Targeting Metabolic Syndrome in Hidradenitis Suppurativa by Phytochemicals as a Potential Complementary Therapeutic Strategy
Abstract
1. Hidradenitis Suppurativa
2. Metabolic Alterations in HS
3. Proinflammatory Mechanisms of Obesity
4. HS Therapy: Time for a New Perspective?
5. Phytotherapy—A Therapeutic Concept with a Long History
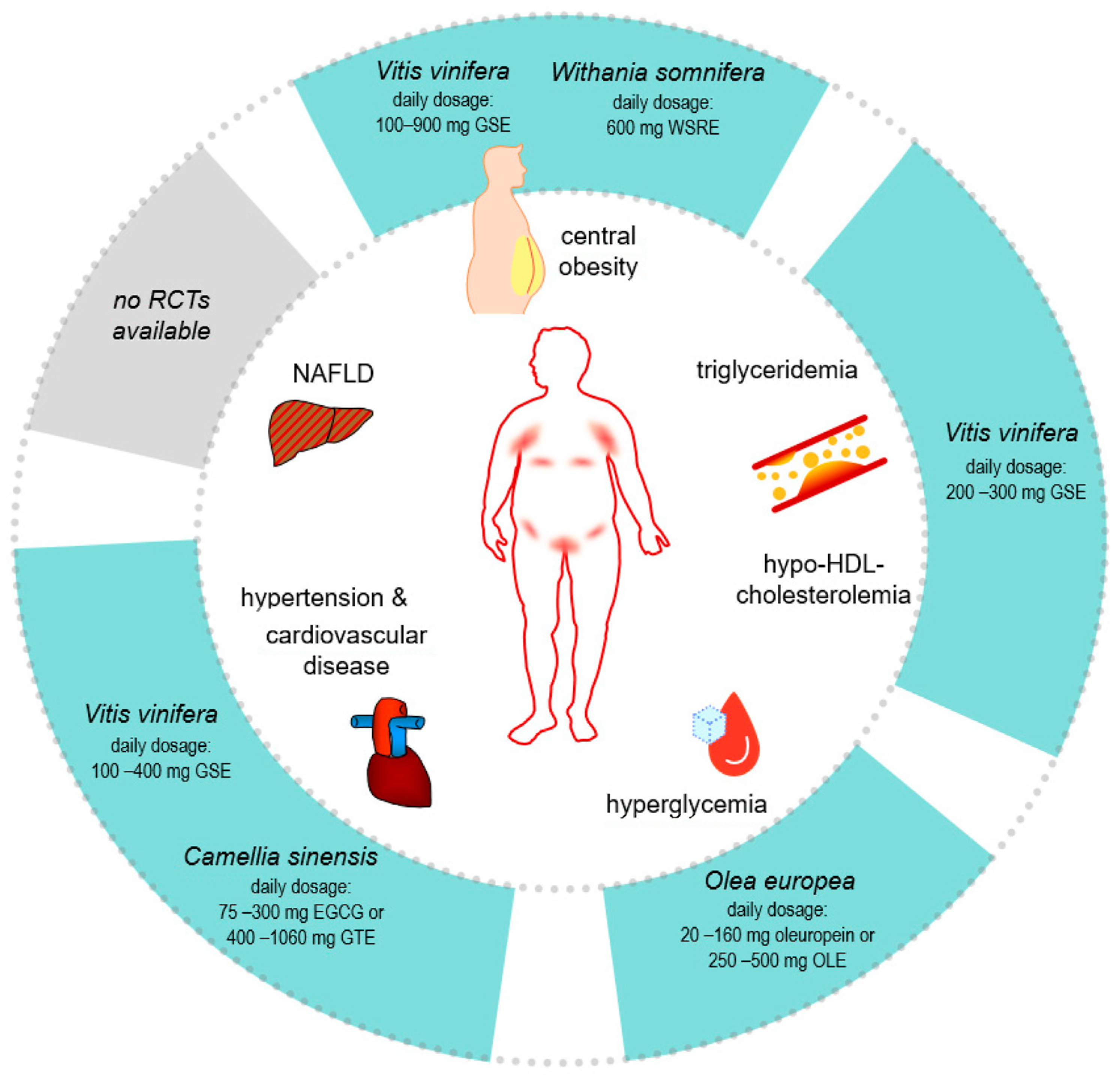
6. Potential Phytotherapeutic Options for MetS in HS Patients
6.1. Olea europea
6.2. Withania somnifera
6.3. Vitis vinifera
6.4. Camellia sinensis
7. General Aspects of the Future Use of Phytochemicals in HS Patients
8. Safety and Drug Interaction
9. Recommendations for Integrated Phytotherapy Targeting MetS Parameters in HS Patients
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabat, R.; Jemec, G.B.E.; Matusiak, L.; Kimball, A.B.; Prens, E.; Wolk, K. Hidradenitis suppurativa. Nat. Rev. Dis. Prim. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Alsadhan, H.; Alfawzan, A.I.; Yaqoub, A.; Almoneef, A.; Almohideb, M. Hidradenitis Suppurativa: Estimated Prevalence, Clinical Features, and Risk Factors in Riyadh, Saudi Arabia. Cureus 2022, 14, e23029. [Google Scholar] [CrossRef]
- Hagan, P.G.; Bouazzi, D.; Nyarko, G.; Dartey, E.S.; Nunoo-Ghartey, K.B.; Nkum, D.; Ansu, P.; Boakye, J.Y.; Andersen, R.K.; Boer, J.; et al. Prevalence of Hidradenitis Suppurativa in Berekum, Ghana. Br. J. Dermatol. 2022, 187, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Avila, D.G.; Anzola, L.C.; Cardona, L.P.G. Prevalence of Hidradenitis Suppurativa in Colombia According to Data from the National Health Registry. Skinmed 2021, 19, 369–373. [Google Scholar] [PubMed]
- Han, H.R.; Choi, C.E.E.; Nagad, M.; Patwardhan, K.R.; Boer, J.; Jemec, G.B.E.; Chandran, N.S. Prevalence and perceptions towards hidradenitis suppurativa: A cross-sectional study in a non-dermatological outpatient population. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e392–e394. [Google Scholar] [CrossRef] [PubMed]
- Prens, L.M.; Bouwman, K.; Troelstra, L.D.; Prens, E.P.; Alizadeh, B.Z.; Horvath, B. New insights in hidradenitis suppurativa from a population-based Dutch cohort: Prevalence, smoking behaviour, socioeconomic status and comorbidities. Br. J. Dermatol. 2022, 186, 814–822. [Google Scholar] [CrossRef]
- Kokolakis, G.; Wolk, K.; Schneider-Burrus, S.; Kalus, S.; Barbus, S.; Gomis-Kleindienst, S.; Sabat, R. Delayed Diagnosis of Hidradenitis Suppurativa and Its Effect on Patients and Healthcare System. Dermatology 2020, 236, 421–430. [Google Scholar] [CrossRef]
- Liakou, A.I.; Papadakis, M.; Tsantes, A.G.; Tsante, K.A.; Kontochristopoulos, G.; Marnelakis, I.; Katoulis, A.; Grigoriou, S.; Rigopoulos, D. Perception and Knowledge of Hidradenitis Suppurativa in Greece: A Cross-Sectional Study of 1301 Individuals. Indian J. Dermatol. 2022, 67, 835. [Google Scholar] [CrossRef]
- Sabat, R.; Tsaousi, A.; Ghoreschi, K.; Wolk, K.; Schneider-Burrus, S. Sex-disaggregated population analysis in patients with hidradenitis suppurativa. Front. Med. 2022, 9, 1028943. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Lux, G.; van der Linde, K.; Barbus, S.; Huss-Marp, J.; Tsaousi, A.; Wasem, J.; Wolff, B.; Sabat, R. Hidradenitis suppurativa—Prevalence analyses of German statutory health insurance data. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e32–e35. [Google Scholar] [CrossRef]
- Sokumbi, O.; Hodge, D.O.; Ederaine, S.A.; Alavi, A.; Alikhan, A. Comorbid diseases of hidradenitis suppurativa: A 15-year population-based study in Olmsted County, Minnesota, USA. Int. J. Dermatol. 2022, 61, 1372–1379. [Google Scholar] [CrossRef]
- Liang, Y.T.; Yeh, C.J.; Huang, J.Y.; Wei, J.C. Epidemiology of hidradenitis suppurativa in Taiwan: A 14-year nationwide population-based study. J. Dermatol. 2021, 48, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Heo, Y.W.; Lee, J.H.; Lee, S. Epidemiology and comorbidity of hidradenitis suppurativa in Korea for 17 years: A nationwide population-based cohort study. J. Dermatol. 2023, 50, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Burrus, S.; Tsaousi, A.; Barbus, S.; Huss-Marp, J.; Witte, K.; Wolk, K.; Fritz, B.; Sabat, R. Features Associated With Quality of Life Impairment in Hidradenitis Suppurativa Patients. Front. Med. 2021, 8, 676241. [Google Scholar] [CrossRef]
- Glowaczewska, A.; Reszke, R.; Szepietowski, J.C.; Matusiak, L. Indirect Self-Destructiveness in Hidradenitis Suppurativa Patients. J. Clin. Med. 2021, 10, 4194. [Google Scholar] [CrossRef] [PubMed]
- Rymaszewska, J.E.; Krajewski, P.K.; Szczech, J.; Szepietowski, J.C. Depression and anxiety in hidradenitis suppurativa patients: A cross-sectional study among Polish patients. Postep. Dermatol. Alergol. 2023, 40, 35–39. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Jost, A.; Peters, E.M.J.; Witte-Haendel, E.; Sterry, W.; Sabat, R. Association of Hidradenitis Suppurativa With Body Image. JAMA Dermatol. 2018, 154, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, G.; Yildiz, I.; Karaismailoglu, E.; Esme, P. Disease severity and poor mental health are the main predictors of stigmatization in patients with hidradenitis suppurativa. Dermatol. Ther. 2021, 34, e14910. [Google Scholar] [CrossRef]
- Rymaszewska, J.E.; Krajewski, P.K.; Matusiak, L.; Maj, J.; Szepietowski, J.C. Satisfaction with Life and Coping Strategies among Patients with Hidradenitis Suppurativa: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 2755. [Google Scholar] [CrossRef]
- Singh, R.; Kelly, K.A.; Senthilnathan, A.; Feldman, S.R.; Pichardo, R.O. Stigmatization, a social perception which may have a debilitating impact on hidradenitis suppurativa patients: An observational study. Arch. Dermatol. Res. 2023, 315, 1049–1052. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Kalus, S.; Fritz, B.; Wolk, K.; Gomis-Kleindienst, S.; Sabat, R. The impact of hidradenitis suppurativa on professional life. Br. J. Dermatol. 2023, 188, 122–130. [Google Scholar] [CrossRef]
- Wolk, K.; Join-Lambert, O.; Sabat, R. Aetiology and pathogenesis of hidradenitis suppurativa. Br. J. Dermatol. 2020, 183, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Hana, A.; Booken, D.; Henrich, C.; Gratchev, A.; Maas-Szabowski, N.; Goerdt, S.; Kurzen, H. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci. 2007, 80, 2214–2220. [Google Scholar] [CrossRef]
- Radek, K.A.; Elias, P.M.; Taupenot, L.; Mahata, S.K.; O’Connor, D.T.; Gallo, R.L. Neuroendocrine nicotinic receptor activation increases susceptibility to bacterial infections by suppressing antimicrobial peptide production. Cell Host Microbe 2010, 7, 277–289. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, Y.; Xu, T.; Zhang, Q.Z.; Bai, J.; Wang, J.; Zhu, T.; Lou, Q.; Gotz, F.; Qu, D.; et al. Nicotine Enhances Staphylococcus epidermidis Biofilm Formation by Altering the Bacterial Autolysis, Extracellular DNA Releasing, and Polysaccharide Intercellular Adhesin Production. Front. Microbiol. 2018, 9, 2575. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Warszawska, K.; Hoeflich, C.; Witte, E.; Schneider-Burrus, S.; Witte, K.; Kunz, S.; Buss, A.; Roewert, H.J.; Krause, M.; et al. Deficiency of IL-22 contributes to a chronic inflammatory disease: Pathogenetic mechanisms in acne inversa. J. Immunol. 2011, 186, 1228–1239. [Google Scholar] [CrossRef]
- Witte-Handel, E.; Wolk, K.; Tsaousi, A.; Irmer, M.L.; Mossner, R.; Shomroni, O.; Lingner, T.; Witte, K.; Kunkel, D.; Salinas, G.; et al. The IL-1 Pathway Is Hyperactive in Hidradenitis Suppurativa and Contributes to Skin Infiltration and Destruction. J. Investig. Dermatol. 2019, 139, 1294–1305. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Szepietowski, J.C.; Martorell, A. Tunnels in Hidradenitis Suppurativa: Active Inflammatory Entities with Specific Molecular and Genetic Profiles—A Narrative Review. Dermatology 2023, 239, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.L.; Karl, I.; Giner, T.; Poppe, H.; Schmidt, M.; Presser, D.; Goebeler, M.; Bauer, B. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br. J. Dermatol. 2016, 174, 514–521. [Google Scholar] [CrossRef]
- Moran, B.; Smith, C.M.; Zabarowski, A.; Ryan, M.; Karman, J.; Dunstan, R.W.; Smith, K.M.; Hambly, R.; Musilova, J.; Petrasca, A.; et al. Targeting the NLRP3 inflammasome reduces inflammation in hidradenitis suppurativa skin. Br. J. Dermatol. 2023, ljad184. [Google Scholar] [CrossRef]
- Sabat, R.; Simaite, D.; Gudjonsson, J.E.; Brembach, T.C.; Witte, K.; Krause, T.; Kokolakis, G.; Bartnik, E.; Nikolaou, C.; Rill, N.; et al. Neutrophilic granulocyte-derived B-cell activating factor supports B cells in skin lesions in hidradenitis suppurativa. J. Allergy Clin. Immunol. 2023, 151, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Tsaousi, A.; Witte, E.; Witte, K.; Rowert-Huber, H.J.; Volk, H.D.; Sterry, W.; Wolk, K.; Schneider-Burrus, S.; Sabat, R. MMP8 Is Increased in Lesions and Blood of Acne Inversa Patients: A Potential Link to Skin Destruction and Metabolic Alterations. Mediat. Inflamm. 2016, 2016, 4097574. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Brembach, T.C.; Simaite, D.; Bartnik, E.; Cucinotta, S.; Pokrywka, A.; Irmer, M.L.; Triebus, J.; Witte-Handel, E.; Salinas, G.; et al. Activity and components of the granulocyte colony-stimulating factor pathway in hidradenitis suppurativa. Br. J. Dermatol. 2021, 185, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Wenzel, J.; Tsaousi, A.; Witte-Handel, E.; Babel, N.; Zelenak, C.; Volk, H.D.; Sterry, W.; Schneider-Burrus, S.; Sabat, R. Lipocalin-2 is expressed by activated granulocytes and keratinocytes in affected skin and reflects disease activity in acne inversa/hidradenitis suppurativa. Br. J. Dermatol. 2017, 177, 1385–1393. [Google Scholar] [CrossRef]
