Highlights
What are the main findings?
- Women in the Study of Women's Health Across the Nation who consumed the highest amount of dietary phosphorus (>1800 mg) compared to the reference amount (800-1000 mg) had a 2.3 increased risk of breast cancer incidence over 10 years of follow-up.
- The highest amount of dietary phosphorus in this study is approximately equal to the phosphorus levels in menus promoted by the United States Department of Agriculture.
What is the implication of the main finding?
- Although the findings are not statistically significant, likely due to the small sample size, the effects are clinically significant, and more studies should investigate the reduction of dietary phosphorus in women with breast cancer.
- The high amount of phosphorus in diets recommended by the United States Department of Agriculture should be reassessed based on its associated risk with breast cancer.
Abstract
Research has shown that high amounts of dietary phosphorus that are twice the amount of the U.S. dietary reference intake of 700 mg for adults are associated with all-cause mortality, phosphate toxicity, and tumorigenesis. The present nested case–control study measured the relative risk of self-reported breast cancer associated with dietary phosphate intake over 10 annual visits in a cohort of middle-aged U.S. women from the Study of Women’s Health Across the Nation. Analyzing data from food frequency questionnaires, the highest level of daily dietary phosphorus intake, >1800 mg of phosphorus, was approximately equivalent to the dietary phosphorus levels in menus promoted by the United States Department of Agriculture. After adjusting for participants’ energy intake, this level of dietary phosphorus was associated with a 2.3-fold increased risk of breast cancer incidence compared to the reference dietary phosphorus level of 800 to 1000 mg, which is based on recommendations from the U.S. National Kidney Foundation, (RR: 2.30, 95% CI: 0.94–5.61, p = 0.07). Despite the lack of statistical significance, likely due to the small sample size of the cohort, the present nested case–control study’s clinically significant effect size, dose–response, temporality, specificity, biological plausibility, consistency, coherence, and analogy with other research findings meet the criteria for inferred causality in observational studies, warranting further investigations. Furthermore, these findings suggest that a low-phosphate diet should be tested on patients with breast cancer.
1. Introduction
As global populations increasingly transition to the risk factor profile of Western nations, “dramatic changes in lifestyle” are affecting the prevalence of risk factors for breast cancer and other cancers [1]. For example, a recent meta-analysis found that the highest dietary intake of a Western dietary pattern, including red or processed meats, high-fat dairy products, potatoes, and sweets, was associated with a 14% increased risk of breast cancer compared to the lowest intake [2]. The same study found that the highest intake of a “prudent” dietary pattern, containing fruits and vegetables, fish, whole grains, and low-fat dairy, was associated with an 18% reduced risk of breast cancer compared to the lowest intake. Of relevance, the plant-based foods that predominate in a prudent dietary pattern tend to be lower in the essential mineral phosphorus than the animal-based foods typically found in a Western dietary pattern [3].
Inorganic phosphate (Pi) metabolism is regulated in the body with a sensitive network of endocrine hormones released by the kidney–bone–parathyroid–intestine axis [4]. The accumulation of excess Pi in the tissues of the body due to dysregulated phosphate metabolism can produce a condition known as phosphate toxicity, and evidence supports the association of phosphate toxicity with tumorigenesis [5]. For example, animal studies have shown that excessive dietary phosphate increases cell signaling in the promotion of cancer cell growth [6,7]. Notably, a “regulation-based model” of cancer research proposed by Schipper et al., in The Lancet in 1996 [8], suggests that cancer is a disease of dysregulated metabolism and may be reversible. In general, metabolomics is currently contributing to the discovery of important metabolic alterations in the growth of cancer cells, with potential applications for clinical oncology [9].
Phosphorus in the form of dietary phosphate is plentiful in the dietary pattern eaten by contemporary Western populations, including in Canada and the United States [10]. Phosphate intake is also rising as people increase their consumption of foods processed with phosphate additives [11]. Dietary sources contributing the greatest amount of phosphorus in the food Americans eat are milk and dairy products (cheese, ice cream, and yogurt), bakery products (bread, rolls, and tortillas), vegetables (starchy), chicken, “Mexican dishes” (nachos, burritos, and tacos), and pizza [12]. Gastrointestinal bioavailability of phosphorus also varies in different dietary sources. For example, phosphorus in meat and dairy has a higher absorption rate (40–60%) compared to phosphorus bound to phytate in whole grains (20–50%), while phosphate additives widely used by the food industry in ultra-processed food have 90–100% bioavailability [13]. Relatedly, recent systematic reviews and meta-analyses found an increased risk of breast cancer and other cancers associated with increased intake of ultra-processed food [14,15]. Another study found an increased risk of mortality from ovarian cancer and breast cancer associated with ultra-processed food intake [16].
