Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review
Abstract
1. Introduction
2. Method
2.1. The Relationship between Depression and Nutritional Deficiencies
2.2. Folate: Its Role in Human Nutrition and Health
2.3. The Link between Folate and Depressive Disorders
2.4. The Potential of Folic Acid as a Neuro-Nutraceutical for Depression
2.5. Exploring Folate and New Therapeutic Avenues in the Treatment of Depression
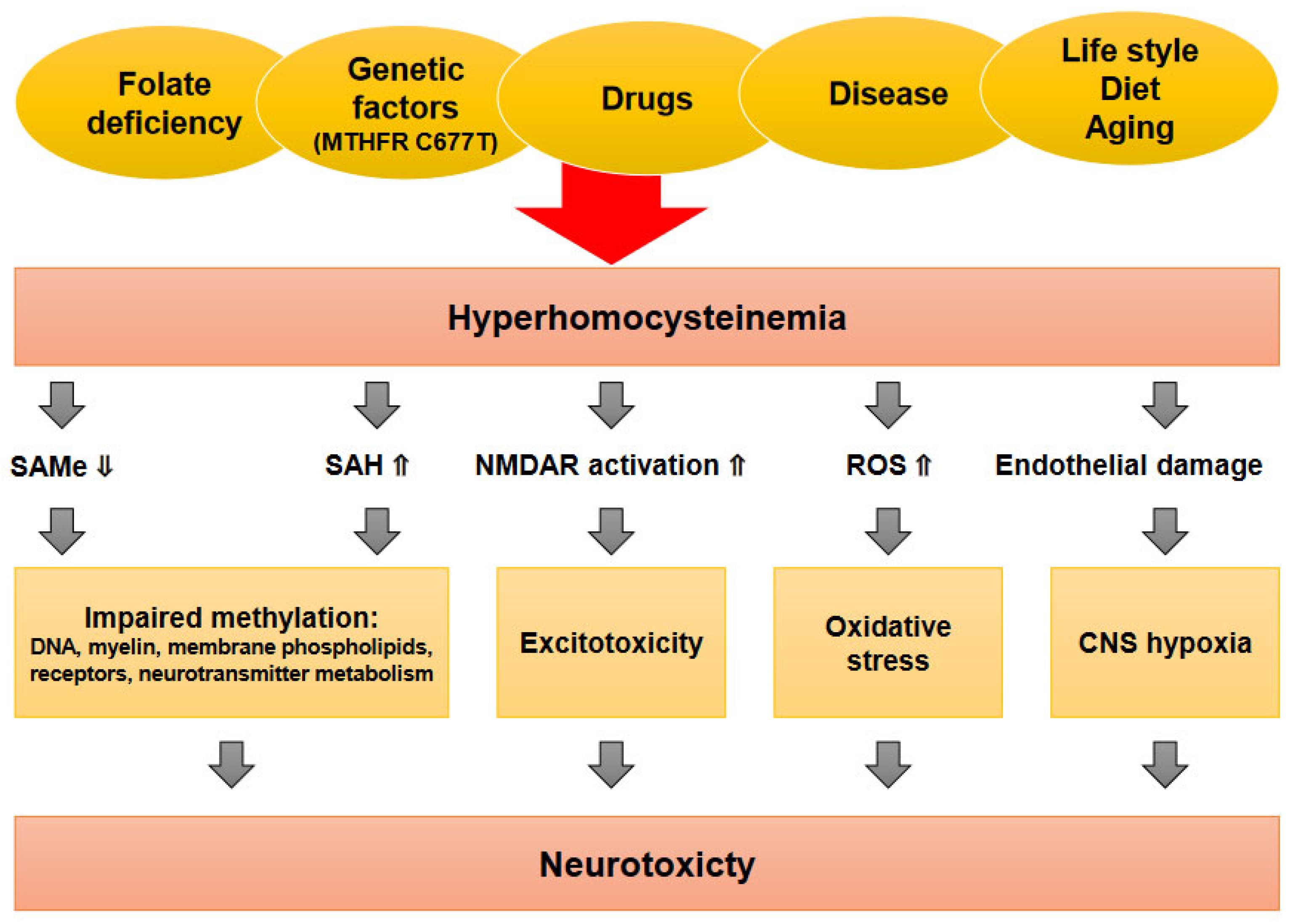
2.6. N-Methyl-D-Aspartate Receptor Modulation
2.7. Anti-Inflammatory Agents
2.8. Gut–Brain Axis
2.9. The Protective Effects of Folic Acid against Suicide
2.10. Population Suicide Risk: Exploring Latitude Variation, Genetic Influences, Diet, and Folate Status
2.11. Discussion and Prospects for Further Study
3. Conclusions
Funding
Conflicts of Interest
References
- World Health Organization Facts Sheets: Depressive Disorder (Depression); WHO: Geneva, Switzerland, 2023.
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Publishing: Arlington, TX, USA, 2013.
- Marcus, M.; Yasamy, M.T.; van Ommeren, M.V.; Chisholm, D.; Saxena, S. Depression: A Global Public Health Concern; World Federation of Mental Health, WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Wittchen, H.U.; Jacobi, F.; Rehm, J.; Gustavsson, A.; Svensson, M.; Jönsson, B.; Olesen, J.; Allgulander, C.; Alonso, J.; Faravelli, C.; et al. The Size and Burden of Mental Disorders and Other Disorders of the Brain in Europe 2010. Eur. Neuropsychopharmacol. 2011, 21, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Takaesu, Y.; Nakai, Y.; Shimura, A.; Ono, Y.; Murakoshi, A.; Matsumoto, Y.; Tanabe, H.; Kusumi, I.; Inoue, T. Associations among Depressive Symptoms, Childhood Abuse, Neuroticism, and Adult Stressful Life Events in the General Adult Population. Neuropsychiatr. Dis. Treat. 2017, 13, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A Systematic Review and Meta-Regression of the Prevalence and Incidence of Perinatal Depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef]
- Institute of Health Metrics and Evaluation Global Health Data Exchange (GHDx). Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 1 July 2023).