- Kanni, T.; Zenker, O.; Habel, M.; Riedemann, N.; Giamarellos-Bourboulis, E.J. Complement activation in hidradenitis suppurativa: A new pathway of pathogenesis? Br. J. Dermatol. 2018, 179, 413–419. [Google Scholar] [CrossRef]
- Macchiarella, G.; Cornacchione, V.; Cojean, C.; Riker, J.; Wang, Y.; Te, H.; Ceci, M.; Gudjonsson, J.E.; Gaulis, S.; Goetschy, J.F.; et al. Disease Association of Anti–Carboxyethyl Lysine Autoantibodies in Hidradenitis Suppurativa. J. Investig. Dermatol. 2023, 143, 273–283 e212. [Google Scholar] [CrossRef]
- Oliveira, C.B.; Byrd, A.S.; Okoye, G.A.; Kaplan, M.J.; Carmona-Rivera, C. Neutralizing Anti–DNase 1 and –DNase 1L3 Antibodies Impair Neutrophil Extracellular Traps Degradation in Hidradenitis Suppurativa. J. Investig. Dermatol. 2023, 143, 57–66. [Google Scholar] [CrossRef]
- Sabat, R.; Grutz, G.; Warszawska, K.; Kirsch, S.; Witte, E.; Wolk, K.; Geginat, J. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010, 21, 331–344. [Google Scholar] [CrossRef]
- Wolk, K.; Docke, W.; von Baehr, V.; Volk, H.; Sabat, R. Comparison of monocyte functions after LPS- or IL-10-induced reorientation: Importance in clinical immunoparalysis. Pathobiology 1999, 67, 253–256. [Google Scholar] [CrossRef]
- Sabat, R.; Chanwangpong, A.; Schneider-Burrus, S.; Metternich, D.; Kokolakis, G.; Kurek, A.; Philipp, S.; Uribe, D.; Wolk, K.; Sterry, W. Increased prevalence of metabolic syndrome in patients with acne inversa. PLoS ONE 2012, 7, e31810. [Google Scholar] [CrossRef]
- Damiani, G.; Leone, S.; Fajgenbaum, K.; Bragazzi, N.L.; Pacifico, A.; Conic, R.R.; Pigatto, P.D.; Maiorana, C.; Poli, P.; Berti, E.; et al. Nonalcoholic fatty liver disease prevalence in an Italian cohort of patients with hidradenitis suppurativa: A multi-center retrospective analysis. World J. Hepatol. 2019, 11, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Deckers, I.E.; Benhadou, F.; Koldijk, M.J.; Del Marmol, V.; Horvath, B.; Boer, J.; van der Zee, H.H.; Prens, E.P. Inflammatory bowel disease is associated with hidradenitis suppurativa: Results from a multicenter cross-sectional study. J. Am. Acad. Dermatol. 2017, 76, 49–53. [Google Scholar] [CrossRef]
- Duran-Vian, C.; Arias-Loste, M.T.; Hernandez, J.L.; Fernandez, V.; Gonzalez, M.; Iruzubieta, P.; Rasines, L.; Gonzalez-Vela, C.; Vaque, J.P.; Blanco, R.; et al. High prevalence of non-alcoholic fatty liver disease among hidradenitis suppurativa patients independent of classic metabolic risk factors. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Gislason, G.H.; Hansen, P.R. Risk of Major Adverse Cardiovascular Events and All-Cause Mortality in Patients With Hidradenitis Suppurativa. JAMA Dermatol. 2016, 152, 429–434. [Google Scholar] [CrossRef]
- Egeberg, A.; Jemec, G.B.E.; Kimball, A.B.; Bachelez, H.; Gislason, G.H.; Thyssen, J.P.; Mallbris, L. Prevalence and Risk of Inflammatory Bowel Disease in Patients with Hidradenitis Suppurativa. J. Investig. Dermatol. 2017, 137, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Villanueva, I.; DeGracia, C.; Planells, M.; Poveda, I.; Alvarez, P.; Schneller-Pavalescu, L.; Betlloch, I.; Jemec, G.B.E.; Ramos, J.M.; Pascual, J.C. Hidradenitis Suppurativa is Associated with Non-alcoholic Fatty Liver Disease: A Cross-sectional Study. Acta Derm. Venereol. 2020, 100, adv00239. [Google Scholar] [CrossRef]
- Richette, P.; Molto, A.; Viguier, M.; Dawidowicz, K.; Hayem, G.; Nassif, A.; Wendling, D.; Aubin, F.; Liote, F.; Bachelez, H. Hidradenitis suppurativa associated with spondyloarthritis—Results from a multicenter national prospective study. J. Rheumatol. 2014, 41, 490–494. [Google Scholar] [CrossRef]
- Schneider-Burrus, S.; Witte-Haendel, E.; Christou, D.; Rigoni, B.; Sabat, R.; Diederichs, G. High Prevalence of Back Pain and Axial Spondyloarthropathy in Patients with Hidradenitis Suppurativa. Dermatology 2016, 232, 606–612. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Sanchez-Pino, M.D.; Richardson, W.S.; Zabaleta, J.; Puttalingaiah, R.T.; Chapple, A.G.; Liu, J.; Kim, Y.; Ponder, M.; DeArmitt, R.; Baiamonte, L.B.; et al. Increased inflammatory low-density neutrophils in severe obesity and effect of bariatric surgery: Results from case-control and prospective cohort studies. EBioMedicine 2022, 77, 103910. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef]
- Soehnlein, O.; Steffens, S.; Hidalgo, A.; Weber, C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017, 17, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nagai, Y.; Honda, H.; Okamoto, N.; Yanagibashi, T.; Ogasawara, M.; Yamamoto, S.; Imamura, R.; Takasaki, I.; Hara, H.; et al. Bidirectional crosstalk between neutrophils and adipocytes promotes adipose tissue inflammation. FASEB J. 2019, 33, 11821–11835. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Sabat, R. Adipokines in psoriasis: An important link between skin inflammation and metabolic alterations. Rev. Endocr. Metab. Disord. 2016, 17, 305–317. [Google Scholar] [CrossRef]
- Gonzalez-Lopez, M.A.; Vilanova, I.; Ocejo-Vinals, G.; Arlegui, R.; Navarro, I.; Guiral, S.; Mata, C.; Perez-Paredes, M.G.; Portilla, V.; Corrales, A.; et al. Circulating levels of adiponectin, leptin, resistin and visfatin in non-diabetics patients with hidradenitis suppurativa. Arch. Dermatol. Res. 2020, 312, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Malara, A.; Hughes, R.; Jennings, L.; Sweeney, C.M.; Lynch, M.; Awdeh, F.; Timoney, I.; Tobin, A.M.; Lynam-Loane, K.; Tobin, L.; et al. Adipokines are dysregulated in patients with hidradenitis suppurativa. Br. J. Dermatol. 2018, 178, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, P.K.; Matusiak, L.; Szepietowski, J.C. Adipokines as an important link between hidradenitis suppurativa and obesity: A narrative review. Br. J. Dermatol. 2023, 188, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak, A.M.; Jalilvand, A.; Hsueh, W.A. Adipocytes, Innate Immunity and Obesity: A Mini-Review. Front. Immunol. 2021, 12, 650768. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Schneider-Burrus, S.; Salinas, G.; Mossner, R.; Ghoreschi, K.; Wolk, K.; Sabat, R. Blood T Helper Memory Cells: A Tool for Studying Skin Inflammation in HS? Int. J. Mol. Sci. 2023, 24, 8854. [Google Scholar] [CrossRef]
- Kadowaki, S.; Okamura, T.; Hozawa, A.; Kadowaki, T.; Kadota, A.; Murakami, Y.; Nakamura, K.; Saitoh, S.; Nakamura, Y.; Hayakawa, T.; et al. Relationship of elevated casual blood glucose level with coronary heart disease, cardiovascular disease and all-cause mortality in a representative sample of the Japanese population. NIPPON DATA80. Diabetologia 2008, 51, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Sukhorukov, V.N.; Orekhov, A.N. Effect of Glucose Levels on Cardiovascular Risk. Cells 2022, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Riise, H.K.R.; Igland, J.; Sulo, G.; Graue, M.; Haltbakk, J.; Tell, G.S.; Iversen, M.M. Casual blood glucose and subsequent cardiovascular disease and all-cause mortality among 159 731 participants in Cohort of Norway (CONOR). BMJ Open Diabetes Res. Care 2021, 9, e001928. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Miller, I.M.; Ellervik, C.; Vinding, G.R.; Zarchi, K.; Ibler, K.S.; Knudsen, K.M.; Jemec, G.B. Association of metabolic syndrome and hidradenitis suppurativa. JAMA Dermatol. 2014, 150, 1273–1280. [Google Scholar] [CrossRef]
- Kromann, C.B.; Ibler, K.S.; Kristiansen, V.B.; Jemec, G.B. The influence of body weight on the prevalence and severity of hidradenitis suppurativa. Acta Derm. Venereol. 2014, 94, 553–557. [Google Scholar] [CrossRef]
- Sivanand, A.; Gulliver, W.P.; Josan, C.K.; Alhusayen, R.; Fleming, P.J. Weight Loss and Dietary Interventions for Hidradenitis Suppurativa: A Systematic Review. J. Cutan. Med. Surg. 2020, 24, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Stagakis, I.; Bertsias, G.; Karvounaris, S.; Kavousanaki, M.; Virla, D.; Raptopoulou, A.; Kardassis, D.; Boumpas, D.T.; Sidiropoulos, P.I. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res. Ther. 2012, 14, R141. [Google Scholar] [CrossRef]
- Tiri, H.; Jokelainen, J.; Timonen, M.; Tasanen, K.; Huilaja, L. Substantially reduced life expectancy in patients with hidradenitis suppurativa: A Finnish nationwide registry study. Br. J. Dermatol. 2019, 180, 1543–1544. [Google Scholar] [CrossRef] [PubMed]
