Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. HC Preparation
2.2. Nutritional Component, Mineral, and Heavy Metal Analyses
2.3. Animals and Ethical Statement
2.4. Behavioral Tests
2.4.1. Step-Down Test
2.4.2. MWM Test
2.5. Hematoylin and Eosin (H&E) and Immunohistochemistry Staining
2.6. Gut Microbiota Analysis
2.7. Non-Targeted Metabolomics Analysis of Serum Samples
2.8. Determination of Biochemical Indexes
2.9. Immunofluorescence
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. The Main Composition of HC
3.2. Effects of HC Treatment on Cognitive Impairments in APP/PS1 Mice
3.3. HC Treatment Alleviated Aβ and p-tau Deposition in APP/PS1 Mice
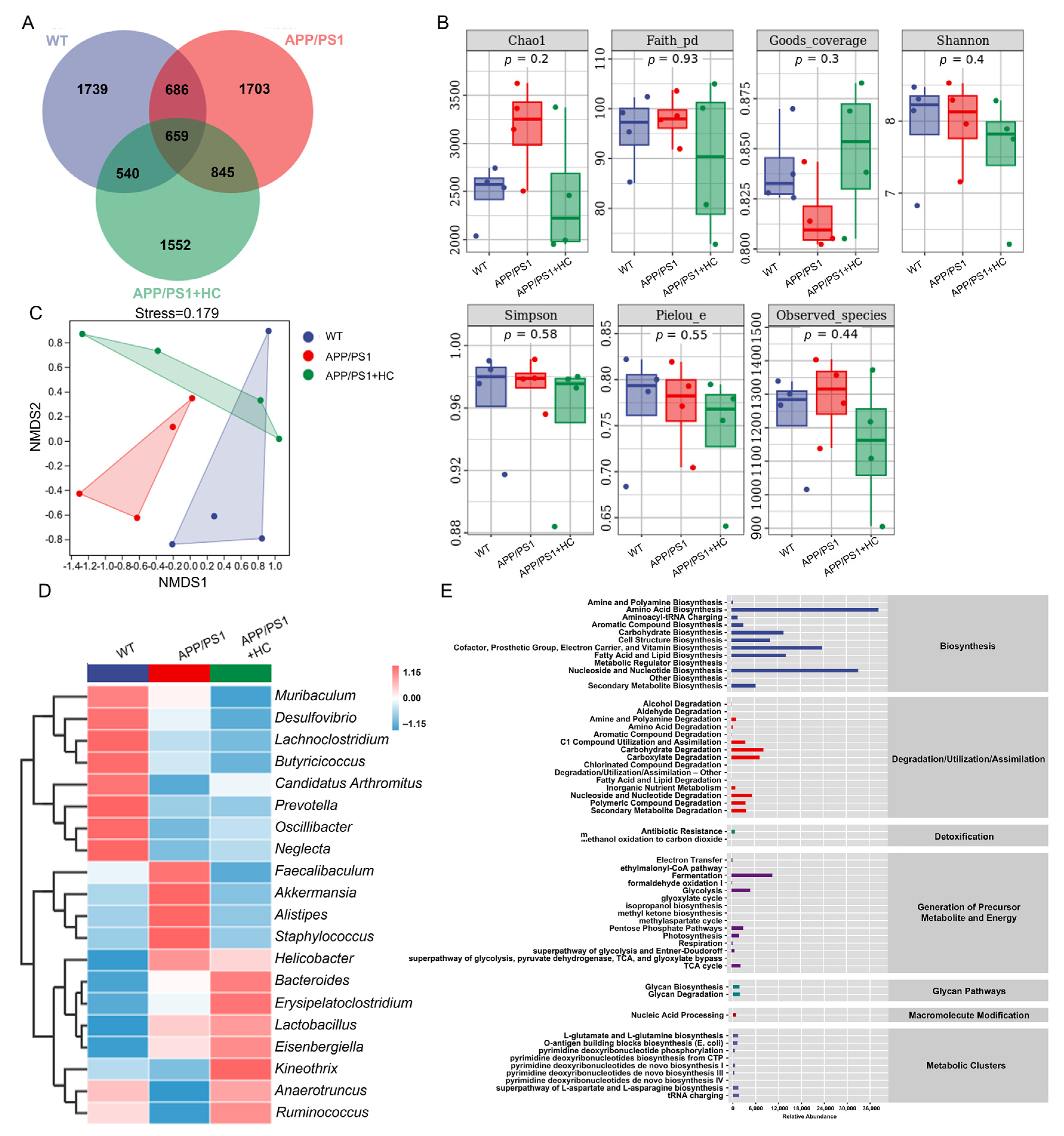
3.4. HC Regulated the Gut Microbiota in APP/PS1 Mice
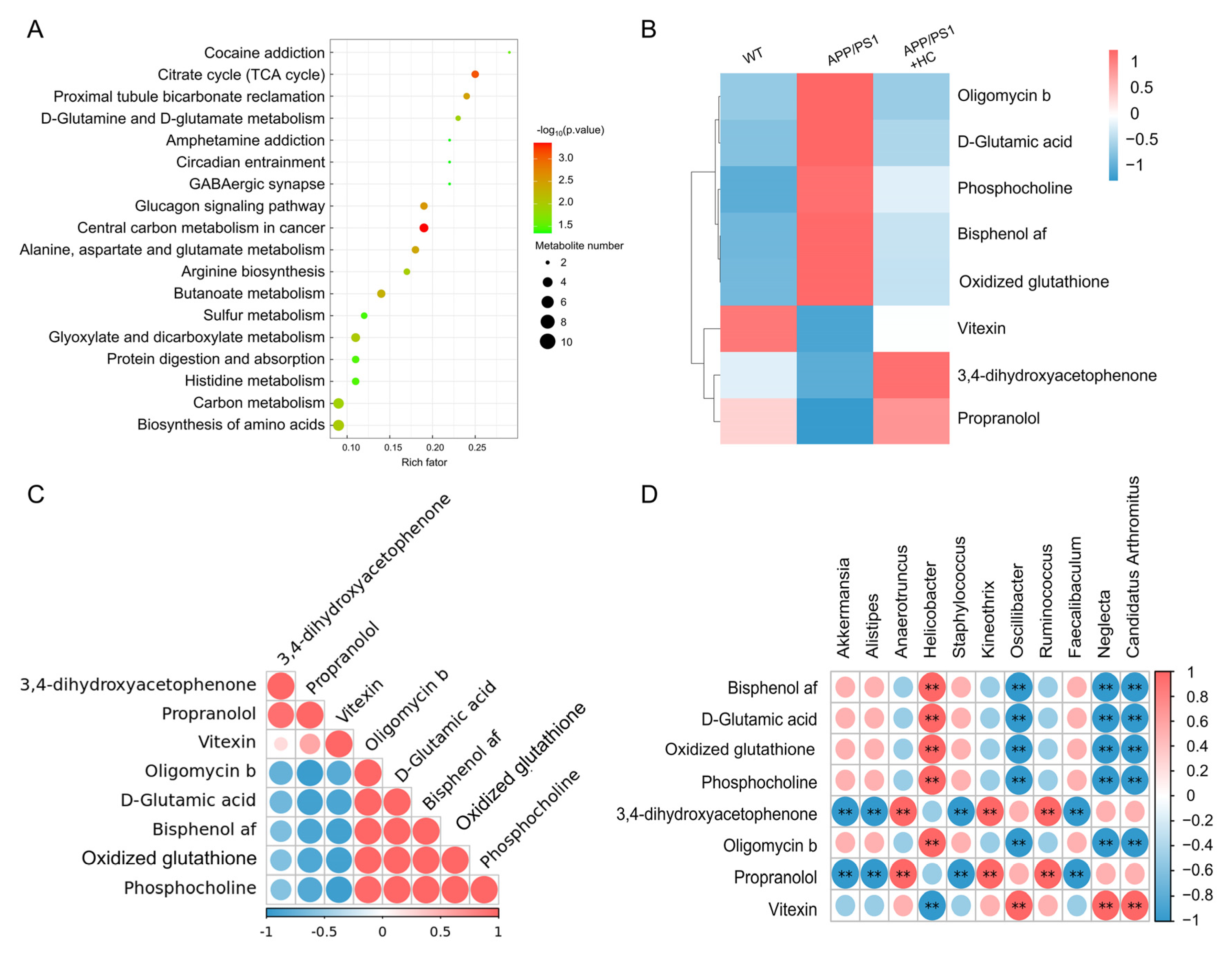
3.5. HC Regulated the Levels of Oxidative Stress-Related Metabolites in Serum
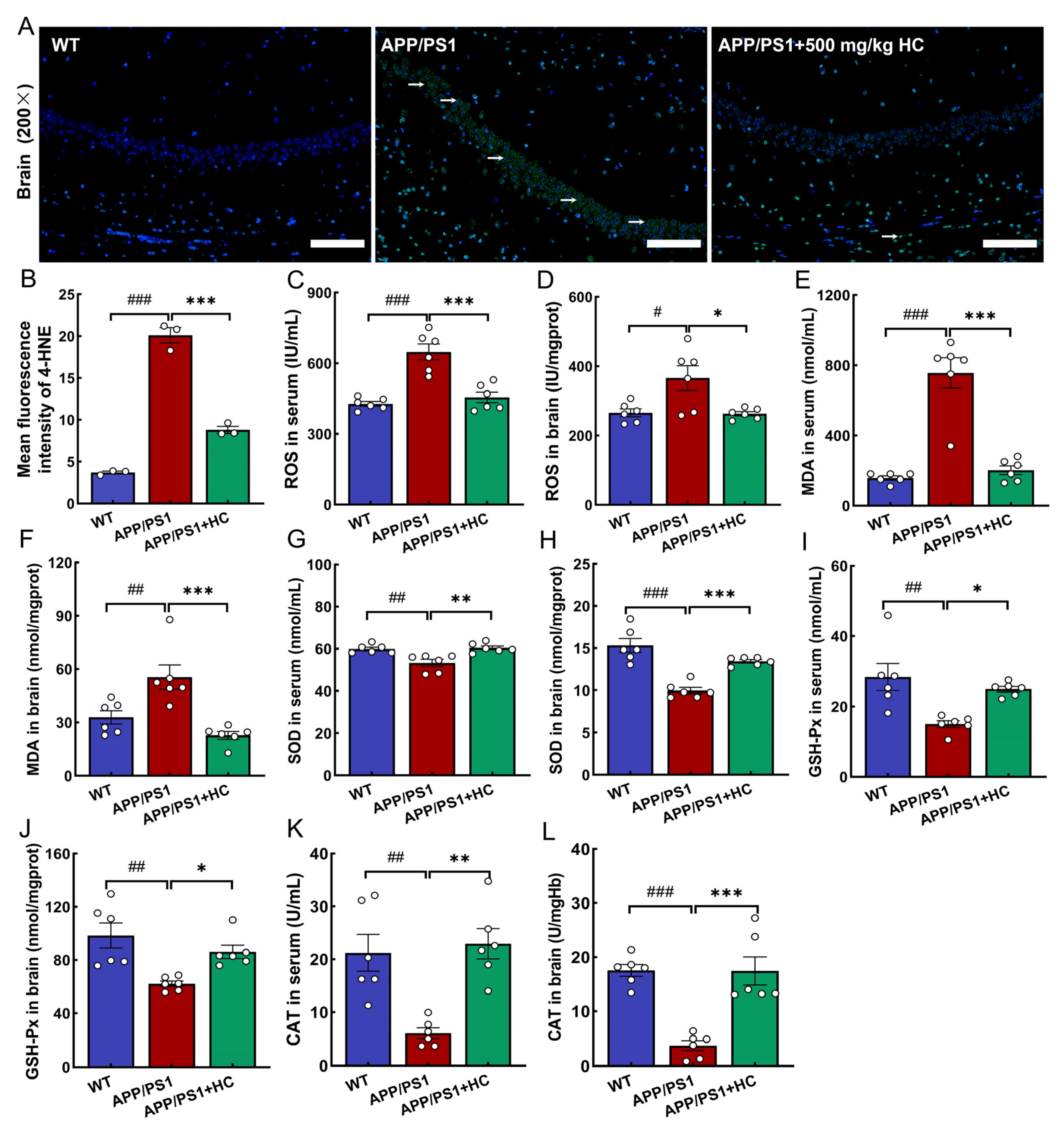
3.6. Anti-Oxidative Stress Effects of HC Treatment Were Mediated by the Nrf2 Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huynh, K.; Lim, W.L.F.; Giles, C.; Jayawardana, K.S.; Salim, A.; Mellett, N.A.; Smith, A.A.T.; Olshansky, G.; Drew, B.G.; Chatterjee, P.; et al. Concordant peripheral lipidome signatures in two large clinical studies of Alzheimer’s disease. Nat. Commun. 2020, 11, 5698. [Google Scholar] [CrossRef] [PubMed]
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021, 17, 327–406. [CrossRef] [PubMed]
- Arriagada, P.V.; Growdon, J.H.; Hedley-Whyte, E.T.; Hyman, B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992, 42, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Janus, C.; Pearson, J.; McLaurin, J.; Mathews, P.M.; Jiang, Y.; Schmidt, S.D.; Chishti, M.A.; Horne, P.; Heslin, D.; French, J.; et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature 2000, 408, 979–982. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, L.; Li, H.; Xia, W.; Liu, Q.; Zhou, X.; Dong, L.; Fu, X. Update on new trend and progress of the mechanism of polysaccharides in the intervention of Alzheimer’s disease, based on the new understanding of relevant theories: A review. Int. J. Biol. Macromol. 2022, 218, 720–738. [Google Scholar] [CrossRef]