- Cuijpers, P.; Beekman, A.T.F.; Reynolds, C.F. Preventing Depression. JAMA 2012, 307, 1033. [Google Scholar] [CrossRef]
- Greenberg, P.E.; Fournier, A.-A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef]
- Herrman, H.; Patel, V.; Kieling, C.; Berk, M.; Buchweitz, C.; Cuijpers, P.; Furukawa, T.A.; Kessler, R.C.; Kohrt, B.A.; Maj, M.; et al. Time for United Action on Depression: A Lancet–World Psychiatric Association Commission. Lancet 2022, 399, 957–1022. [Google Scholar] [CrossRef] [PubMed]
- Skundberg-Kletthagen, H.; Hall-Lord, M.L.; Hedelin, B.; Wangensteen, S. Relatives of Inpatients Suffering from Severe Depression: Their Burden and Encounters with the Psychiatric Health Services. Issues Ment. Health Nurs. 2016, 37, 293–298. [Google Scholar] [CrossRef]
- Marquez, P.V.; Saxena, S. Making Mental Health a Global Priority. Cerebrum 2016, 2016, cer-10-16. [Google Scholar]
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-Economic Variations in the Mental Health Treatment Gap for People with Anxiety, Mood, and Substance Use Disorders: Results from the WHO World Mental Health (WMH) Surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef]
- Holahan, C.J.; Pahl, S.A.; Cronkite, R.C.; Holahan, C.K.; North, R.J.; Moos, R.H. Depression and Vulnerability to Incident Physical Illness across 10 years. J. Affect. Disord. 2010, 123, 222–229. [Google Scholar] [CrossRef]
- Bschor, T.; Bauer, M.; Adli, M. Chronic and Treatment Resistant Depression. Dtsch. Arztebl. Int. 2014, 111, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, T.; Pilling, S.; Mavranezouli, I.; Megnin-Viggars, O.; Ruane, C.; Eadon, H.; Kapur, N. Management of Depression in Adults: Summary of Updated NICE Guidance. Br. Med. J. 2022, 378, o1557. [Google Scholar] [CrossRef]
- Plöderl, M.; Hengartner, M.P. Guidelines for the Pharmacological Acute Treatment of Major Depression: Conflicts with Current Evidence as Demonstrated with the German S3-Guidelines. BMC Psychiatry 2019, 19, 265. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts; American Psychological Association: Washington, DC, USA, 2019.
- Guideline Development Panel for the Treatment of Depressive Disorders. Summary of the Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts. Am. Psychol. 2021, 77, 770–780. [CrossRef]
- Travica, N.; Teasdale, S.; Marx, W. Nutraceuticals in Mood Disorders: Current Knowledge and Future Directions. Curr. Opin. Psychiatry 2023, 36, 54–59. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Petersen, T.; Mischoulon, D.; Green, C.H.; Nierenberg, A.A.; Bottiglieri, T.; Rosenbaum, J.F.; Alpert, J.E.; Fava, M. Serum Folate, Vitamin B12, and Homocysteine in Major Depressive Disorder, Part 2. J. Clin. Psychiatry 2004, 65, 1096–1098. [Google Scholar] [CrossRef]
- Gilbody, S.; Lightfoot, T.; Sheldon, T. Is Low Folate a Risk Factor for Depression? A Meta-Analysis and Exploration of Heterogeneity. J. Epidemiol. Community Health 2007, 61, 631–637. [Google Scholar] [CrossRef]
- Molendijk, M.; Molero, P.; Ortuño Sánchez-Pedreño, F.; Van der Does, W.; Angel Martínez-González, M. Diet Quality and Depression Risk: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and Depression: Exploring the Biological Mechanisms of Action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Hagan, K.E.; Kingston, N. The Association of Folate and Depression: A Meta-Analysis. J. Psychiatr. Res. 2017, 95, 9–18. [Google Scholar] [CrossRef]
- Lee, S.; Chow, C.C.; Shek, C.C.; Wing, Y.K.; Chen, C.-N. Folate Concentration in Chinese Psychiatric Outpatients on Long-Term Lithium Treatment. J. Affect. Disord. 1992, 24, 265–270. [Google Scholar] [CrossRef]
- Abou-Saleh, M.T.; Coppen, A. Psychiatric Progress. J. Psychiatr. Res. 1986, 20, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Ramos, M.I.; Allen, L.H.; Haan, M.N.; Green, R.; Miller, J.W. Plasma Folate Concentrations Are Associated with Depressive Symptoms in Elderly Latina Women despite Folic Acid Fortification. Am. J. Clin. Nutr. 2004, 80, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.V.; Balneaves, L.G.; Faulkner, G.; Ortiz, A.; McIntosh, D.; Morehouse, R.L.; Ravindran, L.; Yatham, L.N.; Kennedy, S.H.; Lam, R.W.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can. J. Psychiatry 2016, 61, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician Guidelines for the Treatment of Psychiatric Disorders with Nutraceuticals and Phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Handbook for Systematic Reviews of Interventions: Version 6.4; The Cochrane Collaboration and John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023.
- Baxter, A.J.; Patton, G.; Scott, K.M.; Degenhardt, L.; Whiteford, H.A. Global Epidemiology of Mental Disorders: What Are We Missing? PLoS ONE 2013, 8, e65514. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A Meta-review of “Lifestyle Psychiatry”: The Role of Exercise, Smoking, Diet and Sleep in the Prevention and Treatment of Mental Disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional Medicine as Mainstream in Psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and Behavioral Health Disorders: Depression and Anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Paul Amminger, G.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. International Society for Nutritional Psychiatry Research Consensus Position Statement: Nutritional Medicine in Modern Psychiatry. World Psychiatry 2015, 14, 370–371. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Milte, C.M.; Meyer, B.J. Nutritional Modulation of Cognitive Function and Mental Health. J. Nutr. Biochem. 2013, 24, 725–743. [Google Scholar] [CrossRef]
- Selhub, E.M.; Logan, A.C.; Bested, A.C. Fermented Foods, Microbiota, and Mental Health: Ancient Practice Meets Nutritional Psychiatry. J. Physiol. Anthropol. 2014, 33, 2. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A Review of Lifestyle Factors That Contribute to Important Pathways Associated with Major Depression: Diet, Sleep and Exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef]
- Ménard, C.; Hodes, G.E.; Russo, S.J. Pathogenesis of Depression: Insights from Human and Rodent Studies. Neuroscience 2016, 321, 138–162. [Google Scholar] [CrossRef]
- Begdache, L.; Chaar, M.; Sabounchi, N.; Kianmehr, H. Assessment of Dietary Factors, Dietary Practices and Exercise on Mental Distress in Young Adults versus Matured Adults: A Cross-Sectional Study. Nutr. Neurosci. 2019, 22, 488–498. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Opie, R.S.; O’Neil, A.; Itsiopoulos, C.; Jacka, F.N. The Impact of Whole-of-Diet Interventions on Depression and Anxiety: A Systematic Review of Randomised Controlled Trials. Public Health Nutr. 2015, 18, 2074–2093. [Google Scholar] [CrossRef]
- Popper, C.W. Single-Micronutrient and Broad-Spectrum Micronutrient Approaches for Treating Mood Disorders in Youth and Adults. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 591–672. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Pérez-Cornago, A.; Zazpe, I.; Santiago, S.; Lahortiga, F.; Martínez-González, M.A. Micronutrient Intake Adequacy and Depression Risk in the SUN Cohort Study. Eur. J. Nutr. 2018, 57, 2409–2419. [Google Scholar] [CrossRef]
- Scaglione, F.; Panzavolta, G. Folate, Folic Acid and 5-Methyltetrahydrofolate Are Not the Same Thing. Xenobiotica 2014, 44, 480–488. [Google Scholar] [CrossRef]
- Bailey, L.B.; Caudill, M.A. Folate. In Present Knowledge in Nutrition; Erdman, J.W., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Washington, DC, USA, 2012; pp. 321–342. [Google Scholar]
- Institute of Medicine. Food and Nutrition Board Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- National Institutes of Health. Fact Sheet for Health Professionals: Folate. Available online: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed on 1 July 2023).