- Scholl, L.; Schneider-Burrus, S.; Fritz, B.; Sabat, R.; Bechara, F.G. The impact of surgical interventions on the psychosocial well-being of patients with hidradenitis suppurativa. J. Dtsch. Dermatol. Ges. 2023, 21, 131–139. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schon, M.P.; Stander, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Price, K.N.; Collier, E.K.; Grogan, T.; Fernandez, J.M.; Alhusayen, R.; Alavi, A.; Hamzavi, I.H.; Lowes, M.A.; Porter, M.J.; Hsiao, J.L.; et al. Physician perspectives on complementary and alternative medicine in hidradenitis suppurativa. Dermatol. Ther. 2021, 34, e14851. [Google Scholar] [CrossRef] [PubMed]
- Price, K.N.; Thompson, A.M.; Rizvi, O.; Hendricks, A.J.; Alavi, A.; Hsiao, J.L.; Shi, V.Y. Complementary and Alternative Medicine Use in Patients With Hidradenitis Suppurativa. JAMA Dermatol. 2020, 156, 345–348. [Google Scholar] [CrossRef]
- Hassan, H.M. A Short History of the Use of Plants as Medicines from Ancient Times. Chimia 2015, 69, 622–623. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, P.; Brand, E. A concise classification of bencao (materia medica). Chin. Med. 2018, 13, 18. [Google Scholar] [CrossRef]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Calisher, C.H. Taxonomy: What’s in a name? Doesn’t a rose by any other name smell as sweet? Croat. Med. J. 2007, 48, 268–270. [Google Scholar]
- Witte, K.; Sabat, R.; Witte-Handel, E.; Ghoreschi, K.; Wolk, K. Phytotherapeuthics Affecting the IL-1/IL-17/G-CSF Axis: A Complementary Treatment Option for Hidradenitis Suppurativa? Int. J. Mol. Sci. 2022, 23, 9057. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Revel, J.S.; Maier, C.S. Mitochondria-Centric Review of Polyphenol Bioactivity in Cancer Models. Antioxid. Redox Signal. 2018, 29, 1589–1611. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.A.; Lopez-Villodres, J.A.; Asensi, R.; Espartero, J.L.; Rodriguez-Gutierez, G.; De La Cruz, J.P. Virgin olive oil polyphenol hydroxytyrosol acetate inhibits in vitro platelet aggregation in human whole blood: Comparison with hydroxytyrosol and acetylsalicylic acid. Br. J. Nutr. 2009, 101, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Vari, R.; D’Archivio, M.; Di Benedetto, R.; Matarrese, P.; Malorni, W.; Scazzocchio, B.; Giovannini, C. Extra virgin olive oil biophenols inhibit cell-mediated oxidation of LDL by increasing the mRNA transcription of glutathione-related enzymes. J. Nutr. 2004, 134, 785–791. [Google Scholar] [CrossRef]
- Singh, I.; Mok, M.; Christensen, A.M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 127–132. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Rosello-Catafau, J.; Pinto, X.; Mitjavila, M.T.; Moreno, J.J. Polyphenol fraction of extra virgin olive oil protects against endothelial dysfunction induced by high glucose and free fatty acids through modulation of nitric oxide and endothelin-1. Redox Biol. 2014, 2, 971–977. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Ma, Y.; Wen, D. Hydroxytyrosol ameliorates insulin resistance by modulating endoplasmic reticulum stress and prevents hepatic steatosis in diet-induced obesity mice. J. Nutr. Biochem 2018, 57, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, C.; Mariyam, Z.; Jiang, P.; Zhou, M.; Zeb, F.; Haq, I.U.; Chen, A.; Feng, Q. Acrolein-induced atherogenesis by stimulation of hepatic flavin containing monooxygenase 3 and a protection from hydroxytyrosol. J. Cell. Physiol. 2018, 234, 475–485. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Scoditti, E.; Calabriso, N.; Massaro, M.; Pellegrino, M.; Storelli, C.; Martines, G.; De Caterina, R.; Carluccio, M.A. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: A potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch. Biochem. Biophys. 2012, 527, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Coni, E.; Di Benedetto, R.; Di Pasquale, M.; Masella, R.; Modesti, D.; Mattei, R.; Carlini, E.A. Protective effect of oleuropein, an olive oil biophenol, on low density lipoprotein oxidizability in rabbits. Lipids 2000, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Mahmoudi, A.; Chamkha, M.; Isoda, H.; Sayadi, S. Olive Leaves Extract and Oleuropein Improve Insulin Sensitivity in 3T3-L1 Cells and in High-Fat Diet-Treated Rats via PI3K/AkT Signaling Pathway. Oxid. Med. Cell. Longev. 2023, 2023, 6828230. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef]
- Jemai, H.; El Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Khalili, A.; Nekooeian, A.A.; Khosravi, M.B. Oleuropein improves glucose tolerance and lipid profile in rats with simultaneous renovascular hypertension and type 2 diabetes. J. Asian Nat. Prod. Res. 2017, 19, 1011–1021. [Google Scholar] [CrossRef]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sanchez, M.; Toral, M.; Martin-Garcia, B.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Liu, Z.; Liu, L.; Yang, K.; Wei, Y.; Liu, Y.; Chen, X.; Sun, X.; Wen, D. Hydroxytyrosol prevents PM2.5-induced adiposity and insulin resistance by restraining oxidative stress related NF-kappaB pathway and modulation of gut microbiota in a murine model. Free Radic. Biol. Med. 2019, 141, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative Study on Beneficial Effects of Hydroxytyrosol- and Oleuropein-Rich Olive Leaf Extracts on High-Fat Diet-Induced Lipid Metabolism Disturbance and Liver Injury in Rats. BioMed Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverria, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-gamma and NF-kappaB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Lemonakis, N.; Efentakis, P.; Gikas, E.; Halabalaki, M.; Andreadou, I.; Skaltsounis, L.; Brown, L. Hydroxytyrosol ameliorates metabolic, cardiovascular and liver changes in a rat model of diet-induced metabolic syndrome: Pharmacological and metabolism-based investigation. Pharmacol. Res. 2017, 117, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Stefanon, B. Different anti-adipogenic effects of bio-compounds on primary visceral pre-adipocytes and adipocytes. EXCLI J. 2016, 15, 362–377. [Google Scholar] [CrossRef]
- Drira, R.; Sakamoto, K. Hydroxytyrosol stimulates lipolysis via A-kinase and extracellular signal-regulated kinase activation in 3T3-L1 adipocytes. Eur. J. Nutr. 2014, 53, 743–750. [Google Scholar] [CrossRef]
- Stefanon, B.; Colitti, M. Original Research: Hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation. Exp. Biol. Med. 2016, 241, 1796–1802. [Google Scholar] [CrossRef]
- Scoditti, E.; Massaro, M.; Carluccio, M.A.; Pellegrino, M.; Wabitsch, M.; Calabriso, N.; Storelli, C.; De Caterina, R. Additive regulation of adiponectin expression by the mediterranean diet olive oil components oleic Acid and hydroxytyrosol in human adipocytes. PLoS ONE 2015, 10, e0128218. [Google Scholar] [CrossRef]
- Lopez-Villodres, J.A.; Abdel-Karim, M.; De La Cruz, J.P.; Rodriguez-Perez, M.D.; Reyes, J.J.; Guzman-Moscoso, R.; Rodriguez-Gutierrez, G.; Fernandez-Bolanos, J.; Gonzalez-Correa, J.A. Effects of hydroxytyrosol on cardiovascular biomarkers in experimental diabetes mellitus. J. Nutr. Biochem. 2016, 37, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Iliodromitis, E.K.; Mikros, E.; Constantinou, M.; Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Kamber, E.; Tsantili-Kakoulidou, A.; Kremastinos, D.T. The olive constituent oleuropein exhibits anti-ischemic, antioxidative, and hypolipidemic effects in anesthetized rabbits. J. Nutr. 2006, 136, 2213–2219. [Google Scholar] [CrossRef]
- Dub, A.M.; Dugani, A.M. Antithrombotic effect of repeated doses of the ethanolic extract of local olive (Olea europaea L.) leaves in rabbits. Libyan J. Med. 2013, 8, 20947. [Google Scholar] [CrossRef] [PubMed]
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Oleuropein offers cardioprotection in rats with simultaneous type 2 diabetes and renal hypertension. Indian J. Pharmacol. 2014, 46, 398–403. [Google Scholar] [CrossRef]
- Nekooeian, A.A.; Khalili, A.; Khosravi, M.B. Effects of oleuropein in rats with simultaneous type 2 diabetes and renal hypertension: A study of antihypertensive mechanisms. J. Asian Nat. Prod. Res. 2014, 16, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.H.; Chen, J.; Xie, L.; Cai, X.M.; Yang, R.H.; Wang, X.; Gong, J.B. Hydroxytyrosol Protects against Myocardial Ischemia/Reperfusion Injury through a PI3K/Akt-Dependent Mechanism. Mediat. Inflamm. 2016, 2016, 1232103. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Toral, M.; Gomez-Guzman, M.; Jimenez, R.; Galindo, P.; Sanchez, M.; Olivares, M.; Galvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol Plays Antiatherosclerotic Effects through Regulating Lipid Metabolism via Inhibiting the p38 Signal Pathway. BioMed Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Stojiljkovic, N.; Stojanovic, N.; Stoiljkovic, M.; Mitic, K.; Salinger-Martinovic, S.; Randjelovic, P. Effects of oleuropein on rat’s atria and thoracic aorta: A study of antihypertensive mechanisms. Can. J. Physiol. Pharmacol. 2021, 99, 110–114. [Google Scholar] [CrossRef]