- Khan, S.; Barve, K.H.; Kumar, M.S. Recent Advancements in Pathogenesis, Diagnostics and Treatment of Alzheimer’s Disease. Curr. Neuropharmacol. 2020, 18, 1106–1125. [Google Scholar] [CrossRef]
- Chen, L.-L.; Fan, Y.-G.; Zhao, L.-X.; Zhang, Q.; Wang, Z.-Y. The metal ion hypothesis of Alzheimer’s disease and the anti-neuroinflammatory effect of metal chelators. Bioorg. Chem. 2023, 131, 106301. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and Bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1–42) injected rats. Appl. Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef]
- Vitheejongjaroen, P.; Kasorn, A.; Puttarat, N.; Loison, F.; Taweechotipatr, M. Bifidobacterium animalis MSMC83 Improves Oxidative Stress and Gut Microbiota in D-Galactose-Induced Rats. Antioxidants 2022, 11, 2146. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, C. The role of short-chain fatty acids in central nervous system diseases. Mol. Cell Biochem. 2022, 477, 2595–2607. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Villavicencio-Tejo, F.; Quintanilla, R.A. Chapter 3—Reducing neurodegeneration and oxidative damage in Alzheimer’s disease: Role of the Nrf2 pathway activation by natural compounds. In Treatments, Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 47–63. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Shang, H.M.; Song, H.; Wang, L.N.; Wu, B.; Ding, G.D.; Jiang, Y.Y.; Yao, X.; Shen, S.J. Effects of dietary polysaccharides from the submerged fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on performance, gut microflora, and cholesterol metabolism in broiler chickens. Livest. Sci. 2014, 167, 276–285. [Google Scholar] [CrossRef]
- Kushairi, N.; Tarmizi, N.A.K.A.; Phan, C.W.; Macreadie, I.; Sabaratnam, V.; Naidu, M.; David, P. Modulation of neuroinflammatory pathways by medicinal mushrooms, with particular relevance to Alzheimer’s disease. Trends Food Sci. Technol. 2020, 104, 153–162. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, H.; Zhang, X. Erinacerins, Novel Glioma Inhibitors from Hericium erinaceus, Induce Apoptosis of U87 Cells through Bax/Capase-2 Pathway. Anticancer Agents Med. Chem. 2020, 20, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H2O2-Induced Oxidative Damage and LPS-Induced Inflammation in HT22 Hippocampal Neurons and BV2 Microglia. Antioxidants 2019, 8, 261. [Google Scholar] [CrossRef]
