Outcome-Specific Efficacy of Different Probiotic Strains and Mixtures in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
- RCTs that compared the efficacy of probiotics with placebo or different probiotics for IBS;
- Participants were diagnosed with IBS based on Rome I, II, III, or IV criteria, Manning criteria, or physician’s opinion;
- Patients in the test group received single or multistrain probiotics;
- Patients in the control group received placebo or another probiotic;
- RCTs should report at least one of the targeted outcomes (see 2.2 Outcome assessment). Outcomes should be reported as data at baseline and endpoint, or as absolute changes during the study;
- The treatment duration was at least two weeks.
- Open-label trials, single-arm studies, nonrandomized trials, reviews, protocols, and letters. Crossover RCTs that did not report data from the first stage were excluded;
- Duplicate study;
- Studies involving pregnant or lactating mothers, patients with a history of gastrointestinal surgery, and patients aged < 18 years;
- Studies involving patients who received combined treatments, such as synbiotics, antibiotics, antidepressants, and psychological therapy.
2.2. Outcome Assessment
2.3. Data Extraction
2.4. Risk of Bias and Evidence Quality
2.5. Statistical Analysis
2.6. Efficacy Classification
- Level A (among the most effective): probiotics that are significantly superior to placebo and at least one probiotic at Level B;
- Level B: probiotics that are more effective than placebo, but not superior to any other probiotic(s) superior to placebo;
- Level C (among the least effective): probiotics with no significant difference compared with placebo.
3. Results
3.1. Characteristics of Included Studies
3.2. Risk of Bias within Studies
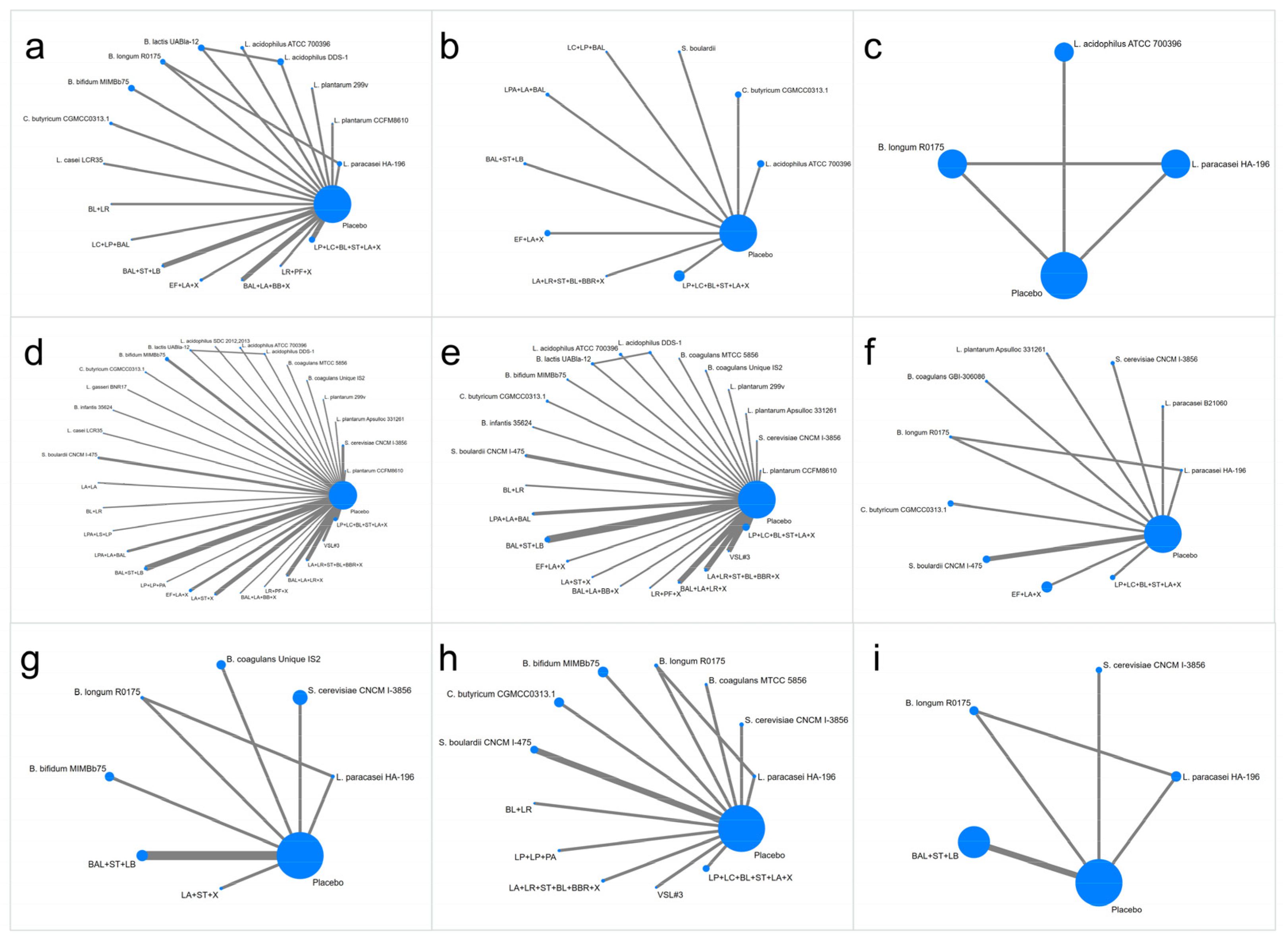
3.3. Critical Results of Network Meta-Analysis
3.3.1. IBS-SSS
3.3.2. IBS-QOL
3.3.3. HADS Score
3.3.4. Abdominal Pain Score
3.3.5. Abdominal Bloating Score
3.3.6. Bowel Movement Frequency (Per Week) in the IBS-D and IBS-C Groups
3.3.7. Bristol Stool form Scale in IBS-D and IBS-C
3.4. Adverse Events
3.5. Heterogeneity and Inconsistency
3.6. Quality of Evidence
3.7. Alteration of Gut Microbiota
4. Discussion
4.1. Key Findings
4.2. Associations with Current Studies
4.3. Study Merits and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Functional Gastrointestinal Disorders 2 Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.S.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Ng, C.E.; Black, C.J.; Ford, A.C. Direct healthcare costs of Rome IV or Rome III-defined irritable bowel syndrome in the United Kingdom. Aliment. Pharmacol. Ther. 2022, 56, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiang, W.; Li, C.Y.; Li, S.C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 2016, 22, 10450–10460. [Google Scholar] [CrossRef]
- Canavan, C.; West, J.; Card, T. Review article: The economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2014, 40, 1023–1034. [Google Scholar] [CrossRef]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef]
- Frandemark, A.; Tornblom, H.; Jakobsson, S.; Simren, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef]
- Drossman, D.A.; Chang, L.; Schneck, S.; Blackman, C.; Norton, W.F.; Norton, N.J. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig. Dis. Sci. 2009, 54, 1532–1541. [Google Scholar] [CrossRef]
- Raskov, H.; Burcharth, J.; Pommergaard, H.-C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes 2016, 7, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Bai, T.; Xu, Z.; Xia, P.; Feng, Y.; Liu, B.; Liu, H.; Chen, Y.; Yan, G.; Lv, B.; Yan, Z.; et al. The Short-Term Efficacy of Bifidobacterium Quadruple Viable Tablet in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: Potentially Mediated by Metabolism Rather Than Diversity Regulation. Am. J. Gastroenterol. 2022, 118, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Konstantis, G.; Efstathiou, S.; Pourzitaki, C.; Kitsikidou, E.; Germanidis, G.; Chourdakis, M. Efficacy and safety of probiotics in the treatment of irritable bowel syndrome: A systematic review and meta-analysis of randomised clinical trials using ROME IV criteria. Clin. Nutr. 2023, 42, 800–809. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Su, G.L.; Ko, C.W.; Bercik, P.; Falck-Ytter, Y.; Sultan, S.; Weizman, A.V.; Morgan, R.L. AGA Clinical Practice Guidelines on the Role of Probiotics in the Management of Gastrointestinal Disorders. Gastroenterology 2020, 159, 697–705. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.Y.; Morris, J.; Whorwell, P.J. The irritable bowel severity scoring system: A simple method of monitoring irritable bowel syndrome and its progress. Aliment. Pharmacol. Ther. 1997, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.L.; Drossman, D.A.; Frederick, I.O.; Dicesare, J.; Puder, K.L. Quality of life in persons with irritable bowel syndrome: Development and validation of a new measure. Dig. Dis. Sci. 1998, 43, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the Quality of Evidence from a Network Meta-Analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [PubMed]
- White, I.R. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef]
- Chaimani, A.; Salanti, G. Visualizing assumptions and results in network meta-analysis: The network graphs package. Stata J. 2015, 15, 905–950. [Google Scholar] [CrossRef]
- Morgan, R.L.; Preidis, G.A.; Kashyap, P.C.; Weizman, A.V.; Sadeghirad, B.; McMaster Probiotic Prebiotic, S. Probiotics Reduce Mortality and Morbidity in Preterm, Low-Birth-Weight Infants: A Systematic Review and Network Meta-analysis of Randomized Trials. Gastroenterology 2020, 159, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Mourey, F.; Decherf, A.; Jeanne, J.-F.; Clement-Ziza, M.; Grisoni, M.-L.; Machuron, F.; Legrain-Raspaud, S.; Bourreille, A.; Desreumaux, P. Saccharomyces cerevisiae I-3856 in irritable bowel syndrome with predominant constipation. World J. Gastroenterol. 2022, 28, 2509–2522. [Google Scholar] [CrossRef]
- Jung, K.; Kim, A.; Lee, J.-H.; Cho, D.; Seo, J.; Jung, E.S.; Kang, H.-j.; Roh, J.; Kim, W. Effect of Oral Intake of Lactiplantibacillus plantarum APsulloc 331261 (GTB1 (TM)) on Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 2015. [Google Scholar] [CrossRef]
- Xu, H.; Ma, C.; Zhao, F.; Chen, P.; Liu, Y.; Sun, Z.; Cui, L.; Kwok, L.-Y.; Zhang, H. Adjunctive treatment with probiotics partially alleviates symptoms and reduces inflammation in patients with irritable bowel syndrome. Eur. J. Nutr. 2021, 60, 2553–2565. [Google Scholar] [CrossRef]
- Skrzydlo-Radomanska, B.; Prozorow-Krol, B.; Cichoz-Lach, H.; Majsiak, E.; Bierla, J.B.; Kanarek, E.; Sowniska, A.; Cukrowska, B. The Effectiveness and Safety of Multi-Strain Probiotic Preparation in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Study. Nutrients 2021, 13, 756. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; Xue, Z.; Zhai, Q.; et al. Lactobacillus plantarum CCFM8610 Alleviates Irritable Bowel Syndrome and Prevents Gut Microbiota Dysbiosis: A Randomized, Double-Blind, Placebo-Controlled, Pilot Clinical Trial. Engineering 2021, 7, 376–385. [Google Scholar] [CrossRef]
- Gupta, A.K.; Maity, C. Efficacy and safety of Bacillus coagulans LBSC in irritable bowel syndrome A prospective, interventional, randomized, double-blind, placebo-controlled clinical study CONSORT Compliant. Medicine 2021, 100, e23641. [Google Scholar] [CrossRef]
- Barraza-Ortiz, D.A.; Perez-Lopez, N.; Medina-Lopez, V.M.; Minero-Alfaro, J.I.; Zamarripa-Dorsey, F.; Fernandez-Martinez, N.d.C.; Llorente-Ramon, A.; Ramos-Aguilar, G.A. Combination of a Probiotic and an Antispasmodic Increases Quality of Life and Reduces Symptoms in Patients with Irritable Bowel Syndrome: A Pilot Study. Dig. Dis. 2021, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sadrin, S.; Sennoune, S.; Gout, B.; Marque, S.; Moreau, J.; Zinoune, K.; Grillasca, J.-P.; Pons, O.; Maixent, J.-M. A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome: A placebo-controlled randomized clinical trial. Dig. Liver Dis. 2020, 52, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Srivastava, S.; Leyer, G.J. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients 2020, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Piano, A.; Bhardwaj, R.; Tompkins, T.A.; Evans, M. Efficacy of Lactobacillus paracasei HA-196 and Bifidobacterium longum R0175 in Alleviating Symptoms of Irritable Bowel Syndrome (IBS): A Randomized, Placebo-Controlled Study. Nutrients 2020, 12, 1159. [Google Scholar] [CrossRef]