- Gonzalez-Correa, J.A.; Navas, M.D.; Munoz-Marin, J.; Trujillo, M.; Fernandez-Bolanos, J.; de la Cruz, J.P. Effects of hydroxytyrosol and hydroxytyrosol acetate administration to rats on platelet function compared to acetylsalicylic acid. J. Agric. Food Chem. 2008, 56, 7872–7876. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B.; et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef]
- Valenzuela, R.; Echeverria, F.; Ortiz, M.; Rincon-Cervera, M.A.; Espinosa, A.; Hernandez-Rodas, M.C.; Illesca, P.; Valenzuela, A.; Videla, L.A. Hydroxytyrosol prevents reduction in liver activity of Delta-5 and Delta-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis. 2017, 16, 64. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- de Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Nyambe-Silavwe, H.; Pyner, A.; Oladele, E.; Gauer, J.S.; Stevens, Y.; Williamson, G. Nutritional implications of olives and sugar: Attenuation of post-prandial glucose spikes in healthy volunteers by inhibition of sucrose hydrolysis and glucose transport by oleuropein. Eur. J. Nutr. 2019, 58, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; D’Avino, M.; Buonomo, G.; Caruso, G.; Ciampaglia, R.; Schiano, E.; Maisto, M.; Annunziata, G.; Novellino, E. A Pilot Screening of Agro-Food Waste Products as Sources of Nutraceutical Formulations to Improve Simulated Postprandial Glycaemia and Insulinaemia in Healthy Subjects. Nutrients 2020, 12, 1292. [Google Scholar] [CrossRef]
- Javadi, H.; Yaghoobzadeh, H.; Esfahani, Z.; Reza Memarzadeh, M.; Mehdi Mirhashemi, S. Effects of Olive Leaf Extract on Metabolic Response, Liver and Kidney Functions and Inflammatory Biomarkers in Hypertensive Patients. Pak. J. Biol. Sci. 2019, 22, 342–348. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Stevens, Y.; Winkens, B.; Jonkers, D.; Masclee, A. The effect of olive leaf extract on cardiovascular health markers: A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2021, 60, 2111–2120. [Google Scholar] [CrossRef]
- Pyner, A.; Chan, S.Y.; Tumova, S.; Kerimi, A.; Williamson, G. Indirect Chronic Effects of an Oleuropein-Rich Olive Leaf Extract on Sucrase-Isomaltase In Vitro and In Vivo. Nutrients 2019, 11, 1505. [Google Scholar] [CrossRef]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.J.; Coxam, V. Twelve-month consumption of a polyphenol extract from olive (Olea europaea) in a double blind, randomized trial increases serum total osteocalcin levels and improves serum lipid profiles in postmenopausal women with osteopenia. J. Nutr. Health Aging 2015, 19, 77–86. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Lockyer, S.; Corona, G.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Secoiridoids delivered as olive leaf extract induce acute improvements in human vascular function and reduction of an inflammatory cytokine: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2015, 114, 75–83. [Google Scholar] [CrossRef]
- Gorelick, J.; Rosenberg, R.; Smotrich, A.; Hanus, L.; Bernstein, N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry 2015, 116, 283–289. [Google Scholar] [CrossRef] [PubMed]
- SoRelle, J.A.; Itoh, T.; Peng, H.; Kanak, M.A.; Sugimoto, K.; Matsumoto, S.; Levy, M.F.; Lawrence, M.C.; Naziruddin, B. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia 2013, 56, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Khalilpourfarshbafi, M.; Devi Murugan, D.; Abdul Sattar, M.Z.; Sucedaram, Y.; Abdullah, N.A. Withaferin A inhibits adipogenesis in 3T3-F442A cell line, improves insulin sensitivity and promotes weight loss in high fat diet-induced obese mice. PLoS ONE 2019, 14, e0218792. [Google Scholar] [CrossRef] [PubMed]
- Batumalaie, K.; Amin, M.A.; Murugan, D.D.; Sattar, M.Z.; Abdullah, N.A. Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci. Rep. 2016, 6, 27236. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Azmi, M.N.; Shariff, K.A.; Tan, J.S. Withaferin A Protects Against High-Fat Diet-Induced Obesity Via Attenuation of Oxidative Stress, Inflammation, and Insulin Resistance. Appl. Biochem. Biotechnol. 2019, 188, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Shiragannavar, V.D.; Sannappa Gowda, N.G.; Puttahanumantharayappa, L.D.; Karunakara, S.H.; Bhat, S.; Prasad, S.K.; Kumar, D.P.; Santhekadur, P.K. The ameliorating effect of withaferin A on high-fat diet-induced non-alcoholic fatty liver disease by acting as an LXR/FXR dual receptor activator. Front. Pharmacol. 2023, 14, 1135952. [Google Scholar] [CrossRef]
- Tekula, S.; Khurana, A.; Anchi, P.; Godugu, C. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed. Pharmacother. 2018, 106, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liu, J.; Wang, B.; Zhang, C.; Su, Z.; Zhao, M.; Qin, L.; Zhang, W.; Zheng, R. Withaferin A Promotes White Adipose Browning and Prevents Obesity Through Sympathetic Nerve-Activated Prdm16-FATP1 Axis. Diabetes 2022, 71, 249–263. [Google Scholar] [CrossRef]
- Lee, D.H.; Ahn, J.; Jang, Y.J.; Seo, H.D.; Ha, T.Y.; Kim, M.J.; Huh, Y.H.; Jung, C.H. Withania somnifera Extract Enhances Energy Expenditure via Improving Mitochondrial Function in Adipose Tissue and Skeletal Muscle. Nutrients 2020, 12, 431. [Google Scholar] [CrossRef]
- Lee, J.; Liu, J.; Feng, X.; Salazar Hernandez, M.A.; Mucka, P.; Ibi, D.; Choi, J.W.; Ozcan, U. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat. Med. 2016, 22, 1023–1032. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, S.H.; Lee, E.; Seo, H.D.; Ahn, J.; Jang, Y.J.; Ha, T.Y.; Im, S.S.; Jung, C.H. Withaferin A exerts an anti-obesity effect by increasing energy expenditure through thermogenic gene expression in high-fat diet-fed obese mice. Phytomedicine 2021, 82, 153457. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mohanty, I.; Talwar, K.K.; Dinda, A.; Joshi, S.; Bansal, P.; Saxena, A.; Arya, D.S. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: A hemodynamic, biochemical and histopathological assessment. Mol. Cell. Biochem. 2004, 260, 39–47. [Google Scholar] [CrossRef]
- Hamza, A.; Amin, A.; Daoud, S. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol. Toxicol. 2008, 24, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, N.; Samuel, S.S.; Bora, H.K.; Sharma, S.; Pachauri, S.D.; Dwivedi, A.K.; Siddiqui, H.H.; Hanif, K. Withania somnifera shows a protective effect in monocrotaline-induced pulmonary hypertension. Pharm. Biol. 2015, 53, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.R.; Arya, D.S.; Gupta, S.K. Withania somnifera provides cardioprotection and attenuates ischemia-reperfusion induced apoptosis. Clin. Nutr. 2008, 27, 635–642. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, R.; Gan, L.; Lau, W.B.; Cao, X.; Zhao, J.; Ma, X.; Christopher, T.A.; Lopez, B.L.; Wang, Y. Withaferin A inhibits apoptosis via activated Akt-mediated inhibition of oxidative stress. Life Sci. 2018, 211, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Yan, T.; Kim, D.; Dias, H.B.; Krausz, K.W.; Kimura, S.; Gonzalez, F.J. Withaferin A Improves Nonalcoholic Steatohepatitis in Mice. J. Pharmacol. Exp. Ther. 2019, 371, 360–374. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Urrunaga, N.H.; Dash, S.; Khurana, S.; Saxena, N.K. Withaferin-A Reduces Acetaminophen-Induced Liver Injury in Mice. Biochem. Pharmacol. 2015, 97, 122–132. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, P.; Yan, N.; Gonzalez, F.J.; Yan, T. Withaferin A alleviates fulminant hepatitis by targeting macrophage and NLRP3. Cell Death Dis. 2021, 12, 174. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults Under Chronic Stress Through Treatment With Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Evid. Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef]
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2015, 12, 43. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, S.K.; Pathak, A.K. A double-blind, randomized, placebo-controlled trial on the effect of Ashwagandha (Withania somnifera dunal.) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults. J. Ethnopharmacol. 2021, 272, 113929. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ambiye, V.R.; Langade, D.; Dongre, S.; Aptikar, P.; Kulkarni, M.; Dongre, A. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evid. Based Complement. Altern. Med. 2013, 2013, 571420. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Rajender, S.; Shankhwar, S.N.; Singh, V.; Dalela, D. Withania somnifera Improves Semen Quality in Stress-Related Male Fertility. Evid. Based Complement. Altern. Med. 2009, 2011, 576962. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Shah, B.; Shenoy, S.; Chauhan, S.; Lavekar, G.S.; Padhi, M.M. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int. J. Ayurveda Res. 2010, 1, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Blay, M.; Pujadas, G.; Garcia-Vallve, S.; Pinent, M.; Ardevol, A. Grape seed-derived procyanidins decrease dipeptidyl-peptidase 4 activity and expression. J. Agric. Food Chem. 2012, 60, 9055–9061. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Margalef, M.; Blay, M.; Arola-Arnal, A.; Muguerza, B.; Ardevol, A.; Pinent, M. A grape seed extract increases active glucagon-like peptide-1 levels after an oral glucose load in rats. Food Funct. 2014, 5, 2357–2364. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Wei, S.; Zheng, Y.; Zhang, M.; Zheng, H.; Yan, P. Grape seed procyanidin extract inhibits adipogenesis and stimulates lipolysis of porcine adipocytes in vitro. J. Anim. Sci. 2018, 96, 2753–2762. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor gamma with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017, 86, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Burton-Freeman, B.; Tissa Kappagoda, C. Mechanism of the endothelium-dependent relaxation evoked by a grape seed extract. Clin. Sci. 2008, 114, 331–337. [Google Scholar] [CrossRef]
