day 3 50 kHz-Whole Body Phase Angle Δ50 kHz-Whole Body Phase Angle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, K.-Y.; Chen, T.-H.; Lee, Y.-F.; Fang, W.-F. Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study. Nutrients 2023, 15, 3814. https://doi.org/10.3390/nu15173814
Hung K-Y, Chen T-H, Lee Y-F, Fang W-F. Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study. Nutrients. 2023; 15(17):3814. https://doi.org/10.3390/nu15173814
Chicago/Turabian StyleHung, Kai-Yin, Tzu-Hsiu Chen, Ya-Fen Lee, and Wen-Feng Fang. 2023. "Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study" Nutrients 15, no. 17: 3814. https://doi.org/10.3390/nu15173814
APA StyleHung, K.-Y., Chen, T.-H., Lee, Y.-F., & Fang, W.-F. (2023). Using Body Composition Analysis for Improved Nutritional Intervention in Septic Patients: A Prospective Interventional Study. Nutrients, 15(17), 3814. https://doi.org/10.3390/nu15173814