- Mitchell, L.E.; Adzick, N.S.; Melchionne, J.; Pasquariello, P.S.; Sutton, L.N.; Whitehead, A.S. Spina Bifida. Lancet 2004, 364, 1885–1895. [Google Scholar] [CrossRef]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F.; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of Nutrition for Development—Folate Review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef]
- Stover, P.J. Folic Acid. In Modern Nutrition in Health and Disease; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2012; pp. 358–368. [Google Scholar]
- Yetley, E.A.; Pfeiffer, C.M.; Phinney, K.W.; Fazili, Z.; Lacher, D.A.; Bailey, R.L.; Blackmore, S.; Bock, J.L.; Brody, L.C.; Carmel, R.; et al. Biomarkers of Folate Status in NHANES: A Roundtable Summary. Am. J. Clin. Nutr. 2011, 94, 303S–312S. [Google Scholar] [CrossRef]
- Pei, P.; Cheng, X.; Yu, J.; Shen, J.; Li, X.; Wu, J.; Wang, S.; Zhang, T. Folate Deficiency Induced H2A Ubiquitination to Lead to Downregulated Expression of Genes Involved in Neural Tube Defects. Epigenetics Chromatin 2019, 12, 69. [Google Scholar] [CrossRef]
- van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic Acid and Primary Prevention of Neural Tube Defects: A Review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Peng, X.; Zhang, S.; Wang, X.; Zhu, C. Folic Acid and Risk of Preterm Birth: A Meta-Analysis. Front. Neurosci. 2019, 13, 1284. [Google Scholar] [CrossRef] [PubMed]
- Carney, M.W. Serum Folate Values in 423 Psychiatric Patients. Br. Med. J. 1967, 4, 512–516. [Google Scholar] [CrossRef][Green Version]
- Morris, M.S.; Fava, M.; Jacques, P.F.; Selhub, J.; Rosenberg, I.H. Depression and Folate Status in the US Population. Psychother. Psychosom. 2003, 72, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H.; Preece, J.M.; Bailey, J.; Coppen, A. Folate Deficiency in Depressive Illness. Br. J. Psychiatry 1970, 117, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Raab, M.F. The Role of Folate in Depression and Dementia. J. Clin. Psychiatry 2007, 68, 28–33. [Google Scholar] [PubMed]
- Fava, M.; Borus, J.S.; Alpert, J.E.; Nierenberg, A.A.; Rosenbaum, J.F.; Bottiglieri, T. Folate, Vitamin B12, and Homocysteine in Major Depressive Disorder. Am. J. Psychiatry 1997, 154, 426–428. [Google Scholar]
- Sachdev, P.S.; Parslow, R.A.; Lux, O.; Salonikas, C.; Wen, W.; Naidoo, D.; Christensen, H.; Jorm, A.F. Relationship of Homocysteine, Folic Acid and Vitamin B 12 with Depression in a Middle-Aged Community Sample. Psychol. Med. 2005, 35, 529–538. [Google Scholar] [CrossRef]
- Levitt, A.J.; Joffe, R.T. Folate, B12, and Life Course of Depressive Illness. Biol. Psychiatry 1989, 25, 867–872. [Google Scholar] [CrossRef]
- Miller, A.L. The Methylation, Neurotransmitter, and Antioxidant Connections between Folate and Depression. Altern. Med. Rev. 2008, 13, 216–226. [Google Scholar] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef]
- Alpert, J.E.; Fava, M. Nutrition and Depression: The Role of Folate. Nutr. Rev. 2009, 55, 145–149. [Google Scholar] [CrossRef]
- Alpert, J.E.; Mischoulon, D.; Nierenberg, A.A.; Fava, M. Nutrition and Depression: Focus on Folate. Nutrition 2000, 16, 544–546. [Google Scholar] [CrossRef]
- Coppen, A.; Bolander-Gouaille, C. Treatment of Depression: Time to Consider Folic Acid and Vitamin B12. J. Psychopharmacol. 2005, 19, 59–65. [Google Scholar] [CrossRef]
- Coppen, A.; Swade, C.; Jones, S.A.; Armstrong, R.A.; Blair, J.A.; Leeming, R.J. Depression and Tetrahydrobiopterin: The Folate Connection. J. Affect. Disord. 1989, 16, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Hamon, C.G.B.; Blair, J.A.; Barford, P.A. The Effect of Tetrahydrofolate on Tetrahydrobiopterin Metabolism. J. Intellect. Disabil. Res. 2008, 30, 179–183. [Google Scholar] [CrossRef]
- Bottiglieri, T. Homocysteine, Folate, Methylation, and Monoamine Metabolism in Depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef]
- Kennedy, B.P.; Bottiglieri, T.; Arning, E.; Ziegler, M.G.; Hansen, L.A.; Masliah, E. Elevated S-Adenosylhomocysteine in Alzheimer Brain: Influence on Methyltransferases and Cognitive Function. J. Neural Transm. 2004, 111, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W. Excitatory Amino Acids and Neuropsychiatrie Disorders. Biol. Psychiatry 1989, 26, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Kim, W.-K.; Choi, Y.-B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity Associated with Dual Actions of Homocysteine at the N-Methyl-D-Aspartate Receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Dayal, S.; Brown, K.L.; Weydert, C.J.; Oberley, L.W.; Arning, E.; Bottiglieri, T.; Faraci, F.M.; Lentz, S.R. Deficiency of Glutathione Peroxidase-1 Sensitizes Hyperhomocysteinemic Mice to Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1996–2002. [Google Scholar] [CrossRef]
- Bottiglieri, T. Homocysteine and Folate Metabolism in Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2005, 29, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Lawlor, D.A.; Davey Smith, G.; Araya, R.; Timpson, N.; Day, I.N.M.; Ebrahim, S. The Thermolabile Variant of MTHFR Is Associated with Depression in the British Women’s Heart and Health Study and a Meta-Analysis. Mol. Psychiatry 2006, 11, 352–360. [Google Scholar] [CrossRef][Green Version]
- Lok, A.; Bockting, C.L.H.; Koeter, M.W.J.; Snieder, H.; Assies, J.; Mocking, R.J.T.; Vinkers, C.H.; Kahn, R.S.; Boks, M.P.; Schene, A.H. Interaction between the MTHFR C677T Polymorphism and Traumatic Childhood Events Predicts Depression. Transl. Psychiatry 2013, 3, e288. [Google Scholar] [CrossRef]