- Kim, J.; Cho, K.; Kim, J.S.; Jung, H.C.; Kim, B.; Park, M.S.; Ji, G.E.; Cho, J.-Y.; Hong, K.S. Probiotic treatment induced change of inflammation related metabolites in IBS-D patients/double-blind, randomized, placebo-controlled trial. Food Sci. Biotechnol. 2020, 29, 837–844. [Google Scholar] [CrossRef]
- Gayathri, R.; Aruna, T.; Malar, S.; Shilpa, B.; Dhanasekar, K.R. Efficacy of Saccharomyces cerevisiae CNCM I-3856 as an add-on therapy for irritable bowel syndrome. Int. J. Color. Dis. 2020, 35, 139–145. [Google Scholar] [CrossRef]
- Bonfrate, L.; Di Palo, D.M.; Celano, G.; Albert, A.; Vitellio, P.; De Angelis, M.; Gobbetti, M.; Portincasa, P. Effects of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 in IBS patients. Eur. J. Clin. Investig. 2020, 50, e13201. [Google Scholar] [CrossRef]
- Andresen, V.; Gschossmann, J.; Layer, P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: A multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol. Hepatol. 2020, 5, 658–666. [Google Scholar] [CrossRef]
- Oh, J.H.; Jang, Y.S.; Kang, D.; Chang, D.K.; Min, Y.W. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2019, 11, 2887. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Randomized clinical trial: The effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci. Rep. 2019, 9, 12210. [Google Scholar] [CrossRef] [PubMed]
- Ashtiani, S.Y.; Amery, M. Effect of Multispecies Probiotic Supplementation on Irritable Bowel Syndrome. J. Pharm. Res. Int. 2019, 28, 1–9. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Li, M.; Li, Y.-Y.; Li, L.-X.; Zhai, W.-Z.; Wang, P.; Yang, X.-X.; Gu, X.; Song, L.-J.; Li, Z.; et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. Sci. Rep. 2018, 8, 2964. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.P.; Choi, Y.M.; Kim, W.H.; Hong, S.P.; Park, J.-M.; Kim, J.; Kwon, O.; Lee, E.H.; Hahm, K.B. A double blind, placebo-controlled, randomized clinical trial that breast milk derived Lactobacillus gasseri BNR17 mitigated diarrhea-dominant irritable bowel syndrome. J. Clin. Biochem. Nutr. 2018, 62, 179–186. [Google Scholar] [CrossRef]
- Preston, K.; Krumian, R.; Hattner, J.; de Montigny, D.; Stewart, M.; Gaddam, S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: A double-blind, randomised, placebo-controlled study. Benef. Microbes 2018, 9, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, Y.J.; Lee, H.J.; Park, M.Y.; Kwon, O. Effect of Lactobacillus gasseri BNR17 on irritable bowel syndrome: A randomized, double-blind, placebo-controlled, dose-finding trial. Food Sci. Biotechnol. 2018, 27, 853–857. [Google Scholar] [CrossRef]
- Ishaque, S.M.; Khosruzzaman, S.M.; Ahmed, D.S.; Sah, M.P. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult (R)) in the management of diarrhea- predominant irritable bowel syndrome. BMC Gastroenterol. 2018, 18, 71. [Google Scholar] [CrossRef]
- Cremon, C.; Guglielmetti, S.; Gargari, G.; Taverniti, V.; Castellazzi, A.M.; Valsecchi, C.; Tagliacarne, C.; Fiore, W.; Bellini, M.; Bertani, L.; et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: A pilot randomized clinical trial. United Eur. Gastroenterol. J. 2018, 6, 604–613. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.-P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459. [Google Scholar] [CrossRef]
- Nobutani, K.; Sawada, D.; Fujiwara, S.; Kuwano, Y.; Nishida, K.; Nakayama, J.; Kutsumi, H.; Azuma, T.; Rokutan, K. The effects of administration of the Lactobacillus gasseri strain CP2305 on quality of life, clinical symptoms and changes in gene expression in patients with irritable bowel syndrome. J. Appl. Microbiol. 2017, 122, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Hod, K.; Sperber, A.D.; Ron, Y.; Boaz, M.; Dickman, R.; Berliner, S.; Halpern, Z.; Maharshak, N.; Dekel, R. A double-blind, placebo-controlled study to assess the effect of a probiotic mixture on symptoms and inflammatory markers in women with diarrhea-predominant IBS. Neurogastroenterol. Motil. 2017, 29, e13037. [Google Scholar] [CrossRef]