- Hu, W.; Song, M.; Wang, C.; Guo, Z.; Li, Y.; Wang, D. Structural characterization of polysaccharide purified from Hericium erinaceus fermented mycelium and its pharmacological basis for application in Alzheimer’s disease: Oxidative stress related calcium homeostasis. Int. J. Biol. Macromol. 2021, 193, 358–369. [Google Scholar] [CrossRef]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A-C, Nerve Growth and Brain-Derived Neurotrophic Factor Inducing Metabolites from the Mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, F.; Alvandi, H.; Hatamian-Zarmi, A.; Kalitukha, L.; Aghajani, H.; Ebrahimi-Hosseinzadeh, B. Antioxidant activity and cytotoxicity of exopolysaccharide from mushroom Hericium coralloides in submerged fermentation. Biomass Convers. Biorefin. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Zhao, L.; Shui, X.; Wang, L.A.; Wu, Y. Antioxidant and Anti-Aging Activities of Ethyl Acetate Extract of the Coral Tooth Mushroom, Hericium coralloides (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Zhang, Y.; Zhang, Y.; Liu, S.; Zhang, N.; Li, Y.; Wang, D. Protective effect of Gloeostereum incarnatum on ulcerative colitis via modulation of Nrf2/NF-κB signaling in C57BL/6 mice. Mol. Med. Rep. 2020, 22, 3418–3428. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, J.; Liu, Z.; Ma, X.; Feng, Y.; Teng, L.; Li, Y.; Wang, D. Anti-obesity effects of Grifola frondosa through the modulation of lipid metabolism via ceramide in mice fed a high-fat diet. Food Funct. 2021, 12, 6725–6739. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Brimacombe, J.S. Methods in carbohydrate chemistry. Vol. I: Analysis and preparation of sugars: Edited by Roy L. Whistler, Department of Biochemistry, Purdue University, Lafayette, Indiana; and M. L. Wolfrom, Department of Chemistry, the Ohio State University, Columbus, Ohio. Arch. Biochem. Biophys. 1963, 100, 162–163. [Google Scholar] [CrossRef]
- Lindsay, H. A colorimetric estimation of reducing sugars in potatoes with 3,5-dinitrosalicylic acid. Potato Res. 1973, 16, 176–179. [Google Scholar] [CrossRef]