- Kelly, C.B.; Mcdonnell, A.P.; Johnston, T.G.; Mulholland, C.; Cooper, S.J.; Mcmaster, D.; Evans, A.; Whitehead, A.S. The MTHFR C677T Polymorphism Is Associated with Depressive Episodes in Patients from Northern Ireland. J. Psychopharmacol. 2004, 18, 567–571. [Google Scholar] [CrossRef]
- Wan, L.; Li, Y.; Zhang, Z.; Sun, Z.; He, Y.; Li, R. Methylenetetrahydrofolate Reductase and Psychiatric Diseases. Transl. Psychiatry 2018, 8, 242. [Google Scholar] [CrossRef]
- Gilbody, S.; Lewis, S.; Lightfoot, T. Methylenetetrahydrofolate Reductase (MTHFR) Genetic Polymorphisms and Psychiatric Disorders: A HuGE Review. Am. J. Epidemiol. 2006, 165, 1–13. [Google Scholar] [CrossRef]
- Choi, S.-W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef]
- Juruena, M.F.; Gadelrab, R.; Cleare, A.J.; Young, A.H. Epigenetics: A Missing Link between Early Life Stress and Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110231. [Google Scholar] [CrossRef] [PubMed]
- Sugden, C. One-Carbon Metabolism in Psychiatric Illness. Nutr. Res. Rev. 2006, 19, 117–136. [Google Scholar] [CrossRef]
- Shelton, R.C.; Manning, J.S.; Barrentine, L.W.; Tipa, E.V. Assessing Effects of l-Methylfolate in Depression Management. Prim. Care Companion CNS Disord. 2013, 25, 23103498. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D.; et al. L-Methylfolate as Adjunctive Therapy for SSRI-Resistant Major Depression: Results of Two Randomized, Double-Blind, Parallel-Sequential Trials. Am. J. Psychiatry 2012, 169, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Altaf, R.; Gonzalez, I.; Rubino, K.; Nemec, E.C. Folate as Adjunct Therapy to SSRI/SNRI for Major Depressive Disorder: Systematic Review & Meta-Analysis. Complement. Ther. Med. 2021, 61, 102770. [Google Scholar] [CrossRef]
- Al Maruf, A.; Poweleit, E.A.; Brown, L.C.; Strawn, J.R.; Bousman, C.A. Systematic Review and Meta-Analysis of L-Methylfolate Augmentation in Depressive Disorders. Pharmacopsychiatry 2022, 55, 139–147. [Google Scholar] [CrossRef]
- Reynolds, E.; Crellin, R.; Bottiglieri, T.; Laundy, M.; Toone, B.; Carney, M. Methylfolate as Monotherapy in Depression. A Pilot Randomised Controlled Trial. J. Neurol. Psychol. 2015, 3, 5–9. [Google Scholar]
- Roberts, E.; Carter, B.; Young, A.H. Caveat Emptor: Folate in Unipolar Depressive Illness, a Systematic Review and Meta-Analysis. J. Psychopharmacol. 2018, 32, 377–384. [Google Scholar] [CrossRef]
- Coppen, A.; Bailey, J. Enhancement of the Antidepressant Action of Fluoxetine by Folic Acid: A Randomised, Placebo Controlled Trial. J. Affect. Disord. 2000, 60, 121–130. [Google Scholar] [CrossRef]
- Resler, G.; Lavie, R.; Campos, J.; Mata, S.; Urbina, M.; García, A.; Apitz, R.; Lima, L. Effect of Folic Acid Combined with Fluoxetine in Patients with Major Depression on Plasma Homocysteine and Vitamin B12, and Serotonin Levels in Lymphocytes. Neuroimmunomodulation 2008, 15, 145–152. [Google Scholar] [CrossRef]
- Godfrey, P.S.A.; Toone, B.K.; Bottiglien, T.; Laundy, M.; Reynolds, E.H.; Carney, M.W.P.; Flynn, T.G.; Chanarin, I. Enhancement of Recovery from Psychiatric Illness by Methylfolate. Lancet 1990, 336, 392–395. [Google Scholar] [CrossRef]
- Schefft, C.; Kilarski, L.L.; Bschor, T.; Köhler, S. Efficacy of Adding Nutritional Supplements in Unipolar Depression: A Systematic Review and Meta-Analysis. Eur. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.S.K.; Long, X.X.; Li, X.; Saad, M.; Lim, F.; Doery, J.C.; Griffin, R.C.; Galletly, C. The Potential Use of Folate and Its Derivatives in Treating Psychiatric Disorders: A Systematic Review. Biomed. Pharmacother. 2022, 146, 112541. [Google Scholar] [CrossRef]
- Dartois, L.L.; Stutzman, D.L.; Morrow, M. L-Methylfolate Augmentation to Antidepressants for Adolescents with Treatment-Resistant Depression: A Case Series. J. Child Adolesc. Psychopharmacol. 2019, 29, 386–391. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, S.; Kim, J.H. Real-World Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Ford, A.H.; Flicker, L. Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials of Folate and Vitamin B12 for Depression. Int. Psychogeriatr. 2015, 27, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Geddes, J.R.; Gardiner, A.; Rendell, J.; Voysey, M.; Tunbridge, E.; Hinds, C.; Yu, L.-M.; Hainsworth, J.; Attenburrow, M.-J.; Simon, J.; et al. Comparative Evaluation of Quetiapine plus Lamotrigine Combination versus Quetiapine Monotherapy (and Folic Acid versus Placebo) in Bipolar Depression (CEQUEL): A 2 × 2 Factorial Randomised Trial. Lancet Psychiatry 2016, 3, 31–39. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neurocircuitry of Mood Disorders. Neuropsychopharmacology 2010, 35, 192–216. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic Plasticity and Depression: New Insights from Stress and Rapid-Acting Antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- Marwaha, S.; Palmer, E.; Suppes, T.; Cons, E.; Young, A.H.; Upthegrove, R. Novel and Emerging Treatments for Major Depression. Lancet 2023, 401, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.S.; Bell, C.E.; Pollard, D.A. Revisiting the Monoamine Hypothesis of Depression: A New Perspective. Perspect. Med. Chem. 2014, 6, 1–8. [Google Scholar] [CrossRef]
- Meldrum, B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef]