- Thijssen, A.Y.; Clemens, C.H.M.; Vankerckhoven, V.; Goossens, H.; Jonkers, D.M.A.E.; Masclee, A.A.M. Efficacy of Lactobacillus casei Shirota for patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2016, 28, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R.; Pelerin, F.; Decherf, A.C.; Maudet, C.; Housez, B.; Cazaubiel, M.; Justen, P. Randomized double blind placebo-controlled trial of Saccharomyces cerevisiae CNCM I-3856 in irritable bowel syndrome: Improvement in abdominal pain and bloating in those with predominant constipation. United Eur. Gastroenterol. J. 2016, 4, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. BioMed Res. Int. 2016, 2016, 4740907. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: A double blind randomized placebo controlled pilot clinical study. Nutr. J. 2016, 21, 15–25. [Google Scholar] [CrossRef]
- Lyra, A.; Hillila, M.; Huttunen, T.; Mannikko, S.; Taalikka, M.; Tennila, J.; Tarpila, A.; Lahtinen, S.; Ouwehand, A.C.; Veijola, L. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J. Gastroenterol. 2016, 22, 10631–10642. [Google Scholar] [CrossRef]
- Yoon, H.; Park, Y.S.; Lee, D.H.; Seo, J.-G.; Shin, C.M.; Kim, N. Effect of administering a multi-species probiotic mixture on the changes in fecal microbiota and symptoms of irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Clin. Biochem. Nutr. 2015, 57, 129–134. [Google Scholar] [CrossRef]
- Wong, R.K.; Yang, C.; Song, G.-H.; Wong, J.; Ho, K.-Y. Melatonin Regulation as a Possible Mechanism for Probiotic (VSL#3) in Irritable Bowel Syndrome: A Randomized Double-Blinded Placebo Study. Dig. Dis. Sci. 2015, 60, 186–194. [Google Scholar] [CrossRef]
- de Chambrun, G.P.; Neut, C.; Chau, A.; Cazaubiel, M.; Pelerin, F.; Justen, P.; Desreumaux, P. A randomized clinical trial of Saccharomyces cerevisiae versus placebo in the irritable bowel syndrome. Dig. Liver Dis. 2015, 47, 119–124. [Google Scholar] [CrossRef]
- Yoon, J.S.; Sohn, W.; Lee, O.Y.; Lee, S.P.; Lee, K.N.; Jun, D.W.; Lee, H.L.; Yoon, B.C.; Choi, H.S.; Chung, W.-S.; et al. Effect of multispecies probiotics on irritable bowel syndrome: A randomized, double-blind, placebo-controlled trial. J. Gastroenterol. Hepatol. 2014, 29, 52–59. [Google Scholar] [CrossRef]
- Stevenson, C.; Blaauw, R.; Fredericks, E.; Visser, J.; Roux, S. Randomized clinical trial: Effect of Lactobacillus plantarum 299 v on symptoms of irritable bowel syndrome. Nutrition 2014, 30, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Sisson, G.; Ayis, S.; Sherwood, R.A.; Bjarnason, I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome—A 12 week double-blind study. Aliment. Pharmacol. Ther. 2014, 40, 51–62. [Google Scholar] [CrossRef]
- Shavakhi, A.; Minakari, M.; Farzamnia, S.; Peykar, M.S.; Taghipour, G.; Tayebi, A.; Hashemi, H.; Shavakhi, S. The effects of multi-strain probiotic compound on symptoms and quality-of-life in patients with irritable bowel syndrome: A randomized placebo-controlled trial. Adv. Biomed. Res. 2014, 3, 140. [Google Scholar] [CrossRef]
- Ludidi, S.; Jonkers, D.M.; Koning, C.J.; Kruimel, J.W.; Mulder, L.; Van der Vaart, I.B.; Conchillo, J.M.; Masclee, A.A.M. Randomized clinical trial on the effect of a multispecies probiotic on visceroperception in hypersensitive IBS patients. Neurogastroenterol. Motil. 2014, 26, 705–714. [Google Scholar] [CrossRef]
- Lorenzo-Zuniga, V.; Llop, E.; Suarez, C.; Alvarez, B.; Abreu, L.; Espadaler, J.; Serra, J.I. 31, a new combination of probiotics, improves irritable bowel syndrome-related quality of life. World J. Gastroenterol. 2014, 20, 8709–8716. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Vahedi, H.; Merat, S.; Momtahen, S.; Riahi, A. Therapeutic Effects, Tolerability and Safety of a Multi-strain Probiotic in Iranian Adults with Irritable Bowel Syndrome and Bloating. Arch. Iran. Med. 2014, 17, 466–470. [Google Scholar] [PubMed]
- Abbas, Z.; Yakoob, J.; Jafri, W.; Ahmad, Z.; Azam, Z.; Usman, M.W.; Shamim, S.; Islam, M. Cytokine and clinical response to Saccharomyces boulardii therapy in diarrhea-dominant irritable bowel syndrome: A randomized trial. Eur. J. Gastroenterol. Hepatol. 2014, 26, 630–639. [Google Scholar] [CrossRef]