As the intake of nutrients like phosphorus rises above optimal levels for health, the increased concentration may eventually become toxic and even result in death [17]. Although the U.S. dietary reference intake (DRI) for phosphorus is 700 mg/day in adult women and men [18], the 2015–2016 National Health and Nutrition Examination Survey (NHANES) reported that women, on average, consume 1189 mg, and men consume 1596 mg of dietary phosphorus/day [19]. By comparison, guidelines from the U.S. National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (K/DOQI) recommend that patients with progressive kidney disease restrict phosphorus intake to 800–1000 mg/day, depending on protein requirements [20]. Indeed, higher dietary phosphorus intake starting at about 1400 mg per day has been associated with increased all-cause mortality in the U.S. population [21]. Furthermore, based on Dietary Guidelines for Americans, 2020–2025, published by the United States Department of Agriculture (USDA), a MyPlate 2000-calorie daily menu that includes whole grains and fat-free milk provides approximately 1800 mg phosphorous [22], well above the 1400 mg of dietary phosphorus associated with increased mortality risk [21].
Three cups of fat-free milk, as recommended by USDA menu plans, supplies more than 700 mg of phosphorus, which is sufficient to meet adult requirements but provides only about 13% of calories in a 2000-calorie diet [23]; as a result, overall phosphorus intake would quite reasonably be expected to be even higher when other foods are included. Furthermore, a recent study funded by the U.S. National Cancer Institute found a 50% increased risk of breast cancer incidence associated with the highest milk intake compared to the lowest milk intake [24], possibly related to milk’s high phosphorus content. Three cups of milk a day was also associated with a 44% increased risk of cancer mortality compared to one cup [25]. Also, a systematic review and meta-analysis found that dietary acid load is associated with a 58% increased relative risk of cancer [26], and dietary acid load and phosphorus intake were lower in participants in a randomized controlled trial who consumed a vegan diet compared to a meat-rich diet [27].
The purpose of the present study is to investigate associations of breast cancer incidence with discrete categories of dietary phosphate levels. Phosphate levels are based on dietary guidelines from U.S. health organizations and government agencies, and categories also include levels of phosphate associated with disease in the research literature. The hypothesis in the present study is that the relative risk of breast cancer incidence is more strongly associated with high levels of dietary phosphate compared to low levels of phosphate. The rationale for selecting the National Kidney Foundation (NKF) guidelines for dietary phosphate intake as the reference level in this study is based on numerous findings implicating chronic kidney disease as a risk factor for cancer, such as Lees et al. [28], Wong et al. [29], Stengel [30], Tendulkar et al. [31], Kitchlu et al. [32], Hu et al. [33], Movahhed et al. [34], Wei et al. [35], Guo et al. [36], Na et al. [37], and Yu et al. [38]. Additionally, high serum phosphate levels associated with tumorigenesis [5] are also prevalent in chronic kidney disease [39,40]. Conceivably, a low dietary level of phosphate that is least harmful in chronic kidney disease may also reduce the risk of cancer. Therefore, the hypothesis of the study posits that the lowest level of phosphate intake, represented by the NKF recommendations (800–1000 mg), will reduce the relative risk of breast cancer in the cohort compared to higher dietary phosphate levels.
2. Materials and Methods
The present study used a nested case–control design to conduct a secondary analysis of cohort data from the Study of Women’s Health Across the Nation (SWAN) [41]. SWAN is funded by the U.S. National Institutes of Health, the National Institute on Aging, the National Institute of Nursing Research, the National Center for Complementary and Alternative Medicine, and the Office of Research on Women’s Health, and the open access dataset for the SWAN study, along with demographic information of the cohort, is freely available online at the study website [42]. SWAN study participants included 3302 multi-ethnic middle-aged American women from a multi-site longitudinal sample. “At the time of enrollment, women were premenopausal, not taking hormones and between 42–52 years of age.” Figure 1 shows the proportion of participants who identified themselves as African American, Caucasian, Chinese, Hispanic, or Japanese [43].
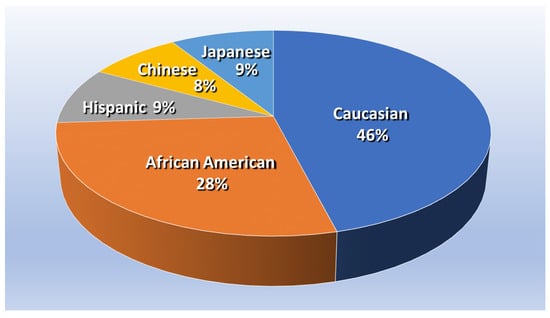
Figure 1.
Proportion of SWAN participants, based on About SWAN—Study of Women’s Health Across the Nation, swanstudy.org (accessed on 2 July 2023) [44].