- Feng, Z.; Wei, R.B.; Hong, Q.; Cui, S.Y.; Chen, X.M. Grape seed extract enhances eNOS expression and NO production through regulating calcium-mediated AKT phosphorylation in H2O2-treated endothelium. Cell Biol. Int. 2010, 34, 1055–1061. [Google Scholar] [CrossRef]
- Decorde, K.; Teissedre, P.L.; Sutra, T.; Ventura, E.; Cristol, J.P.; Rouanet, J.M. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol. Nutr. Food Res. 2009, 53, 659–666. [Google Scholar] [CrossRef]
- Meeprom, A.; Sompong, W.; Suwannaphet, W.; Yibchok-anun, S.; Adisakwattana, S. Grape seed extract supplementation prevents high-fructose diet-induced insulin resistance in rats by improving insulin and adiponectin signalling pathways. Br. J. Nutr. 2011, 106, 1173–1181. [Google Scholar] [CrossRef]
- Ohyama, K.; Furuta, C.; Nogusa, Y.; Nomura, K.; Miwa, T.; Suzuki, K. Catechin-rich grape seed extract supplementation attenuates diet-induced obesity in C57BL/6J mice. Ann. Nutr. Metab. 2011, 58, 250–258. [Google Scholar] [CrossRef]
- Rajasekhar, S.; Subramanyam, M.V.V.; Asha Devi, S. Grape seed proanthocyanidin extract suppresses oxidative stress in the rat pancreas of type-1 diabetes. Arch. Physiol. Biochem. 2021, 1–13. [Google Scholar] [CrossRef]
- Suwannaphet, W.; Meeprom, A.; Yibchok-Anun, S.; Adisakwattana, S. Preventive effect of grape seed extract against high-fructose diet-induced insulin resistance and oxidative stress in rats. Food Chem. Toxicol. 2010, 48, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Shen, S.; Jiang, J.; Tan, D.; Jiang, D.; Bai, B.; Sun, X.; Fu, S. Protective effects of grape seed extract fractions with different degrees of polymerisation on blood glucose, lipids and hepatic oxidative stress in diabetic rats. Nat. Prod. Res. 2015, 29, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Martinez-Micaelo, N.; Blay, M.; Ardevol, A.; Pinent, M. Grape-seed procyanidins prevent the cafeteria-diet-induced decrease of glucagon-like peptide-1 production. J. Agric. Food Chem. 2014, 62, 1066–1072. [Google Scholar] [CrossRef]
- Castell-Auvi, A.; Cedo, L.; Pallares, V.; Blay, M.; Pinent, M.; Ardevol, A. Grape seed procyanidins improve beta-cell functionality under lipotoxic conditions due to their lipid-lowering effect. J. Nutr. Biochem. 2013, 24, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Charradi, K.; Sebai, H.; Elkahoui, S.; Ben Hassine, F.; Limam, F.; Aouani, E. Grape seed extract alleviates high-fat diet-induced obesity and heart dysfunction by preventing cardiac siderosis. Cardiovasc. Toxicol. 2011, 11, 28–37. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Blade, C.; Suarez, M.; Arola-Arnal, A. Grape Seed Proanthocyanidins Improve White Adipose Tissue Expansion during Diet-Induced Obesity Development in Rats. Int. J. Mol. Sci. 2018, 19, 2632. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, M.; Xu, L.; Fang, Z.; Wu, W.; Zhang, Q.; Chung, P.; Lin, Y.; Wang, S.; Zhang, Y. miR-96 and autophagy are involved in the beneficial effect of grape seed proanthocyanidins against high-fat-diet-induced dyslipidemia in mice. Phytother. Res. 2019, 33, 1222–1232. [Google Scholar] [CrossRef]
- Sierra-Cruz, M.; Miguens-Gomez, A.; Grau-Bove, C.; Rodriguez-Gallego, E.; Blay, M.; Pinent, M.; Ardevol, A.; Terra, X.; Beltran-Debon, R. Grape-Seed Proanthocyanidin Extract Reverts Obesity-Related Metabolic Derangements in Aged Female Rats. Nutrients 2021, 13, 2059. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Sreeja, S.; Geetha, R.; Radika, M.K.; Anuradha, C.V. Grape seed proanthocyanidin rescues rats from steatosis: A comparative and combination study with metformin. J. Lipids 2013, 2013, 153897. [Google Scholar] [CrossRef]
- Mokrani, M.; Charradi, K.; Limam, F.; Aouani, E.; Urdaci, M.C. Grape seed and skin extract, a potential prebiotic with anti-obesity effect through gut microbiota modulation. Gut Pathog. 2022, 14, 30. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Marti, A.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardevol, A. Acutely administered grape-seed proanthocyanidin extract acts as a satiating agent. Food Funct. 2016, 7, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Zhang, Z.; Yu, H.; Huang, Y.; Chen, Z.Y. Hypocholesterolemic activity of grape seed proanthocyanidin is mediated by enhancement of bile acid excretion and up-regulation of CYP7A1. J. Nutr. Biochem. 2010, 21, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape seed proanthocyanidins reduced the overweight of C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, J.; Gao, H.; Wang, Q.; Zhang, J.; Qiu, J. Beneficial effects of grape seed proanthocyanidin extract on arterial remodeling in spontaneously hypertensive rats via protecting against oxidative stress. Mol. Med. Rep. 2016, 14, 3711–3718. [Google Scholar] [CrossRef]
- Ruan, Y.; Jin, Q.; Zeng, J.; Ren, F.; Xie, Z.; Ji, K.; Wu, L.; Wu, J.; Li, L. Grape Seed Proanthocyanidin Extract Ameliorates Cardiac Remodelling After Myocardial Infarction Through PI3K/AKT Pathway in Mice. Front. Pharmacol. 2020, 11, 585984. [Google Scholar] [CrossRef] [PubMed]
- Sergazy, S.; Shulgau, Z.; Fedotovskikh, G.; Chulenbayeva, L.; Nurgozhina, A.; Nurgaziyev, M.; Krivyh, E.; Kamyshanskiy, Y.; Kushugulova, A.; Gulyayev, A.; et al. Cardioprotective effect of grape polyphenol extract against doxorubicin induced cardiotoxicity. Sci. Rep. 2020, 10, 14720. [Google Scholar] [CrossRef]
- Guisantes-Batan, E.; Mazuecos, L.; Rubio, B.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Andres, A.; Gomez-Alonso, S.; Gallardo, N. Grape seed extract supplementation modulates hepatic lipid metabolism in rats. Implication of PPARbeta/delta. Food Funct. 2022, 13, 11353–11368. [Google Scholar] [CrossRef]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of grape seed extract in Type 2 diabetic subjects at high cardiovascular risk: A double blind randomized placebo controlled trial examining metabolic markers, vascular tone, inflammation, oxidative stress and insulin sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef]
- Mohammad, A.; Shahnaz, T.; Sorayya, K. Effect of 8 weeks’ supplementation grape seed extract on insulin resistance in iranian adolescents with metabolic syndrome: A randomized controlled trial. Diabetes Metab. Syndr. 2021, 15, 197–203. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Choy, Y.Y.; Waterhouse, A.; Burton-Freeman, B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: A randomised, double-blinded, two-arm, parallel, placebo-controlled trial. Br. J. Nutr. 2016, 115, 226–238. [Google Scholar] [CrossRef]
- Argani, H.; Ghorbanihaghjo, A.; Vatankhahan, H.; Rashtchizadeh, N.; Raeisi, S.; Ilghami, H. The effect of red grape seed extract on serum paraoxonase activity in patients with mild to moderate hyperlipidemia. Sao Paulo Med. J. 2016, 134, 234–239. [Google Scholar] [CrossRef]
- Razavi, S.M.; Gholamin, S.; Eskandari, A.; Mohsenian, N.; Ghorbanihaghjo, A.; Delazar, A.; Rashtchizadeh, N.; Keshtkar-Jahromi, M.; Argani, H. Red grape seed extract improves lipid profiles and decreases oxidized low-density lipoprotein in patients with mild hyperlipidemia. J. Med. Food 2013, 16, 255–258. [Google Scholar] [CrossRef]
- Weseler, A.R.; Ruijters, E.J.; Drittij-Reijnders, M.J.; Reesink, K.D.; Haenen, G.R.; Bast, A. Pleiotropic benefit of monomeric and oligomeric flavanols on vascular health—A randomized controlled clinical pilot study. PLoS ONE 2011, 6, e28460. [Google Scholar] [CrossRef]
- Yousefi, R.; Parandoosh, M.; Khorsandi, H.; Hosseinzadeh, N.; Madani Tonekaboni, M.; Saidpour, A.; Babaei, H.; Ghorbani, A. Grape seed extract supplementation along with a restricted-calorie diet improves cardiovascular risk factors in obese or overweight adult individuals: A randomized, placebo-controlled trial. Phytother. Res. 2021, 35, 987–995. [Google Scholar] [CrossRef]
- Preuss, H.G.; Wallerstedt, D.; Talpur, N.; Tutuncuoglu, S.O.; Echard, B.; Myers, A.; Bui, M.; Bagchi, D. Effects of niacin-bound chromium and grape seed proanthocyanidin extract on the lipid profile of hypercholesterolemic subjects: A pilot study. J. Med. 2000, 31, 227–246. [Google Scholar]
- Vogels, N.; Nijs, I.M.; Westerterp-Plantenga, M.S. The effect of grape-seed extract on 24 h energy intake in humans. Eur. J. Clin. Nutr. 2004, 58, 667–673. [Google Scholar] [CrossRef][Green Version]