- Roberts, L.M.; McCahon, D.; Holder, R.; Wilson, S.; Hobbs, F.D.R. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013, 13, 45. [Google Scholar] [CrossRef]
- Lee, J.; Rheem, S.; Yun, B.; Ahn, Y.; Joung, J.; Lee, S.J.; Oh, S.; Chun, T.; Rheem, I.; Yea, H.S.; et al. Effects of probiotic yoghurt on symptoms and intestinal microbiota in patients with irritable bowel syndrome. Int. J. Dairy Technol. 2013, 66, 243–255. [Google Scholar] [CrossRef]
- Charbonneau, D.; Gibb, R.D.; Quigley, E.M.M. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes 2013, 4, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Cappello, C.; Tremolaterra, F.; Pascariello, A.; Ciacci, C.; Iovino, P. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): Effects on symptoms, colonic transit and quality of life. Int. J. Color. Dis. 2013, 28, 349–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Begtrup, L.M.; de Muckadell, O.B.S.; Kjeldsen, J.; Christensen, R.d.; Jarbol, D.E. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome—A randomised, double-blind, placebo controlled trial. Scand. J. Gastroenterol. 2013, 48, 1127–1135. [Google Scholar] [CrossRef]
- Amirimani, B.; Nikfam, S.; Albaji, M.; Vahedi, S.; Nasseri-Moghaddam, S.; Sharafkhah, M.; Ansari, R.; Vahedi, H. Probiotic vs. Placebo in Irritable Bowel Syndrome:A Randomized Controlled Trial. Middle East J. Dig. Dis. 2013, 5, 98–102. [Google Scholar] [PubMed]
- Kruis, W.; Chrubasik, S.; Boehm, S.; Stange, C.; Schulze, J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int. J. Color. Dis. 2012, 27, 467–474. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ducrotte, P.; Sawant, P.; Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Dapoigny, M.; Piche, T.; Ducrotte, P.; Lunaud, B.; Cardot, J.-M.; Bernalier-Donadille, A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: A randomized, double-blind study. World J. Gastroenterol. 2012, 18, 2067–2075. [Google Scholar] [CrossRef]
- Cui, S.; Hu, Y. Multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Int. J. Clin. Exp. Med. 2012, 5, 238–244. [Google Scholar]
- Cha, B.K.; Jung, S.M.; Choi, C.H.; Song, I.-D.; Lee, H.W.; Kim, H.J.; Do, J.H.; Chang, S.K.; Kim, K.; Chung, W.-S.; et al. The Effect of a Multispecies Probiotic Mixture on the Symptoms and Fecal Microbiota in Diarrhea-dominant Irritable Bowel Syndrome A Randomized, Double-blind, Placebo-controlled Trial. J. Clin. Gastroenterol. 2012, 46, 220–227. [Google Scholar] [CrossRef]
- Sondergaard, B.; Olsson, J.; Ohlson, K.; Svensson, U.; Bytzer, P.; Ekesbo, R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: A randomized, placebo-controlled trial. Scand. J. Gastroenterol. 2011, 46, 663–672. [Google Scholar] [CrossRef]
- Michail, S.; Kenche, H. Gut Microbiota is Not Modified by Randomized, Double-Blind, Placebo-Controlled Trial of VSL#3 in Diarrhea-Predominant Irritable Bowel Syndrome. Probiotics Antimicrob. Proteins 2011, 3, 1–7. [Google Scholar] [CrossRef]
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—A double-blind, placebo-controlled study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132. [Google Scholar] [CrossRef]
- Choi, C.H.; Jo, S.Y.; Park, H.J.; Chang, S.K.; Byeon, J.-S.; Myung, S.-J. A Randomized, Double-blind, Placebo-controlled Multicenter Trial of Saccharomyces boulardii in Irritable Bowel Syndrome Effect on Quality of Life. J. Clin. Gastroenterol. 2011, 45, 679–683. [Google Scholar] [CrossRef]
- Simren, M.; Ohman, L.; Olsson, J.; Svensson, U.; Ohlson, K.; Posserud, I.; Strid, H. Clinical trial: The effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome—A randomized, double-blind, controlled study. Aliment. Pharmacol. Ther. 2010, 31, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ligaarden, S.C.; Axelsson, L.; Naterstad, K.; Lydersen, S.; Farup, P.G. A candidate probiotic with unfavourable effects in subjects with irritable bowel syndrome: A randomised controlled trial. BMC Gastroenterol. 2010, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Hun, L. Original Research: Bacillus coagulans Significantly Improved Abdominal Pain and Bloating in Patients with IBS. Postgrad. Med. 2009, 121, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.S.; Kang, H.W.; Im, J.P.; Ji, G.E.; Kim, S.G.; Jung, H.C.; Song, I.S.; Kim, J.S. Effect of Probiotics on Symptoms in Korean Adults with Irritable Bowel Syndrome. Gut Liver 2009, 3, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Zimmermann, K.; Menke, G.; Klosterhalfen, S. Randomized Controlled Treatment Trial of Irritable Bowel Syndrome with a Probiotic E.-coli Preparation (DSM17252) Compared to Placebo. Z. Für Gastroenterol. 2009, 47, 209–214. [Google Scholar] [CrossRef]