- Murrough, J.W.; Abdallah, C.G.; Mathew, S.J. Targeting Glutamate Signalling in Depression: Progress and Prospects. Nat. Rev. Drug Discov. 2017, 16, 472–486. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a Glutamate Hypothesis of Depression. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Canavero, S.; Bonicalzi, V. Antiglutamatergic Agents. In Central Pain Syndrome; Springer International Publishing: Cham, Switzerland, 2018; pp. 275–288. [Google Scholar]
- Moore, T.J.; Alami, A.; Alexander, G.C.; Mattison, D.R. Safety and Effectiveness of NMDA Receptor Antagonists for Depression: A Multidisciplinary Review. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2022, 42, 567–579. [Google Scholar] [CrossRef]
- Liang, X.; Shi, L.; Wang, M.; Zhang, L.; Gong, Z.; Luo, S.; Wang, X.; Zhang, Q.; Zhang, X. Folic Acid Ameliorates Synaptic Impairment Following Cerebral Ischemia/Reperfusion Injury via Inhibiting Excessive Activation of NMDA Receptors. J. Nutr. Biochem. 2023, 112, 109209. [Google Scholar] [CrossRef]
- Poddar, R.; Paul, S. Homocysteine-NMDA Receptor-Mediated Activation of Extracellular Signal-Regulated Kinase Leads to Neuronal Cell Death. J. Neurochem. 2009, 110, 1095–1106. [Google Scholar] [CrossRef]
- Bhatia, P.; Singh, N. Homocysteine Excess: Delineating the Possible Mechanism of Neurotoxicity and Depression. Fundam. Clin. Pharmacol. 2015, 29, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.H.; Flicker, L.; McCaul, K.; van Bockxmeer, F.; Hegarty, S.; Hirani, V.; Fenner, S.; Almeida, O.P. The B-VITAGE Trial: A Randomized Trial of Homocysteine Lowering Treatment of Depression in Later Life. Trials 2010, 11, 8. [Google Scholar] [CrossRef]
- Newport, D.J.; Carpenter, L.L.; McDonald, W.M.; Potash, J.B.; Tohen, M.; Nemeroff, C.B. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am. J. Psychiatry 2015, 172, 950–966. [Google Scholar] [CrossRef]
- von Jauregg, J.W. Fieberbehandlung Bei Psychosen. Wien. Med. Wochenschr. 1926, 76, 79–82. [Google Scholar]
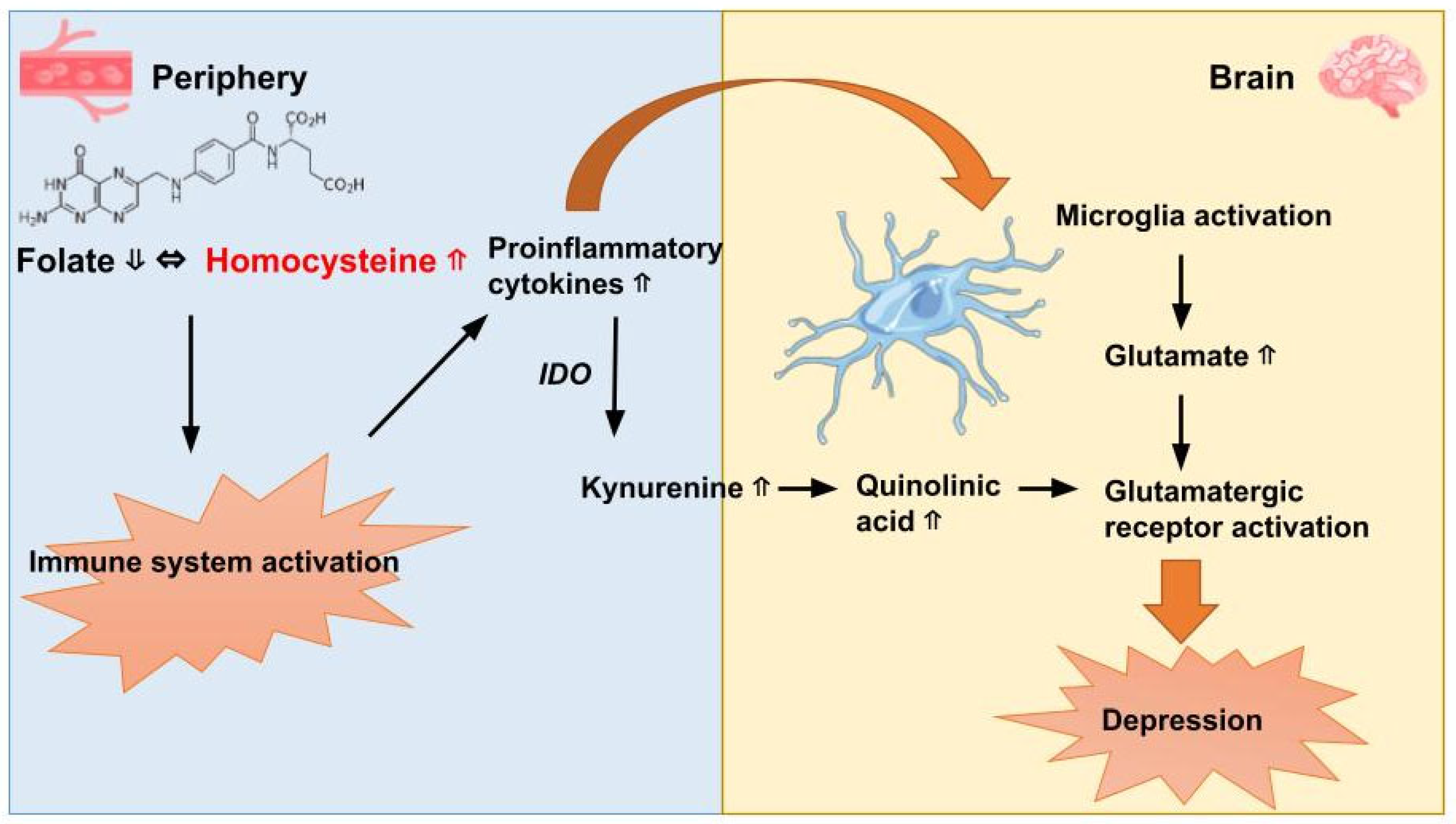
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef]
- Song, C.; Wang, H. Cytokines Mediated Inflammation and Decreased Neurogenesis in Animal Models of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 760–768. [Google Scholar] [CrossRef]
- Kelly, K.M.; Smith, J.A.; Mezuk, B. Depression and Interleukin-6 Signaling: A Mendelian Randomization Study. Brain Behav. Immun. 2021, 95, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pace, T.W.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased Stress-Induced Inflammatory Responses in Male Patients with Major Depression and Increased Early Life Stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef]
- Dantzer, R.; Walker, A.K. Is There a Role for Glutamate-Mediated Excitotoxicity in Inflammation-Induced Depression? J. Neural Transm. 2014, 121, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress Induced Microglial Activation Contributes to Depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Huang, X.-F.; Newell, K.A. The Kynurenine Pathway in Major Depression: What We Know and Where to Next. Neurosci. Biobehav. Rev. 2021, 127, 917–927. [Google Scholar] [CrossRef]
- Khandaker, G.M.; Pearson, R.M.; Zammit, S.; Lewis, G.; Jones, P.B. Association of Serum Interleukin 6 and C-Reactive Protein in Childhood with Depression and Psychosis in Young Adult Life. JAMA Psychiatry 2014, 71, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. The Evolutionary Significance of Depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 2013, 18, 15–37. [Google Scholar] [CrossRef]
- Han, K.-M.; Ham, B.-J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Guo, W.; Feng, Y.; Deng, H.; Li, G.; Nie, H.; Guo, G.; Yu, H.; Ma, Y.; Wang, J.; et al. Efficacy and Safety of Anti-Inflammatory Agents for the Treatment of Major Depressive Disorder: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 21–32. [Google Scholar] [CrossRef]
- Jones, P.; Lucock, M.; Scarlett, C.J.; Veysey, M.; Beckett, E.L. Folate and Inflammation—Links between Folate and Features of Inflammatory Conditions. J. Nutr. Intermed. Metab. 2019, 18, 100104. [Google Scholar] [CrossRef]
- Gisondi, P.; Fantuzzi, F.; Malerba, M.; Girolomoni, G. Folic Acid in General Medicine and Dermatology. J. Dermatol. Treat. 2007, 18, 138–146. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial Dysfunction: The Link Between Homocysteine and Hydrogen Sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Moosavian, S.P.; Nazarian, B.; Afrisham, R.; Kelishadi, M.R.; Wong, A.; Dutheil, F.; Suzuki, K.; et al. Effects of Folic Acid Supplementation on Inflammatory Markers: A Grade-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2327. [Google Scholar] [CrossRef]
- Kruse, J.L.; Congdon, E.; Olmstead, R.; Njau, S.; Breen, E.C.; Narr, K.L.; Espinoza, R.; Irwin, M.R. Inflammation and Improvement of Depression Following Electroconvulsive Therapy in Treatment-Resistant Depression. J. Clin. Psychiatry 2018, 79, 17m11597. [Google Scholar] [CrossRef]
- Nikkheslat, N. Targeting Inflammation in Depression: Ketamine as an Anti-Inflammatory Antidepressant in Psychiatric Emergency. Brain Behav. Immun. Health 2021, 18, 100383. [Google Scholar] [CrossRef] [PubMed]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.J.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Wang, S.; Zhao, Z.; Jian, C.; Li, M.; Qin, X. Microbiome and Metabolome Integrally Reveal the Anti-Depression Effects of Cistanche Deserticola Polysaccharides from the Perspective of Gut Homeostasis. Int. J. Biol. Macromol. 2023, 245, 125542. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, Q.; Wu, W.; Chen, J. Multi-Omics Data Reveals the Important Role of Glycerophospholipid Metabolism in the Crosstalk between Gut and Brain in Depression. J. Transl. Med. 2023, 21, 93. [Google Scholar] [CrossRef]