- Parandoosh, M.; Yousefi, R.; Khorsandi, H.; Nikpayam, O.; Saidpour, A.; Babaei, H. The effects of grape seed extract (Vitis vinifera) supplement on inflammatory markers, neuropeptide Y, anthropometric measures, and appetite in obese or overweight individuals: A randomized clinical trial. Phytother. Res. 2020, 34, 379–387. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Schon, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape Seed Extract Positively Modulates Blood Pressure and Perceived Stress: A Randomized, Double-Blind, Placebo-Controlled Study in Healthy Volunteers. Nutrients 2021, 13, 654. [Google Scholar] [CrossRef]
- Odai, T.; Terauchi, M.; Kato, K.; Hirose, A.; Miyasaka, N. Effects of Grape Seed Proanthocyanidin Extract on Vascular Endothelial Function in Participants with Prehypertension: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2844. [Google Scholar] [CrossRef] [PubMed]
- Dillon, K.N.; Shariffi, B.; Thompson, B.; Steele, R.; Kim, J.K. Effects of Acute Grape Seed Extract Supplementation on Hemodynamics in Normal Body Weight and Obese Males. J. Nutr. Sci. Vitaminol. 2020, 66, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kim, K.A.; Choi, H.M.; Park, S.K.; Stebbins, C.L. Grape Seed Extract Supplementation Attenuates the Blood Pressure Response to Exercise in Prehypertensive Men. J. Med. Food 2018, 21, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Ras, R.T.; Zock, P.L.; Zebregs, Y.E.; Johnston, N.R.; Webb, D.J.; Draijer, R. Effect of polyphenol-rich grape seed extract on ambulatory blood pressure in subjects with pre- and stage I hypertension. Br. J. Nutr. 2013, 110, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Erukainure, O.L.; Sanni, O.; Koorbanally, N.A.; Islam, M.S. Phytochemical properties of black tea (Camellia sinensis) and rooibos tea (Aspalathus linearis); and their modulatory effects on key hyperglycaemic processes and oxidative stress. J. Food Sci. Technol. 2020, 57, 4345–4354. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Sawi, M.R.; El-Missiry, M.A.; Abukhalil, M.H. Epigallocatechin-3-gallate protects against diabetic cardiomyopathy through modulating the cardiometabolic risk factors, oxidative stress, inflammation, cell death and fibrosis in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2017, 94, 362–373. [Google Scholar] [CrossRef]
- Li, W.; Zhu, C.; Liu, T.; Zhang, W.; Liu, X.; Li, P.; Zhu, T. Epigallocatechin-3-gallate ameliorates glucolipid metabolism and oxidative stress in type 2 diabetic rats. Diabetes Vasc. Dis. Res. 2020, 17, 1479164120966998. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, Z.; Lu, Y.; Zhang, R.; Yang, H. Antiglycolipid disorder effect of epigallocatechin3gallate on highfat diet and STZinduced T2DM in mice. Mol. Med. Rep. 2020, 21, 2475–2483. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T.; et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Hu, X.; Xu, Q.; Zhang, Y.; Liu, H.; Diao, Y.; Zhang, X.; Li, L.; Yu, J.; et al. Epigallocatechin-3-gallate prevents inflammation and diabetes -Induced glucose tolerance through inhibition of NLRP3 inflammasome activation. Int. Immunopharmacol. 2021, 93, 107412. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, L.; Cheng, Q.; Ji, B.; Yang, M.; Sanidad, K.Z.; Wang, C.; Zhou, F. Structurally Different Flavonoid Subclasses Attenuate High-Fat and High-Fructose Diet Induced Metabolic Syndrome in Rats. J. Agric. Food Chem. 2018, 66, 12412–12420. [Google Scholar] [CrossRef]
- Park, J.H.; Jin, J.Y.; Baek, W.K.; Park, S.H.; Sung, H.Y.; Kim, Y.K.; Lee, J.; Song, D.K. Ambivalent role of gallated catechins in glucose tolerance in humans: A novel insight into non-absorbable gallated catechin-derived inhibitors of glucose absorption. J. Physiol. Pharmacol. 2009, 60, 101–109. [Google Scholar]
- Li, X.; Li, S.; Chen, M.; Wang, J.; Xie, B.; Sun, Z. (−)-Epigallocatechin-3-gallate (EGCG) inhibits starch digestion and improves glucose homeostasis through direct or indirect activation of PXR/CAR-mediated phase II metabolism in diabetic mice. Food Funct. 2018, 9, 4651–4663. [Google Scholar] [CrossRef]
- Ni, D.; Ai, Z.; Munoz-Sandoval, D.; Suresh, R.; Ellis, P.R.; Yuqiong, C.; Sharp, P.A.; Butterworth, P.J.; Yu, Z.; Corpe, C.P. Inhibition of the facilitative sugar transporters (GLUTs) by tea extracts and catechins. FASEB J. 2020, 34, 9995–10010. [Google Scholar] [CrossRef]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S. Epigallocatechin gallate suppresses hepatic cholesterol synthesis by targeting SREBP-2 through SIRT1/FOXO1 signaling pathway. Mol. Cell. Biochem. 2018, 448, 175–185. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Wang, Y.; Zhang, Q.; Li, J.; Zhong, P.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Inhibition of Apoptosis and Promotion of Autophagy through the ROS/MAPK Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar] [CrossRef]
- Choi, C.; Song, H.D.; Son, Y.; Cho, Y.K.; Ahn, S.Y.; Jung, Y.S.; Yoon, Y.C.; Kwon, S.W.; Lee, Y.H. Epigallocatechin-3-Gallate Reduces Visceral Adiposity Partly through the Regulation of Beclin1-Dependent Autophagy in White Adipose Tissues. Nutrients 2020, 12, 3072. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, C.T.; Kim, Y. Green tea (−)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef]
- Santana, A.; Santamarina, A.; Souza, G.; Mennitti, L.; Okuda, M.; Venancio, D.; Seelaender, M.; do Nascimento, C.O.; Ribeiro, E.; Lira, F.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate improves insulin resistance and metabolic profiles in normolipidic diet—But not high-fat diet-fed mice. J. Nutr. Biochem 2015, 26, 893–902. [Google Scholar] [CrossRef]
- Cui, C.J.; Jin, J.L.; Guo, L.N.; Sun, J.; Wu, N.Q.; Guo, Y.L.; Liu, G.; Dong, Q.; Li, J.J. Beneficial impact of epigallocatechingallate on LDL-C through PCSK9/LDLR pathway by blocking HNF1alpha and activating FoxO3a. J. Transl. Med. 2020, 18, 195. [Google Scholar] [CrossRef]
- Kim, S.N.; Kwon, H.J.; Akindehin, S.; Jeong, H.W.; Lee, Y.H. Effects of Epigallocatechin-3-Gallate on Autophagic Lipolysis in Adipocytes. Nutrients 2017, 9, 680. [Google Scholar] [CrossRef]
- Mi, Y.; Liu, X.; Tian, H.; Liu, H.; Li, J.; Qi, G.; Liu, X. EGCG stimulates the recruitment of brite adipocytes, suppresses adipogenesis and counteracts TNF-alpha-triggered insulin resistance in adipocytes. Food Funct. 2018, 9, 3374–3386. [Google Scholar] [CrossRef]
- Yu, J.; Li, W.; Xiao, X.; Huang, Q.; Yu, J.; Yang, Y.; Han, T.; Zhang, D.; Niu, X. (−)-Epicatechin gallate blocks the development of atherosclerosis by regulating oxidative stress in vivo and in vitro. Food Funct. 2021, 12, 8715–8727. [Google Scholar] [CrossRef]
- Antonello, M.; Montemurro, D.; Bolognesi, M.; Di Pascoli, M.; Piva, A.; Grego, F.; Sticchi, D.; Giuliani, L.; Garbisa, S.; Rossi, G.P. Prevention of hypertension, cardiovascular damage and endothelial dysfunction with green tea extracts. Am. J. Hypertens. 2007, 20, 1321–1328. [Google Scholar] [CrossRef]
- Yin, J.; Huang, F.; Yi, Y.; Yin, L.; Peng, D. EGCG attenuates atherosclerosis through the Jagged-1/Notch pathway. Int. J. Mol. Med. 2016, 37, 398–406. [Google Scholar] [CrossRef]
- Faria, A.M.; Papadimitriou, A.; Silva, K.C.; Lopes de Faria, J.M.; Lopes de Faria, J.B. Uncoupling endothelial nitric oxide synthase is ameliorated by green tea in experimental diabetes by re-establishing tetrahydrobiopterin levels. Diabetes 2012, 61, 1838–1847. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D. (−)-Epigallocatechin-3-Gallate Inhibits eNOS Uncoupling and Alleviates High Glucose-Induced Dysfunction and Apoptosis of Human Umbilical Vein Endothelial Cells by 186PI3K/AKT/eNOS Pathway. Diabetes Metab. Syndr. Obes. 2020, 13, 2495–2504. [Google Scholar] [CrossRef]
- Nan, J.; Nan, C.; Ye, J.; Qian, L.; Geng, Y.; Xing, D.; Rahman, M.S.U.; Huang, M. EGCG protects cardiomyocytes against hypoxia-reperfusion injury through inhibition of OMA1 activation. J. Cell Sci. 2019, 132, jcs220871. [Google Scholar] [CrossRef]
- Shen, K.; Feng, X.; Su, R.; Xie, H.; Zhou, L.; Zheng, S. Epigallocatechin 3-gallate ameliorates bile duct ligation induced liver injury in mice by modulation of mitochondrial oxidative stress and inflammation. PLoS ONE 2015, 10, e0126278. [Google Scholar] [CrossRef]
- Thitimuta, S.; Pithayanukul, P.; Nithitanakool, S.; Bavovada, R.; Leanpolchareanchai, J.; Saparpakorn, P. Camellia sinensis L. Extract and Its Potential Beneficial Effects in Antioxidant, Anti-Inflammatory, Anti-Hepatotoxic, and Anti-Tyrosinase Activities. Molecules 2017, 22, 401. [Google Scholar] [CrossRef] [PubMed]
- Kochi, T.; Shimizu, M.; Terakura, D.; Baba, A.; Ohno, T.; Kubota, M.; Shirakami, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: Suppressing effects of EGCG on the development of liver lesions. Cancer Lett. 2014, 342, 60–69. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef] [PubMed]
- de Morais Junior, A.C.; Schincaglia, R.M.; Passarelli, M.; Pimentel, G.D.; Mota, J.F. Acute Epigallocatechin-3-Gallate Supplementation Alters Postprandial Lipids after a Fast-Food Meal in Healthy Young Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2020, 12, 2533. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Coates, A.M.; Buckley, J.D.; Ross, R.; Thielecke, F.; Howe, P.R. Can EGCG reduce abdominal fat in obese subjects? J. Am. Coll. Nutr. 2007, 26, 396S–402S. [Google Scholar] [CrossRef]