- Dolin, B.J. Effects of a proprietary bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find. Exp. Clin. Pharmacol. 2009, 31, 655–659. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.Q.; Zuo, X.L.; Zhen, Y.B.; Yang, J.; Liu, C.H. Clinical trial: Effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008, 28, 994–1002. [Google Scholar] [CrossRef]
- Williams, E.A.; Stimpson, J.; Wang, D.; Plummer, S.; Garaiova, I.; Barker, M.E.; Corfe, B.M. Clinical trial: A multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2008, 29, 97–103. [Google Scholar] [CrossRef]
- Sinn, D.H.; Song, J.H.; Kim, H.J.; Lee, J.H.; Son, H.J.; Chang, D.K.; Kim, Y.-H.; Kim, J.J.; Rhee, J.C.; Rhee, P.-L. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig. Dis. Sci. 2008, 53, 2714–2718. [Google Scholar] [CrossRef]
- Kajander, K.; Myllyluoma, E.; Rajilic-Stojanovic, M.; Kyronpalo, S.; Rasmussen, M.; Jarvenpaa, S.; Zoetendal, E.G.; De Vos, W.M.; Vapaatalo, H.; Korpela, R. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment. Pharmacol. Ther. 2008, 27, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Enck, P.; Zimmermann, K.; Menke, G.; Mueller-Lissner, S.; Martens, U.; Klosterhalfen, S. A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome—A randomized controlled trial with primary care physicians. Neurogastroenterol. Motil. 2008, 20, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Drouault-Holowacz, S.; Bieuvelet, S.; Burckel, A.; Cazaubiel, M.; Dray, X.; Marteau, P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol. Clin. Et Biol. 2008, 32, 147–152. [Google Scholar] [CrossRef]
- Andriulli, A.; Neri, M.; Loguercio, C.; Terreni, N.; Merla, A.; Cardarella, M.P.; Federico, A.; Chilovi, F.; Milandri, G.L.; De Bona, M.; et al. Clinical trial on the efficacy of a new symbiotic formulation, Flortec, in patients with irritable bowel syndrome—A multicenter, randomized study. J. Clin. Gastroenterol. 2008, 42, S218–S223. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Houghton, L.A.; Morris, J.; Reilly, B.; Guyonnet, D.; Feuillerat, N.G.; Schlumberger, A.; Jakob, S.; Whorwell, P.J. Clinical trial: The effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2008, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, D.; Chassany, O.; Ducrotte, P.; Picard, C.; Mouret, M.; Mercier, C.H.; Matuchansky, C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: A multicentre, randomized, double-blind, controlled trial. Aliment. Pharmacol. Ther. 2007, 26, 475–486. [Google Scholar] [CrossRef]
- Whorwell, P.J.; Altringer, L.; Morel, J.; Bond, Y.; Charbonneau, D.; O’Mahony, L.; Kiely, B.; Shanahan, F.; Quigley, E.M.M. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am. J. Gastroenterol. 2006, 101, 1581–1590. [Google Scholar] [CrossRef]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.Y.; Chen, K.S.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef]
- Niv, E.; Naftali, T.; Hallak, R.; Vaisman, N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome—A double blind, placebo-controlled, randomized study. Clin. Nutr. 2005, 24, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Roque, M.I.V.; Camilleri, M.; Stephens, D.; Burton, D.D.; Baxter, K.; Thomforde, G.; Zinsmeister, A.R. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol. Motil. 2005, 17, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Kajander, K.; Hatakka, K.; Poussa, T.; Markkila, M.; Korpela, R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: A controlled 6-month intervention. Aliment. Pharmacol. Ther. 2005, 22, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Camilleri, M.; McKinzie, S.; Lempke, M.B.; Burton, D.D.; Thomforde, G.M.; Zinsmeister, A.R. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment. Pharmacol. Ther. 2003, 17, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Niedzielin, K.; Kordecki, H.; Birkenfeld, B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur. J. Gastroenterol. Hepatol. 2001, 13, 1143–1147. [Google Scholar] [CrossRef]