- Marichal, M.d.J.; Trujillo, A.I.; Cadenazzi, M.; Arias, G. Fiber analysis: Evaluation of screen printing fabric filters bags by three statistical approaches. Anim. Feed Sci. Technol. 2011, 169, 79–85. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Guo, Y. Rapidly Simultaneous Determination of Six Effective Components in Cistanche tubulosa by Near Infrared Spectroscopy. Molecules 2017, 22, 843. [Google Scholar] [CrossRef] [PubMed]
- Fiot, J.; Jansen, O.; Akhmedjanova, V.; Angenot, L.; Balansard, G.; Ollivier, E. HPLC quantification of alkaloids from Haplophyllum extracts and comparison with their cytotoxic properties. Phytochem. Anal. PCA 2006, 17, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Hu, X.Q.; Zhang, X.F.; Liu, J.J.; Cao, L.S. Study on variation of main ingredients from spores and fruiting bodies of Ganoderma lucidum. China J. Chin. Mater. Medica 2014, 39, 4246–4251. [Google Scholar]
- Araújo, L.B.D.C.; Silva, S.L.; Galvão, M.A.M.; Ferreira, M.R.A.; Araújo, E.L.; Randau, K.P.; Soares, L.A.L. Total phytosterol content in drug materials and extracts from roots of Acanthospermum hispidum by UV-VIS spectrophotometry. Rev. Bras. Farmacogn. 2013, 23, 736–742. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.X.; Wang, S.M.; Liang, S.W. Investigation into the anti-thrombosis effect and contents of total saponins and flavonoids in the bioactive fraction of Naodesheng prescription. J. Ethnopharmacol. 2012, 144, 208–212. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, X.; Teng, S.; Zhang, Y.; Liu, Y.; Li, X.; Li, Y. The Antidiabetic and Antinephritic Activities of Auricularia cornea (An Albino Mutant Strain) via Modulation of Oxidative Stress in the db/db Mice. Front. Immunol. 2019, 10, 1039. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Guo, H.; Jiang, H.; Liu, H.; Fu, H.; Wang, D. Forsythoside A Mitigates Alzheimer’s-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int. J. Biol. Sci. 2022, 18, 2075–2090. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Li, L.; Wang, W.; Tan, H.-Y.; Qu, Y.; Wang, D. The involvement of gut microbiota in the anti-tumor effect of carnosic acid via IL-17 suppression in colorectal cancer. Chem. Biol. Interact. 2022, 365, 110080. [Google Scholar] [CrossRef]