- Yu, S.; Wang, L.; Jing, X.; Wang, Y.; An, C. Features of Gut Microbiota and Short-Chain Fatty Acids in Patients with First-Episode Depression and Their Relationship with the Clinical Symptoms. Front. Psychol. 2023, 14, 1088268. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Fan, Y.; Zhao, D.; Wang, Y.; Lv, M.; Qin, X. Microbiome and Metabolome Reveal the Metabolic and Microbial Variations Induced by Depression and Constipation. Psychogeriatrics 2023, 23, 319–336. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Cheng, R.; Liu, H.; Zhao, Y.; Liu, Y.; Chen, Y.; Sun, Z.; Zhai, Z.; Wu, M.; et al. Alteration of the Gut Microbiome and Correlated Metabolism in a Rat Model of Long-Term Depression. Front. Cell. Infect. Microbiol. 2023, 13, 1116277. [Google Scholar] [CrossRef]
- Jiang, R.; Wang, Y.; Liu, J.; Wu, Z.; Wang, D.; Deng, Q.; Yang, C.; Zhou, Q. Gut Microbiota Is Involved in the Antidepressant Effects of Adipose-Derived Mesenchymal Stem Cells in Chronic Social Defeat Stress Mouse Model. Psychopharmacology 2022, 239, 533–549. [Google Scholar] [CrossRef]
- Chen, J.; Vitetta, L. Mitochondria Could Be a Potential Key Mediator Linking the Intestinal Microbiota to Depression. J. Cell. Biochem. 2020, 121, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Zhang, H.; Chen, X.; Zhang, Y.; Wu, J.; Zhao, L.; Wang, D.; Pu, J.; Ji, P.; et al. Toward a Deeper Understanding of Gut Microbiome in Depression: The Promise of Clinical Applicability. Adv. Sci. 2022, 9, e2203707. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wu, H.; Chen, Z.; Hao, H.; Zheng, X. Gut Microbiome at the Crossroad of Genetic Variants and Behavior Disorders. Gut Microbes 2023, 15, 2201156. [Google Scholar] [CrossRef]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Holscher, H.D. A Review of Dietary and Microbial Connections to Depression, Anxiety, and Stress. Nutr. Neurosci. 2020, 23, 237–250. [Google Scholar] [CrossRef]
- Slyepchenko, A.; Maes, M.; Jacka, F.N.; Köhler, C.A.; Barichello, T.; McIntyre, R.S.; Berk, M.; Grande, I.; Foster, J.A.; Vieta, E.; et al. Gut Microbiota, Bacterial Translocation, and Interactions with Diet: Pathophysiological Links between Major Depressive Disorder and Non-Communicable Medical Comorbidities. Psychother. Psychosom. 2017, 86, 31–46. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Long-Smith, C.; Kennedy, P.; Cryan, J.F.; Cowan, C.S.M.; Cenit, M.C.; van der Kamp, J.-W.; Sanz, Y. Feeding Melancholic Microbes: MyNewGut Recommendations on Diet and Mood. Clin. Nutr. 2019, 38, 1995–2001. [Google Scholar] [CrossRef]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate Production by Probiotic Bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef]
- Hill, M.J. Intestinal Flora and Endogenous Vitamin Synthesis. Eur. J. Cancer Prev. 1997, 6, S43–S45. [Google Scholar] [CrossRef]
- Ichihashi, T.; Takagishi, Y.; Uchida, K.; Yamada, H. Colonic Absorption of Menaquinone-4 and Menaquinone-9 in Rats. J. Nutr. 1992, 122, 506–512. [Google Scholar] [CrossRef]
- Said, H.M.; Mohammed, Z.M. Intestinal Absorption of Water-Soluble Vitamins: An Update. Curr. Opin. Gastroenterol. 2006, 22, 140–146. [Google Scholar] [CrossRef]
- Asrar, F.M.; O’Connor, D.L. Bacterially Synthesized Folate and Supplemental Folic Acid Are Absorbed across the Large Intestine of Piglets. J. Nutr. Biochem. 2005, 16, 587–593. [Google Scholar] [CrossRef]
- Lakoff, A.; Fazili, Z.; Aufreiter, S.; Pfeiffer, C.M.; Connolly, B.; Gregory, J.F.; Pencharz, P.B.; O’Connor, D.L. Folate Is Absorbed across the Human Colon: Evidence by Using Enteric-Coated Caplets Containing 13C-Labeled [6S]-5-Formyltetrahydrofolate. Am. J. Clin. Nutr. 2014, 100, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Pompei, A.; Cordisco, L.; Amaretti, A.; Zanoni, S.; Raimondi, S.; Matteuzzi, D.; Rossi, M. Administration of Folate-Producing Bifidobacteria Enhances Folate Status in Wistar Rats. J. Nutr. 2007, 137, 2742–2746. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.L.; Kim, T.H.; Yang, J.; Darling, P.B. A Large Pool of Available Folate Exists in the Large Intestine of Human Infants and Piglets. J. Nutr. 2004, 134, 1389–1394. [Google Scholar] [CrossRef]
- Schaub, A.-C.; Schneider, E.; Vazquez-Castellanos, J.F.; Schweinfurth, N.; Kettelhack, C.; Doll, J.P.K.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Clinical, Gut Microbial and Neural Effects of a Probiotic Add-on Therapy in Depressed Patients: A Randomized Controlled Trial. Transl. Psychiatry 2022, 12, 227. [Google Scholar] [CrossRef]
- Turpin, W.; Humblot, C.; Thomas, M.; Guyot, J.-P. Lactobacilli as Multifaceted Probiotics with Poorly Disclosed Molecular Mechanisms. Int. J. Food Microbiol. 2010, 143, 87–102. [Google Scholar] [CrossRef]
- Rao, D.R.; Reddy, A.V.; Pulusani, S.R.; Cornwell, P.E. Biosynthesis and Utilization of Folic Acid and Vitamin B12 by Lactic Cultures in Skim Milk. J. Dairy Sci. 1984, 67, 1169–1174. [Google Scholar] [CrossRef]
- Sybesma, W.; Starrenburg, M.; Tijsseling, L.; Hoefnagel, M.H.N.; Hugenholtz, J. Effects of Cultivation Conditions on Folate Production by Lactic Acid Bacteria. Appl. Environ. Microbiol. 2003, 69, 4542–4548. [Google Scholar] [CrossRef]