- Takahashi, M.; Ozaki, M.; Tsubosaka, M.; Kim, H.K.; Sasaki, H.; Matsui, Y.; Hibi, M.; Osaki, N.; Miyashita, M.; Shibata, S. Effects of Timing of Acute and Consecutive Catechin Ingestion on Postprandial Glucose Metabolism in Mice and Humans. Nutrients 2020, 12, 565. [Google Scholar] [CrossRef]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Yu, X.; Li, Y. Dietary epigallocatechin 3-gallate supplement improves maternal and neonatal treatment outcome of gestational diabetes mellitus: A double-blind randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 753–758. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: Randomized controlled trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Fernandes, R.C.; Araujo, V.A.; Giglio, B.M.; Marini, A.C.B.; Mota, J.F.; Teixeira, K.S.; Monteiro, P.A.; Lira, F.S.; Pimentel, G.D. Acute Epigallocatechin 3 Gallate (EGCG) Supplementation Delays Gastric Emptying in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Crossover Study. Nutrients 2018, 10, 1122. [Google Scholar] [CrossRef]
- Hsu, C.H.; Liao, Y.L.; Lin, S.C.; Tsai, T.H.; Huang, C.J.; Chou, P. Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Altern. Med. Rev. 2011, 16, 157–163. [Google Scholar]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef]
- Wu, A.H.; Spicer, D.; Stanczyk, F.Z.; Tseng, C.C.; Yang, C.S.; Pike, M.C. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev. Res. 2012, 5, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Dostal, A.M.; Samavat, H.; Espejo, L.; Arikawa, A.Y.; Stendell-Hollis, N.R.; Kurzer, M.S. Green Tea Extract and Catechol-O-Methyltransferase Genotype Modify Fasting Serum Insulin and Plasma Adiponectin Concentrations in a Randomized Controlled Trial of Overweight and Obese Postmenopausal Women. J. Nutr. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Bazyar, H.; Hosseini, S.A.; Saradar, S.; Mombaini, D.; Allivand, M.; Labibzadeh, M.; Alipour, M. Effects of epigallocatechin-3-gallate of Camellia sinensis leaves on blood pressure, lipid profile, atherogenic index of plasma and some inflammatory and antioxidant markers in type 2 diabetes mellitus patients: A clinical trial. J. Complement. Integr. Med. 2020, 18, 405–411. [Google Scholar] [CrossRef]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Liu, C.Y.; Wang, L.Y.; Huang, C.J.; Hsu, C.H. Effects of green tea extract on overweight and obese women with high levels of low density-lipoprotein-cholesterol (LDL-C): A randomised, double-blind, and cross-over placebo-controlled clinical trial. BMC Complement. Altern. Med. 2018, 18, 294. [Google Scholar] [CrossRef]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef]
- Dostal, A.M.; Arikawa, A.; Espejo, L.; Bedell, S.; Kurzer, M.S.; Stendell-Hollis, N.R. Green tea extract and catechol-O-methyltransferase genotype modify the post-prandial serum insulin response in a randomised trial of overweight and obese post-menopausal women. J. Hum. Nutr. Diet. 2017, 30, 166–176. [Google Scholar] [CrossRef]
- Thielecke, F.; Rahn, G.; Bohnke, J.; Adams, F.; Birkenfeld, A.L.; Jordan, J.; Boschmann, M. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: A pilot study. Eur. J. Clin. Nutr. 2010, 64, 704–713. [Google Scholar] [CrossRef]
- Quezada-Fernandez, P.; Trujillo-Quiros, J.; Pascoe-Gonzalez, S.; Trujillo-Rangel, W.A.; Cardona-Muller, D.; Ramos-Becerra, C.G.; Barocio-Pantoja, M.; Rodriguez-de la Cerda, M.; Nerida Sanchez-Rodriguez, E.; Cardona-Munoz, E.G.; et al. Effect of green tea extract on arterial stiffness, lipid profile and sRAGE in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled trial. Int. J. Food Sci. Nutr. 2019, 70, 977–985. [Google Scholar] [CrossRef]
- Widlansky, M.E.; Hamburg, N.M.; Anter, E.; Holbrook, M.; Kahn, D.F.; Elliott, J.G.; Keaney, J.F., Jr.; Vita, J.A. Acute EGCG supplementation reverses endothelial dysfunction in patients with coronary artery disease. J. Am. Coll. Nutr. 2007, 26, 95–102. [Google Scholar] [CrossRef]
- Lorenz, M.; Rauhut, F.; Hofer, C.; Gwosc, S.; Muller, E.; Praeger, D.; Zimmermann, B.F.; Wernecke, K.D.; Baumann, G.; Stangl, K.; et al. Tea-induced improvement of endothelial function in humans: No role for epigallocatechin gallate (EGCG). Sci. Rep. 2017, 7, 2279. [Google Scholar] [CrossRef]
- Arazi, H.; Samami, N.; Kheirkhah, J.; Taati, B. The effect of three weeks green tea extract consumption on blood pressure, heart rate responses to a single bout resistance exercise in hypertensive women. High Blood Press. Cardiovasc. Prev. 2014, 21, 213–219. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Tzellos, T.; Kyrgidis, A.; Jemec, G.B.E.; Bechara, F.G.; Giamarellos-Bourboulis, E.J.; Ingram, J.R.; Kanni, T.; Karagiannidis, I.; Martorell, A.; et al. Development and validation of the International Hidradenitis Suppurativa Severity Score System (IHS4), a novel dynamic scoring system to assess HS severity. Br. J. Dermatol. 2017, 177, 1401–1409. [Google Scholar] [CrossRef]
- Bentivegna, S.S.; Whitney, K.M. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem. Toxicol. 2002, 40, 1731–1743. [Google Scholar] [CrossRef]
- Durg, S.; Bavage, S.; Shivaram, S.B. Withania somnifera (Indian ginseng) in diabetes mellitus: A systematic review and meta-analysis of scientific evidence from experimental research to clinical application. Phytother. Res. 2020, 34, 1041–1059. [Google Scholar] [CrossRef]
- Goey, A.K.; Meijerman, I.; Beijnen, J.H.; Schellens, J.H. The effect of grape seed extract on the pharmacokinetics of dextromethorphan in healthy volunteers. Eur. J. Clin. Pharmacol. 2013, 69, 1883–1890. [Google Scholar] [CrossRef]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the phenolic compounds of the fruits (drupes) of Olea europaea (olives): Impact on plasma antioxidant status in humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sano, A.; Uchida, R.; Saito, M.; Shioya, N.; Komori, Y.; Tho, Y.; Hashizume, N. Beneficial effects of grape seed extract on malondialdehyde-modified LDL. J. Nutr. Sci Vitaminol. 2007, 53, 174–182. [Google Scholar] [CrossRef]
- Wren, A.F.; Cleary, M.; Frantz, C.; Melton, S.; Norris, L. 90-day oral toxicity study of a grape seed extract (IH636) in rats. J. Agric. Food Chem. 2002, 50, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.A.; Rege, N.N.; Tadvi, F.M.; Solanki, P.V.; Kene, K.R.; Shirolkar, S.G.; Pandey, S.N.; Vaidya, R.A.; Vaidya, A.B. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J. Ayurveda Integr. Med. 2012, 3, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Darweesh, R.S.; El-Elimat, T.; Zayed, A.; Khamis, T.N.; Babaresh, W.M.; Arafat, T.; Al Sharie, A.H. The effect of grape seed and green tea extracts on the pharmacokinetics of imatinib and its main metabolite, N-desmethyl imatinib, in rats. BMC Pharmacol. Toxicol. 2020, 21, 77. [Google Scholar] [CrossRef]
- Etheridge, A.S.; Black, S.R.; Patel, P.R.; So, J.; Mathews, J.M. An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents. Planta Med. 2007, 73, 731–741. [Google Scholar] [CrossRef]
- Malliou, F.; Andriopoulou, C.E.; Gonzalez, F.J.; Kofinas, A.; Skaltsounis, A.L.; Konstandi, M. Oleuropein-Induced Acceleration of Cytochrome P450-Catalyzed Drug Metabolism: Central Role for Nuclear Receptor Peroxisome Proliferator-Activated Receptor alpha. Drug Metab. Dispos. 2021, 49, 833–843. [Google Scholar] [CrossRef]
- Mooiman, K.D.; Maas-Bakker, R.F.; Hendrikx, J.J.; Bank, P.C.; Rosing, H.; Beijnen, J.H.; Schellens, J.H.; Meijerman, I. The effect of complementary and alternative medicines on CYP3A4-mediated metabolism of three different substrates: 7-benzyloxy-4-trifluoromethyl-coumarin, midazolam and docetaxel. J. Pharm. Pharmacol. 2014, 66, 865–874. [Google Scholar] [CrossRef]
- Ray, S.D.; Parikh, H.; Hickey, E.; Bagchi, M.; Bagchi, D. Differential effects of IH636 grape seed proanthocyanidin extract and a DNA repair modulator 4-aminobenzamide on liver microsomal cytochrome 4502E1-dependent aniline hydroxylation. Mol. Cell. Biochem. 2001, 218, 27–33. [Google Scholar] [CrossRef]
- Kumar, S.; Bouic, P.J.; Rosenkranz, B. Investigation of CYP2B6, 3A4 and beta-esterase interactions of Withania somnifera (L.) dunal in human liver microsomes and HepG2 cells. J. Ethnopharmacol. 2021, 270, 113766. [Google Scholar] [CrossRef]
- Savai, J.; Varghese, A.; Pandita, N.; Chintamaneni, M. Investigation of CYP3A4 and CYP2D6 Interactions of Withania somnifera and Centella asiatica in Human Liver Microsomes. Phytother. Res. 2015, 29, 785–790. [Google Scholar] [CrossRef]
- Savai, J.; Varghese, A.; Pandita, N.; Chintamaneni, M. In vitro assessment of CYP1A2 and 2C9 inhibition potential of Withania somnifera and Centella asiatica in human liver microsomes. Drug Metab. Pers. Ther. 2015, 30, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. The Val158Met polymorphism in COMT gene and cancer risk: Role of endogenous and exogenous catechols. Drug Metab. Rev. 2017, 49, 56–83. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (beta-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef] [PubMed]