- Cheng, X.; Tan, Y.; Li, H.; Huang, J.; Zhao, D.; Zhang, Z.; Yi, M.; Zhu, L.; Hui, S.; Yang, J.; et al. Fecal 16S rRNA sequencing and multi-compartment metabolomics revealed gut microbiota and metabolites interactions in APP/PS1 mice. Comput. Biol. Med. 2022, 151, 106312. [Google Scholar] [CrossRef]
- Park, H.-J.; Shabashvili, D.; Nekorchuk, M.D.; Shyqyriu, E.; Jung, J.I.; Ladd, T.B.; Moore, B.D.; Felsenstein, K.M.; Golde, T.E.; Kim, S.-H. Retention in Endoplasmic Reticulum 1 (RER1) Modulates Amyloid-β (Aβ) Production by Altering Trafficking of γ-Secretase and Amyloid Precursor Protein (APP). J. Biol. Chem. 2012, 287, 40629–40640. [Google Scholar] [CrossRef]
- Kim, M.; Bezprozvanny, I. Analysis of Non-Amyloidogenic Mutations in APP Supports Loss of Function Hypothesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 2092. [Google Scholar] [CrossRef]
- Li, Y.; Guan, S.; Liu, C.; Chen, X.; Zhu, Y.; Xie, Y.; Wang, J.; Ji, X.; Li, L.; Li, Z.; et al. Neuroprotective effects of Coptis chinensis Franch polysaccharide on amyloid-beta (Aβ)-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease (AD). Int. J. Biol. Macromol. 2018, 113, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110112. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Arimon, M.; Takeda, S.; Post, K.L.; Svirsky, S.; Hyman, B.T.; Berezovska, O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol. Dis. 2015, 84, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef]
- Tolar, M.; Abushakra, S.; Sabbagh, M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2019, 16, 1553–1560. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Emran, T.B. Clinically important natural products for Alzheimer’s disease. Int. J. Surg. 2022, 104, 106807. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Sabaratnam, V. Edible and Medicinal Mushrooms: Emerging Brain Food for the Mitigation of Neurodegenerative Diseases. J. Med. Food 2017, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Cuzzocrea, S.; Di Paola, R.; Fusco, R.; et al. Key Mechanisms and Potential Implications of Hericium erinaceus in NLRP3 Inflammasome Activation by Reactive Oxygen Species during Alzheimer’s Disease. Antioxidants 2021, 10, 1664. [Google Scholar] [CrossRef]