- Santos, F.; Wegkamp, A.; de Vos, W.M.; Smid, E.J.; Hugenholtz, J. High-Level Folate Production in Fermented Foods by the B 12 Producer Lactobacillus Reuteri JCM1112. Appl. Environ. Microbiol. 2008, 74, 3291–3294. [Google Scholar] [CrossRef]
- Wegkamp, A.; Starrenburg, M.; de Vos, W.M.; Hugenholtz, J.; Sybesma, W. Transformation of Folate-Consuming Lactobacillus Gasseri into a Folate Producer. Appl. Environ. Microbiol. 2004, 70, 3146–3148. [Google Scholar] [CrossRef]
- Wegkamp, A.; Teusink, B.; De Vos, W.M.; Smid, E.J. Development of a Minimal Growth Medium for Lactobacillus Plantarum. Lett. Appl. Microbiol. 2010, 50, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Suicide Worldwide in 2019: Global Health Estimates. Available online: https://apps.who.int/iris/bitstream/handle/10665/341728/9789240026643-eng.pdf2021 (accessed on 1 July 2023).
- Bachmann, S. Epidemiology of Suicide and the Psychiatric Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef]
- Eurostat Just over 56,000 Persons in the EU Committed Suicide. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/DDN-20180716-1 (accessed on 1 July 2023).
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the Prevention of Suicide in Mood Disorders: Updated Systematic Review and Meta-Analysis. Br. Med. J. 2013, 346, f3646. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.T.; Trujillo Diaz, D.; Rupp, Z.W.; Kidambi, A.; Ramirez, K.L.; Flores, J.M.; Avila-Quintero, V.J.; Rhee, T.G.; Olfson, M.; Bloch, M.H. Pharmacological and Somatic Treatment Effects on Suicide in Adults: A Systematic Review and Meta-analysis. Depress. Anxiety 2022, 39, 100–112. [Google Scholar] [CrossRef]
- Wolfersdorf, M.; Keller, F.; Maier, V.; Fröscher, W.; Kaschka, W. Red-Cell and Serum Folate Levels in Depressed Inpatients Who Commit Violent Suicide: A Comparison with Control Groups. Pharmacopsychiatry 1995, 207, 77–79. [Google Scholar] [CrossRef]
- Kim, J.-M.; Kim, H.-Y.; Lee, H.-J.; Kim, J.-W.; Kang, H.-J.; Kim, S.-W.; Shin, I.-S.; Chun, B.J.; Stewart, R. Prediction of Suicidality According to Serum Folate Levels in Depressive Patients Receiving Stepwise Pharmacotherapy. Front. Psychiatry 2021, 12, 747228. [Google Scholar] [CrossRef]
- Gibbons, R.; Hur, K.; Lavigne, J.; Wang, J.; Mann, J.J. Medications and Suicide: High Dimensional Empirical Bayes Screening (IDEAS). Harv. Data Sci. Rev. 2019, 1. [Google Scholar] [CrossRef]
- Gibbons, R.D.; Hur, K.; Lavigne, J.E.; Mann, J.J. Association Between Folic Acid Prescription Fills and Suicide Attempts and Intentional Self-Harm Among Privately Insured US Adults. JAMA Psychiatry 2022, 79, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Bedson, E.; Bell, D.; Carr, D.; Carter, B.; Hughes, D.; Jorgensen, A.; Lewis, H.; Lloyd, K.; McCaddon, A.; Moat, S.; et al. Folate Augmentation of Treatment—Evaluation for Depression (FolATED): Randomised Trial and Economic Evaluation. Health Technol. Assess. 2014, 18, 1–160. [Google Scholar] [CrossRef]
- Carter, G.; Milner, A.; McGill, K.; Pirkis, J.; Kapur, N.; Spittal, M.J. Predicting Suicidal Behaviours Using Clinical Instruments: Systematic Review and Meta-Analysis of Positive Predictive Values for Risk Scales. Br. J. Psychiatry 2017, 210, 387–395. [Google Scholar] [CrossRef]
- An, S.; Lim, S.; Kim, H.-W.; Kim, H.-S.; Lee, D.; Son, E.; Kim, T.W.; Goh, T.S.; Kim, K.; Kim, Y.H. Global Prevalence of Suicide by Latitude: A Systematic Review and Meta-Analysis. Asian J. Psychiatr. 2023, 81, 103454. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, A. Perspective: Suicide in Europe. Suicide Life Threat. Behav. 1997, 27, 127–136. [Google Scholar]
- Voracek, M.; Fisher, M.L.; Marušiš, A. The Finno-Ugrian Suicide Hypothesis: Variation in European Suicide Rates by Latitude and Longitude. Percept. Mot. Ski. 2003, 97, 401–406. [Google Scholar] [CrossRef]
- Voracek, M. Ancestry, Genes, and Suicide: A Test of the Finno-Ugrian Suicide Hypothesis in the United States. Percept. Mot. Ski. 2006, 103, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Voracek, M.; Marušič, A. Testing the Finno-Ugrian Suicide Hypothesis: Geographic Variation of Elderly Suicide Rates across Europe. Nord. J. Psychiatry 2008, 62, 302–308. [Google Scholar] [CrossRef]
- Suzuki, T.A.; Worobey, M. Geographical Variation of Human Gut Microbial Composition. Biol. Lett. 2014, 10, 20131037. [Google Scholar] [CrossRef] [PubMed]
- Vasileska, A.; Rechkoska, G. Global and Regional Food Consumption Patterns and Trends. Procedia Soc. Behav. Sci. 2012, 44, 363–369. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Suzuki, M. The Okinawan Diet: Health Implications of a Low-Calorie, Nutrient-Dense, Antioxidant-Rich Dietary Pattern Low in Glycemic Load. J. Am. Coll. Nutr. 2009, 28, 500S–516S. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; McKeown, R.E. Cross-Sectional Assessment of Diet Quality in Individuals with a Lifetime History of Attempted Suicide. Psychiatry Res. 2009, 165, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Poudel-Tandukar, K.; Noda, M.; Kato, M.; Kurotani, K.; Goto, A.; Oba, S.; Inoue, M.; Tsugane, S. Dietary Patterns and Suicide in Japanese Adults: The Japan Public Health Center-Based Prospective Study. Br. J. Psychiatry 2013, 203, 422–427. [Google Scholar] [CrossRef]