| Study Medication | Study Type | Dose Regimen | Cohort Size (n) | Study Cohort Criteria | Main Study Results Verum vs. Control (Increased: ↑; Decreased: ↓; Unaffected: ≈) | Ref. |
|---|---|---|---|---|---|---|
| 500 mg OLE | pc, db, RCT | daily application (8 weeks) | placebo: 38 verum: 39 | overweight participants age: 56 ± 10 years BMI: 29 ± 2.7 | ≈ fasting glucose, insulin ≈ SBP, DBP ≈ lipid profile | [127] |
| 250 mg OLE | pc, db, RCT | daily application (12 weeks) | 30/group | hypertension participants age: 23.4 ± 1.4 years BMI: 22.7 ± 3.0 | ≈ fasting plasma glucose, insulin ≈ liver enzymes ↓ inflammatory cytokines (TNF-α, IL-8, IL-6) | [125] |
| oleuropein (20 mg) | pc, db, RCT, co | single application | placebo: 20 verum: 20 | healthy participants age: 33.9 ± 6.9 BMI: 20.7 ± 3.7 | ↓ postprandial plasma glucose ↑ postprandial plasma insulin ↓ postprandial oxidative stress ↑ GLP-1, ↓ DPP-4 | [121] |
| 20 mL OLE (136.2 mg oleuropein; 6.4 mg hydroxytyrosol) | pc, db, RCT, co | daily application (6 weeks) | placebo: 60 verum: 60 | PHT participants age: 45.3 ± 12.7 years BMI: 27.0 ± 3.4 | ↓ SBP, DBP (slight reduction) ↓ total CH, LDL-C, TG, IL-8 ≈ vascular function, CRP, adiponectin ≈ fasting glucose, insulin, HOMA-IR, QUICKI, HDL-C | [126] |
| 250 mg OLE (oleuropein ≥ 100 mg) | pc, db, RCT | daily application (12 month) | placebo: 32 verum: 32 | OST participants verum/placebo: age: 59.72/59.35 years BMI: 25.90/27.52 | ↓ total CH, LDL-C, TG ≈ HDL-C | [129] |
| OLE (51.1 mg oleuropein; 9.7 mg hydroxytyrosol) | pc, db, RCT, co | daily application (12 weeks) | placebo: 46 verum: 46 | overweight participants age: 46.4 ± 5.5 years BMI: 28.0 ± 2.0 | ↓ postprandial plasma glucose, insulin ↑ insulin sensitivity (Matsuda index) ↑ pancreatic β-cell function (disposition index) ≈ lipid profile, body fat proportion, ABP | [122] |
| Study Medication | Study Type | Dose Regimen | Cohort Size (n) | Study Cohort Criteria | Main Study Results Verum vs. Control (Increased: ↑; Decreased:↓; Unaffected: ≈) | Ref. |
|---|---|---|---|---|---|---|
| 600 mg WSRE | pc, db, RCT | daily application (8 weeks) | placebo: 25 verum: 25 | healthy athletes age: 18–≤45 years | ↑ cardiorespiratory endurance: (↑ VO2 max outcome; ↑ TQR score; improved RESTQ score) ↑ anti-oxidative capacity | [153] |
| 600 mg WSRE | pc, db, RCT | daily application (8 weeks) | placebo: 25 verum: 25 | subclinical hypothyroid participants verum/placebo: age: 35.6/35.1 years | ↑ T3, T4 ↓ TSH | [154] |
| 600 mg WSRE (5% withanolides) | pc, db, RCT | daily application (8 weeks) | placebo: 25 verum: 25 | chronic stressed, overweight participants | ↓ perceived Stress Scale Score ↓ Food Cravings Questionnaire scores ↑ Oxford Happiness Questionnaire scores ↓ serum cortisol level | [151] |
| 600 mg WSRE (5% withanolides) | pc, db, RCT | daily application (8 weeks) | placebo: 25 verum: 25 | healthy participants undergoing resistance training verum/placebo: age: 28 ± 8/29 ± 9 years | ↑ muscle strength, muscle size (upper body) ↓ body fat percentage | [152] |
| Study Medication | Study Type | Dose Regimen | Cohort Size (n) | Study Cohort Criteria | Main Study Results Verum vs. Control (Increased: ↑; Decreased: ↓; Unaffected: ≈) | Ref. |
|---|---|---|---|---|---|---|
| 300 mg GSE | pc, db, RCT | daily application (16 weeks) | placebo: 38 verum:40 | mild hypertension participants verum/placebo: age: 56.4/56.9 years BMI: 25.2/26.1 | ↓ SBP, DBP (only in male participants) ↓ perceived stress score (PSQ) | [200] |
| 200 mg GSE | pc, db, RCT, co | daily application (8 weeks) | placebo: 45 verum: 45 | mild hyperlipidemia participants age: 48.22 ± 9.07 years | ↓ CH, LDL, ox-LDL | [193] |
| 200 mg GSE | pc, db, RCT | daily application (8 weeks) | placebo: 35 verum: 35 | hyperlipidemia participants verum/placebo age: 46.6/47.3 years | ↑ ApoA1, HDL ↑ PON activity ↓ CH, TG, LDL | [192] |
| 300 mg GSE | pc, db, RCT | daily application (8 weeks) | placebo: 35 verum: 34 | pre- and stage-I hypertension participants verum/placebo: age: 62.9/64.5 years BMI: 25.3/25.7 | ≈ SBP, DBP ≈ vasoactive markers | [204] |
| 600 mg GSE | pc, db, RCT, co | daily application (4 weeks) | placebo: 32 verum: 32 | T2D participants age: 61.8 ± 6.4 years BMI: 30.2 ± 5.9 | ↓ fructosamine, CH, CRP ↑ GSH ≈ fasting glucose, HOMA-IR ≈ endothelial function | [189] |
| 900 mg GSE | pc, db, RCT, co | daily application (3 days) | placebo: 51 verum: 51 | healthy participants age: 48.7 ± 14.3 years BMI: 25.6 ± 2.6 | ↓ 24 h energy intake (only in subjects with ≥7.5 MJ/day) | [197] |
| Study Medication | Study Type | Dose Regimen | Cohort Size (n) | Study Cohort Criteria | Main Study Results Verum vs. Control (Increased: ↑; Decreased: ↓; Unaffected: ≈) | Ref. |
|---|---|---|---|---|---|---|
| 1500 mg GTE (856.8 mg EGCG) | pc, db, RCT, co | daily application (6 weeks) | placebo: 73 verum: 73 | overweight participants age: 18–65 years BMI: ≥27 | ↓ LDL-C ↑ Leptin ≈ CH, TG, HDL | [247] |
| 500 mg EGCG | pc, db, RCT | daily application (until birth) | placebo: 176 verum: 150 | GDM participants verum/placebo: age: 29.6/28.7 years BMI: 25.9/26.2 | ↓ fasting plasma glucose and insulin ↓ HOMA-IR/HOMA-ß scores ↑ QUICK-I index | [238] |
| green tea/GTE/EGCG) (200 mg EGCG each) | pc, RCT, co | single application | placebo: 50 verum: 50 | healthy participants age: 33.9 ± 7.6 years BMI: 23.7 ± 2.5 | ↑ FMD (only in the green tea group) ≈ NMD | [256] |
| GTE (843 mg EGCG; decaffeinated) | pc, db, RCT | daily application (12 month) | placebo: 473 verum: 463 | healthy participants verum/placebo: age: 60.02/59.65 years BMI: 25.16/25.01 | ↓ CH, LDL ↑ TG (mainly obese, statin users) | [248] |
| 1500 mg GTE (856.8 mg EGCG) | pc, db, RCT | daily application (12 weeks) | placebo: 38 verum: 39 | obese participants verum/placebo: age: 44.1/44.9 years BMI: 31/30 | ↓ CH, LDL | [246] |
| 1060 mg GTE (431.5 mg EGCG) | pc, db, RCT, co | daily application (6 weeks) | placebo: 65 verum: 63 | obese participants verum/placebo: age: 49.5/49.4 years BMI: 31.7/31.4 | ≈ blood pressure ≈ body weight (only slight reduction during intervention period 1) | [251] |
| MetS Parameter | Olea europea | Withania somnifera | Vitis vinifera | Camellia sinensis |
|---|---|---|---|---|
| glucose metabolism | improvement of postprandial plasma glucose | only preclinical data available | no clear impact | improvement of glucose metabolism |
| dyslipidemia | improvement of single lipid parameters | only preclinical data available | improvement of single lipid parameters | improvement of single lipid parameters |
| cardiovascular alterations | improvement of vascular function | improved cardiorespiratory endurance | improvement of blood pressure parameters | improvement of cardiovascular parameters |
| no clear impact on blood pressure | ||||
| obesity/ weight management | no clear impact | reduced perceived stress; reduced food craving; enhanced body weight reduction during reistance training | enhanced body weight reduction during caloric restriction | no clear impact |
| NAFLD | only preclinical data available | only preclinical data available | only preclinical data available | only preclinical data available |
| RCT quantity | n = 11 | n = 4 | n = 21 | n = 28 |
 No RCTs or only 1 RCT available for this parameter.
No RCTs or only 1 RCT available for this parameter.  <50% of available RCTs show the effectivity of study medication on the respective parameter.
<50% of available RCTs show the effectivity of study medication on the respective parameter.  =50% of available RCTs show the effectivity of study medication on the respective parameter.
=50% of available RCTs show the effectivity of study medication on the respective parameter.  ≥50% of available RCTs show the effectivity of study medication on the respective parameter.
≥50% of available RCTs show the effectivity of study medication on the respective parameter.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, K.; Wolk, K.; Witte-Händel, E.; Krause, T.; Kokolakis, G.; Sabat, R. Targeting Metabolic Syndrome in Hidradenitis Suppurativa by Phytochemicals as a Potential Complementary Therapeutic Strategy. Nutrients 2023, 15, 3797. https://doi.org/10.3390/nu15173797
Witte K, Wolk K, Witte-Händel E, Krause T, Kokolakis G, Sabat R. Targeting Metabolic Syndrome in Hidradenitis Suppurativa by Phytochemicals as a Potential Complementary Therapeutic Strategy. Nutrients. 2023; 15(17):3797. https://doi.org/10.3390/nu15173797
Chicago/Turabian StyleWitte, Katrin, Kerstin Wolk, Ellen Witte-Händel, Torben Krause, Georgios Kokolakis, and Robert Sabat. 2023. "Targeting Metabolic Syndrome in Hidradenitis Suppurativa by Phytochemicals as a Potential Complementary Therapeutic Strategy" Nutrients 15, no. 17: 3797. https://doi.org/10.3390/nu15173797
APA StyleWitte, K., Wolk, K., Witte-Händel, E., Krause, T., Kokolakis, G., & Sabat, R. (2023). Targeting Metabolic Syndrome in Hidradenitis Suppurativa by Phytochemicals as a Potential Complementary Therapeutic Strategy. Nutrients, 15(17), 3797. https://doi.org/10.3390/nu15173797