- Tsai-Teng, T.; Chin-Chu, C.; Li-Ya, L.; Wan-Ping, C.; Chung-Kuang, L.; Chien-Chang, S.; Chi-Ying, H.F.; Chien-Chih, C.; Shiao, Y.J. Erinacine A-enriched Hericium erinaceus mycelium ameliorates Alzheimer’s disease-related pathologies in APPswe/PS1dE9 transgenic mice. J. Biomed. Sci. 2016, 23, 49. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Essa, M.M.; Rathipriya, A.G.; Bishir, M.; Ray, B.; Mahalakshmi, A.M.; Tousif, A.H.; Sakharkar, M.K.; Kashyap, R.S.; Friedland, R.P.; et al. Gut dysbiosis, defective autophagy and altered immune responses in neurodegenerative diseases: Tales of a vicious cycle. Pharmacol. Ther. 2022, 231, 107988. [Google Scholar] [CrossRef]
- Jia, L.G.; Wang, Y.W.; Fang, W.; Geng, W.T.; Wang, Y.P. Progress in Understanding the Role of Probiotics in Alleviating Alzheimer’s Disease. Food Sci. 2022, 43, 287–295. [Google Scholar]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Zhang, L.; Yang, J.; Meng, H.; Wan, H.; He, Y. Hydroxysafflor yellow A and anhydrosafflor yellow B alleviate ferroptosis and parthanatos in PC12 cells injured by OGD/R. Free Radic. Biol. Med. 2022, 179, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oruc, A.; Oruc, K.Y.; Yanar, K.; Mengi, M.; Caglar, A.; Kurt, B.O.; Altan, M.; Sonmez, O.F.; Cakatay, U.; Uzun, H.; et al. The Role of Glycogen Synthase Kinase-3β in the Zinc-Mediated Neuroprotective Effect of Metformin in Rats with Glutamate Neurotoxicity. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef]
- Sekura, R.; Van Der Werf, P.; Meister, A. Mechanism and significance of the mammalian pathway for elimination of D-glutamate; inhibition of glutathione synthesis by D-glutamate. Biochem. Biophys. Res. Commun. 1976, 71, 11–18. [Google Scholar] [CrossRef]
- Blanc, E.M.; Keller, J.N.; Fernandez, S.; Mattson, M.P. 4-hydroxynonenal, a lipid peroxidation product, impairs glutamate transport in cortical astrocytes. Glia 1998, 22, 149–160. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A.; Reddy, P.H. Defective mitophagy in Alzheimer’s disease. Ageing Res. Rev. 2020, 64, 101191. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Nasser, M.I.; Masood, M.; Adlat, S.; Huang, Y.; Yang, B.; Luo, C.; Jiang, N. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 126, 110074. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Y.; Yang, H.Z.; Wang, Y.J.; Chen, Y. HMGB1 regulates ferroptosis through Nrf2 pathway in mesangial cells in response to high glucose. Biosci. Rep. 2021, 41, BSR20202924. [Google Scholar] [CrossRef] [PubMed]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalases. Cell Mol. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef] [PubMed]



| Compounds | Contents (%) | Compounds | Contents (%) | |
|---|---|---|---|---|
| General nutritional composition | Total sugar | 33.40 | Total triterpenoids | 3.32 |
| Crude protein | 16.00 | Total saponins | 1.10 | |
| Total ash | 11.70 | Total flavonoids | 0.88 | |
| Reducing sugar | 7.95 | Total alkaloids | 0.57 | |
| Crude fat | 6.30 | Total sterol | 0.43 | |
| Crude fiber | 4.60 | Total phenol | 0.18 | |
| Compounds | Contents (mg/kg) | Compounds | Contents (mg/kg) | |
| Minerals | K | 4.02 × 104 | Zn | 64.60 |
| Mg | 1.09 × 103 | Fe | 50.90 | |
| Ca | 280.00 | Mn | 7.97 | |
| Na | 110.00 | Se | 0.04 | |
| Heavy metals | Cu | 113.00 | As | 0.11 |
| Cr | 0.76 | Pb | 0.04 | |
| Cd | 0.20 | Hg | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Shi, D.; Wang, S.; Sun, Y.; Song, W.; Liu, S.; Wang, C. Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota. Nutrients 2023, 15, 3799. https://doi.org/10.3390/nu15173799
Guan Y, Shi D, Wang S, Sun Y, Song W, Liu S, Wang C. Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota. Nutrients. 2023; 15(17):3799. https://doi.org/10.3390/nu15173799
Chicago/Turabian StyleGuan, Yue, Dongyu Shi, Shimiao Wang, Yueying Sun, Wanyu Song, Shuyan Liu, and Chunyue Wang. 2023. "Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota" Nutrients 15, no. 17: 3799. https://doi.org/10.3390/nu15173799
APA StyleGuan, Y., Shi, D., Wang, S., Sun, Y., Song, W., Liu, S., & Wang, C. (2023). Hericium coralloides Ameliorates Alzheimer’s Disease Pathologies and Cognitive Disorders by Activating Nrf2 Signaling and Regulating Gut Microbiota. Nutrients, 15(17), 3799. https://doi.org/10.3390/nu15173799