- Nobaek, S.; Johansson, M.L.; Molin, G.; Ahrne, S.; Jeppsson, B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2000, 95, 1231–1238. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- McFarland, L.V.; Karakan, T.; Karatas, A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Eclinicalmedicine 2021, 41, 101154. [Google Scholar] [CrossRef]
- Drossman, D.A. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology 2016, 150, 1262–1279. [Google Scholar] [CrossRef]
- US Department of Health and Human Services Food and Drug Administration Center for Drug Evalution and Research (CDER). Guidance for industry: Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment. 2012. Available online: https://www.fda.gov/media/78622/download (accessed on 15 August 2023).
- Bijkerk, C.J.; de Wit, N.J.; Muris, J.W.M.; Jones, R.H.; Knottnerus, J.A.; Hoes, A.W. Outcome measures in irritable bowel syndrome: Comparison of psychometric and methodological characteristics. Am. J. Gastroenterol. 2003, 98, 122–127. [Google Scholar] [CrossRef]
- Andrae, D.A.; Patrick, D.L.; Drossman, D.A.; Covington, P.S. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual. Life Outcomes 2013, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Midenfjord, I.; Borg, A.; Tornblom, H.; Simren, M. Cumulative Effect of Psychological Alterations on Gastrointestinal Symptom Severity in Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Lembo, A.; Sultan, S.; Chang, L.; Heidelbaugh, J.J.; Smalley, W.; Verne, G.N. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome with Diarrhea. Gastroenterology 2022, 163, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Sultan, S.; Lembo, A.; Verne, G.N.; Smalley, W.; Heidelbaugh, J.J. AGA Clinical Practice Guideline on the Pharmacological Management of Irritable Bowel Syndrome with Constipation. Gastroenterology 2022, 163, 118–136. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.Z.; Zhang, J.D.; Sun, F.; Duan, L.P. Efficacy of Probiotics for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 349. [Google Scholar] [CrossRef] [PubMed]

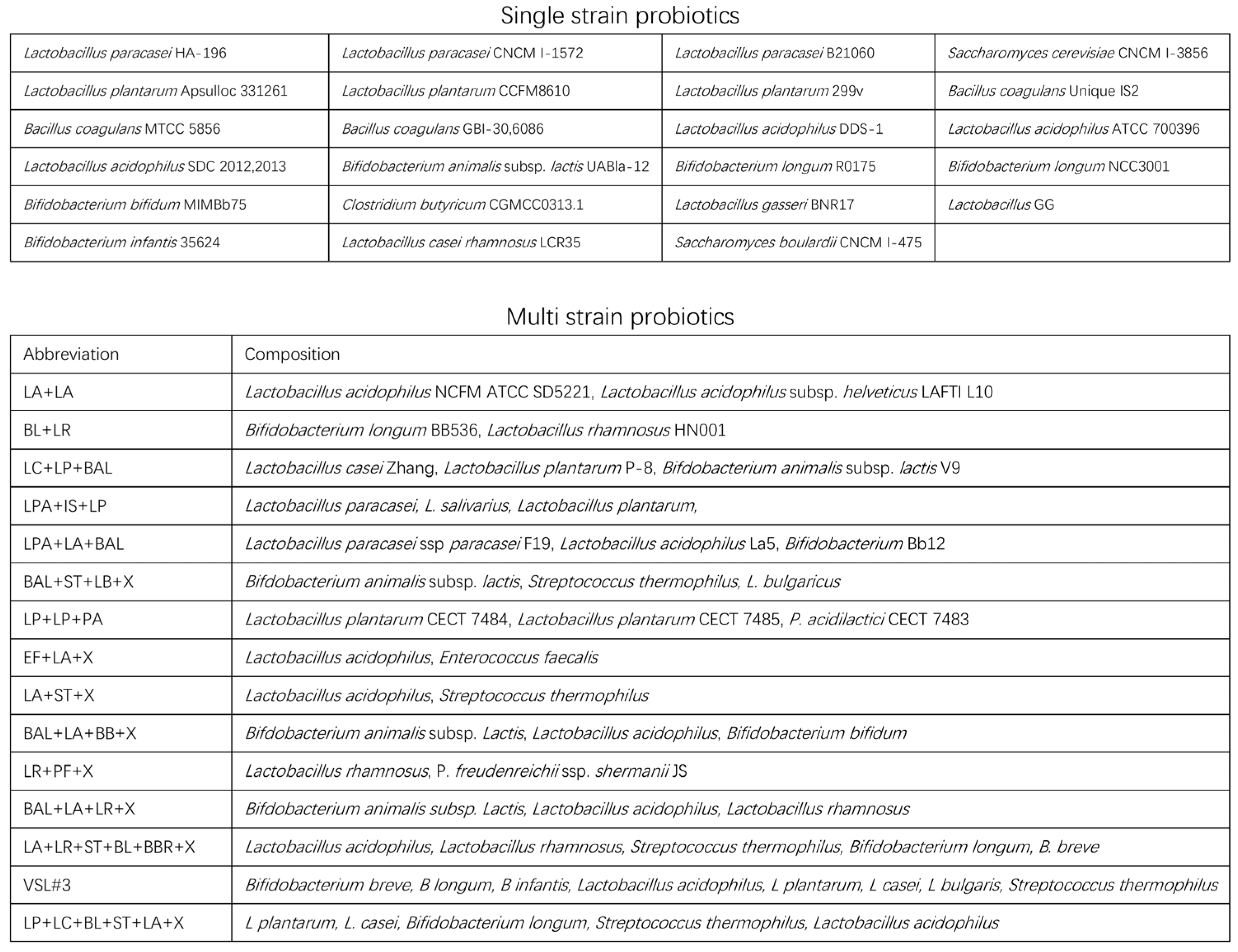
| Outcome | Efficacy Level A 1 | Efficacy Level B | Efficacy Level C | ||||
|---|---|---|---|---|---|---|---|
| Probiotic | NMA 2 | GRADE | Probiotic | NMA | GRADE | Probiotic | |
| IBS-SSS | L. acidophilus DDS-1 | −77.70 (−101.72, −53.68) | M | EF + LA + X | −35.00 (−60.44, −9.56) | M | L. acidophilus ATCC 700396, L. plantarum 299v, L. plantarum CCFM8610, L. casei LCR35, L. paracasei HA-196, B. longum R0175, BAL + ST + LB, LR + PF + X |
| BL + LR | −80.99 (−130.73, −31.26) | M | B. bifidum MIMBb75 | −29.83 (−48.24, −11.42) | H | ||
| LC + LP + BAL | −76.42 (−114.90, −37.95) | M | C. butyricum CGMCC0313.1 | −21.38 (−40.47, −2.29) | H | ||
| LP + LC + BL + ST + LA + X | −63.96 (−78.66, −49.26) | M | BAL + LA + BB + X | −18.86 (−25.88, −11.85) | M | ||
| B. lactis UABla-12 | −48.80 (−73.00, −24.60) | M | |||||
| IBS-QOL | LP + LC + BL + ST + LA + X | 24.80 (20.65, 28.95) | M | C. butyricum CGMCC0313.1 | 4.07 (0.50, 7.65) | H | LP + LC + BL + ST + LA + X, S. boulardii CNCM I-475, LC + LP + BAL, LA + LR + ST + BL + BBR + X, EF + LA + X, BAL + ST + LB, LPA + LA + BAL, L. acidophilus ATCC 700396 |
| HADS-total score | B. longum R0175 | −0.34 (−0.48, −0.20) | H | None | L. paracasei HA-196, L. acidophilus ATCC 700396 | ||
| HADS-anxiety | None | None | B. longum NCC3001, L. acidophilus ATCC 700396, LPA + LA + BAL | ||||
| HADS-depression | B. longum NCC3001 | −3.00 (−4.92, −1.08) | H | None | L. paracasei CNCM I-1572, L. acidophilus ATCC 700396, LPA + LA + BAL | ||
| Outcome | Efficacy Level A 1 | Efficacy Level B | Efficacy Level C | ||||
|---|---|---|---|---|---|---|---|
| Probiotic | NMA 2 | GRADE | Probiotic | NMA | GRADE | Probiotic | |