- Sanada, M.; Imai, T.; Sezaki, A.; Miyamoto, K.; Kawase, F.; Shirai, Y.; Abe, C.; Suzuki, N.; Inden, A.; Kato, T.; et al. Changes in the Association between the Traditional Japanese Diet Score and Suicide Rates over 26 Years: A Global Comparative Study. J. Affect. Disord. 2021, 294, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Yan, H.; Zeng, L.; Wang, Q.; Li, Q.; Xiao, S.; Fan, X. The Status of Vitamin B12 and Folate among Chinese Women: A Population-Based Cross-Sectional Study in Northwest China. PLoS ONE 2014, 9, e112586. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-J.; Pan, W.-H.; Lin, Y.-C.; Lin, B.-F. Trends in Folate Status in the Taiwanese Population Aged 19 Years and Older from the Nutrition and Health Survey in Taiwan 1993–1996 to 2005–2008. Asia Pac. J. Clin. Nutr. 2011, 20, 275–282. [Google Scholar]
- Rogers, L.M.; Cordero, A.M.; Pfeiffer, C.M.; Hausman, D.B.; Tsang, B.L.; De-Regil, L.M.; Rosenthal, J.; Razzaghi, H.; Wong, E.C.; Weakland, A.P.; et al. Global Folate Status in Women of Reproductive Age: A Systematic Review with Emphasis on Methodological Issues. Ann. N. Y. Acad. Sci. 2018, 1431, 35–57. [Google Scholar] [CrossRef]
- Colapinto, C.K.; O’Connor, D.L.; Tremblay, M.S. Folate Status of the Population in the Canadian Health Measures Survey. Can. Med. Assoc. J. 2011, 183, E100–E106. [Google Scholar] [CrossRef] [PubMed]
- Öhrvik, V.; Lemming, E.W.; Nälsén, C.; Becker, W.; Ridefelt, P.; Lindroos, A.K. Dietary Intake and Biomarker Status of Folate in Swedish Adults. Eur. J. Nutr. 2018, 57, 451–462. [Google Scholar] [CrossRef]
- Liao, S.-C.; Chen, W.J.; Lee, M.-B.; Lung, F.-W.; Lai, T.-J.; Liu, C.-Y.; Lin, C.-Y.; Yang, M.-J.; Chen, C.-C. Low Prevalence of Major Depressive Disorder in Taiwanese Adults: Possible Explanations and Implications. Psychol. Med. 2012, 42, 1227–1237. [Google Scholar] [CrossRef]
- Streit, F.; Zillich, L.; Frank, J.; Kleineidam, L.; Wagner, M.; Baune, B.T.; Klinger-König, J.; Grabe, H.J.; Pabst, A.; Riedel-Heller, S.G.; et al. Lifetime and Current Depression in the German National Cohort (NAKO). World J. Biol. Psychiatry 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Statista. Mortality Rate for Suicide in Taiwan from 2010 to 2022. Available online: https://www.statista.com/statistics/860970/taiwan-suicide-mortality-rate/ (accessed on 1 July 2023).
- Federal Statistical Office of Germany. Causes of Death. Available online: https://www.destatis.de/EN/Themes/Society-Environment/Health/Causes-Death/_node.html (accessed on 1 July 2023).
- Nugent, A.C.; Ballard, E.D.; Park, L.T.; Zarate, C.A. Research on the Pathophysiology, Treatment, and Prevention of Suicide: Practical and Ethical Issues. BMC Psychiatry 2019, 19, 332. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.H. Neurometabolic Approach to Treatment-Resistant Depression. Br. J. Psychiatry 2019, 215, 568. [Google Scholar] [CrossRef]
- Martone, G. Enhancement of Recovery from Mental Illness with L-Methylfolate Supplementation. Perspect. Psychiatr. Care 2018, 54, 331–334. [Google Scholar] [CrossRef]
- Tretter, F.; Albus, M. Systems Biology and Psychiatry—Modeling Molecular and Cellular Networks of Mental Disorders. Pharmacopsychiatry 2008, 41, S2–S18. [Google Scholar] [CrossRef]
- Manchia, M.; Pisanu, C.; Squassina, A.; Carpiniello, B. Challenges and Future Prospects of Precision Medicine in Psychiatry. Pharmgenomics Pers. Med. 2020, 13, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, K.; Buchanan, J.; Fermont, J.M.; Dreau, H.; Tilley, M.W.; Taylor, J.M.; Antoniou, P.; Knight, S.J.L.; Camps, C.; Pentony, M.M.; et al. The Complete Costs of Genome Sequencing: A Microcosting Study in Cancer and Rare Diseases from a Single Center in the United Kingdom. Genet. Med. 2020, 22, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kautzky, A.; Lanzenberger, R.; Kasper, S. Big Data Guided Interventions: Predicting Treatment Response. In Personalized Psychiatry; Springer International Publishing: Cham, Switzerland, 2019; pp. 53–76. [Google Scholar]
- Jägerstad, M. Folic Acid Fortification Prevents Neural Tube Defects and May Also Reduce Cancer Risks. Acta Paediatr. 2012, 101, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Folic Acid and Neural Tube Defects. Available online: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/prevention-and-risk-factors/folic-acid-neural-tube-defects_en (accessed on 1 July 2023).
- Harrington, A. “Seeing” the Placebo Effect: Historical Legacies and Present Opportunities. In The Science of the Placebo: Toward an Interdisciplinary Research Agenda; Guess, H., Kleinman, A., Kusek, J., Engel, L., Eds.; BMJ Books: London, UK, 2002; pp. 35–53. [Google Scholar]
- Severus, E.; Laber, E.; Lipkovich, I. Double-Blind Randomized Placebo-Controlled Trials in the Treatment of Affective Disorders: Problems and Alternatives. Curr. Treat. Options Psychiatry 2015, 2, 262–270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liwinski, T.; Lang, U.E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients 2023, 15, 3859. https://doi.org/10.3390/nu15173859
Liwinski T, Lang UE. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients. 2023; 15(17):3859. https://doi.org/10.3390/nu15173859
Chicago/Turabian StyleLiwinski, Timur, and Undine E. Lang. 2023. "Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review" Nutrients 15, no. 17: 3859. https://doi.org/10.3390/nu15173859
APA StyleLiwinski, T., & Lang, U. E. (2023). Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients, 15(17), 3859. https://doi.org/10.3390/nu15173859