| Abdominal pain score | B. coagulans MTCC 5856 | −41.80 (−61.59, −22.00) | M | L. gasseri BNR17 | −36.10 (−64.53, −7.67) | M | BL + LR, L. acidophilus SDC 2012,2013, B. lactis UABla-12, BAL + LA + BB + X, LR + PF + X, LA + LR + ST + BL + BBR + X, B. bifidum MIMBb75, LP + LP + PA, LP + LC + BL + ST + LA + X, LA + LA, C. butyricum CGMCC0313.1, L. plantarum 299v, L. acidophilus ATCC 700396, L. casei LCR35, BAL + ST + LB, LPA + LA + BAL, BAL + LA + LR + X, S. boulardii CNCM I-475, B. infantis 35624, L. plantarum CCFM8610 |
| B. coagulans Unique IS2 | −32.00 (−45.35, −18.65) | M | L. plantarum Apsulloc 331261 | −26.59 (−47.07, −6.11) | M | ||
| L. acidophilus DDS-1 | −19.53 (−33.49, −5.57) | M | |||||
| LPA + LS + LP | −20.00 (−35.97, −4.03) | L | |||||
| S. cerevisiae CNCM I-3856 | −15.24 (−24.62, −5.87) | M | |||||
| VSL#3 | −12.93 (−25.59, −0.26) | L | |||||
| EF + LA + X | −11.37 (−21.68, −1.06) | M | |||||
| LA + ST + X | −8.14 (−15.54, −0.75) | M | |||||
| Abdominal bloating score | None | BL + LR | −34.00 (−56.94, −11.06) | M | B. coagulans MTCC 5856, L. plantarum Apsulloc 331261, L. acidophilus DDS-1, LR + PF + X, LA + ST + X, B. coagulans Unique IS2, S. cerevisiae CNCM I-3856, BAL + LA + BB + X, B. lactis UABla-12, LP + LC + BL + ST + LA + X, EF + LA + X, LA + LR + ST + BL + BBR + X, L. acidophilus ATCC 700396, BAL + LA + LR + X, S. boulardii CNCM I-475, C. butyricum CGMCC0313.1, BAL + ST + LB, B. infantis 35624, LPA + LA + BAL | ||
| L. plantarum CCFM8610 | −19.92 (−34.91, −4.94) | M | |||||
| L. plantarum 299v | −14.79 (−29.11, −0.48) | M | |||||
| VSL#3 | −13.71 (−22.12, −5.30) | L | |||||
| B. bifidum MIMBb75 | −11.83 (−22.93, −0.74) | M | |||||
| Outcome | Efficacy Level A 1 | Efficacy Level B | Efficacy Level C | ||||
|---|---|---|---|---|---|---|---|
| Probiotic | Network Meta-Analysis 2 | GRADE | Probiotic | Network Meta-Analysis | GRADE | Probiotic | |
| Bowel movement frequency (IBS-D) | EF + LA + X | −3.95 (−5.02, −2.88) | M | L. paracasei B21060 | −5.11 (−9.98, −0.24) | H | L. plantarum Apsulloc 331261, LP + LC + BL + ST + LA + X, C. butyricum CGMCC0313.1, S. cerevisiae CNCM I-3856, S. boulardii CNCM I-475 |
| L. paracasei HA-196 | −3.13 (−4.63, −1.62) | H | |||||
| B. coagulans GBI-306086 | −2.11 (−3.00, −1.23) | M | |||||
| B. longum R0175 | −1.95 (−3.45, −0.45) | H | |||||
| Bowel movement frequency (IBS-C) | None | None | B. bifidum MIMBb75, LA + ST + X, S. cerevisiae CNCM I-3856, L. paracasei HA-196, B. coagulans Unique IS2, B. longum R0175, BAL + ST + LB | ||||
| Bristol stool form scale (IBS-D) | B. coagulans MTCC 5856 | −3.28 (−5.21, −1.34) | L | BL + LR | −0.80 (−1.57, −0.03) | L | L. paracasei HA-196, B. bifidum MIMBb75, VSL#3, C. butyricum CGMCC0313.1, B. longum R0175, LP + LC + BL + ST + LA + X, S. boulardii CNCM I-475 |
| S. cerevisiae CNCM I-3856 | −1.24 (−1.63, −0.86) | L | LA + LR + ST + BL + BBR + X | −0.70 (−1.32, −0.08) | M | ||
| LP + LP + PA | −0.50 (−0.76, −0.24) | L | |||||
| Bristol stool form scale (IBS-C) | None | None | L. paracasei HA-196, S. cerevisiae CNCM I-3856, B. longum R0175, BAL + ST + LB | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, P.; Luo, M.; Deng, X.; Fan, J.; Xiong, L. Outcome-Specific Efficacy of Different Probiotic Strains and Mixtures in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Nutrients 2023, 15, 3856. https://doi.org/10.3390/nu15173856
Xie P, Luo M, Deng X, Fan J, Xiong L. Outcome-Specific Efficacy of Different Probiotic Strains and Mixtures in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Nutrients. 2023; 15(17):3856. https://doi.org/10.3390/nu15173856
Chicago/Turabian StyleXie, Peiwei, Mei Luo, Xuehong Deng, Jiahui Fan, and Lishou Xiong. 2023. "Outcome-Specific Efficacy of Different Probiotic Strains and Mixtures in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis" Nutrients 15, no. 17: 3856. https://doi.org/10.3390/nu15173856
APA StyleXie, P., Luo, M., Deng, X., Fan, J., & Xiong, L. (2023). Outcome-Specific Efficacy of Different Probiotic Strains and Mixtures in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. Nutrients, 15(17), 3856. https://doi.org/10.3390/nu15173856