Publicly available data from SWAN used in the present study were collected from baseline interviews and examinations of physical, psychological, biological, and social factors, followed up with 10 annual visits (1997–2007). Food frequency questionnaires (FFQs) were administered to collect dietary data at baseline and at visits 5 and 9. In the present study, each of the 74 breast cancer cases, who self-reported breast cancer during annual follow-up visits, were matched with four controls randomly selected from the cohort, totaling 296 controls consisting of women with similar ages (42–52 years) who were followed over 10 annual assessments. Four controls per case is recommended to increase statistical power in a case–control study, with beyond four matched controls generally leading to negligible increases in power [45]. A list of random numbers was generated using Microsoft Excel to select controls.
2.1. Statistical Analysis
Although an odds ratio is most often used in case–control studies to measure the ratio of disease prevalence between exposed and unexposed groups, the present case–control study is nested within a cohort and measures disease incidence or an incidence rate ratio between exposed and unexposed groups, which is represented in the present paper as a risk ratio [46].
“The numerator of an incidence proportion or rate consists only of persons whose illness began during the specified interval. The numerator for prevalence includes all persons ill from a specified cause during the specified interval regardless of when the illness began” [47].
Additionally, odds ratios in cohort studies overestimate the risk ratio [48]. Relative risk formulas with 95% confidence intervals and p-values were calculated to four decimal places using online MedCalc Software Ltd. [49]. Statistical significance was set at p < 0.05.
2.2. Dietary Assessment
Data of dietary phosphorus intake collected at baseline from food frequency questionnaires (FFQ) were cumulatively averaged with FFQ data collected in visits 5 and 9 according to the cumulative average method used by Wallace et al. [50]. Specifically, the sum of phosphorus from three prior FFQ measures over 10 visits was divided by 3 to provide the final cumulative average. Willett stated, “The use of cumulative average measurements (i.e., the average of all measurements for an individual up to the start of each follow-up interval) takes advantage of all prior data and thus should provide a statistically more powerful test of association with cumulative exposure” [17]. For example, a recent study on dietary flavonoids “used the cumulative average intake of flavonoids and other nutrients calculated by averaging their intake at baseline and each follow-up survey” [51]. The same method to calculate cumulative average was used for calorie intake. Also, Wallace et al. handled missing FFQ data for visits 5 and 9 by imputing previously reported values, which is a single-imputation method known as last observation carry-forward (LOCF) [52]. However, noting LOCF can have problems both with biased estimation and artificial reduction in variance [53], missing data in the present study were handled with procedures for multiple imputation calculated with SAS PROC MI using the Fully Conditional Specification Method (FCS).
Additionally, the adjustment method from the Dietary Assessment Primer of the National Cancer Institute (NCI) [54] was used in the present study to standardize self-reported dietary information by adjusting for energy intake. According to the NCI, the purpose of energy adjustment is to mitigate “the effects of measurement error in data collected using self-reported dietary assessment instruments.” Energy adjustment is based on “the assumption that individuals tend to misreport intakes of most reported foods and beverages to a similar degree and in the same direction” (e.g., less healthy foods are often underreported more than healthy foods). Information biases from underreported calorie and phosphorus intakes in FFQs were adjusted by estimating each participant’s caloric density of phosphorus, calculated by dividing milligrams of phosphorus intake by caloric intake. This nutrient density quotient was then multiplied by 2000 calories needed for average bodyweight maintenance in women.
To analyze breast cancer risk ratios, energy-standardized dietary phosphorus intakes of participants were grouped into six discrete categories, each spanning 200 mg of phosphorus (P), with 800 to 1000 mg of P as the reference category to which the other five categories were compared. As mentioned in the introduction, the reference category is based on NKF guidelines for P dietary intake [20]. The second phosphate category covers the range from >1000 to 1200 mg of P. The third category ranges from >1200 mg to 1400 mg, which is the level associated with increasing all-cause mortality [21]. The fourth and fifth categories range from >1400 mg to 1600 mg and >1600 to 1800, respectively, and the sixth category, >1800 mg of P, is the approximate level of phosphate in menus recommended by the USDA. Supporting data for categorization and multiple imputation of breast cancer cases and controls is available in the Supplementary Materials.
3. Results
Table 1 shows the mean, standard deviation, minimum, and maximum values for P mg intake in the unadjusted and standardized case and control groups, rounded to multiples of 10. Mean unadjusted dietary P levels for the case and control groups are 1120 mg and 1150 mg, respectively, which are approximately equal to the average dietary P intake of 1189 mg reported for U.S. women in the 2015–2016 National Health and Nutrition Examination Survey (NHANES) [55]. Group mean standardized P levels for the case and control groups increased to 1390 mg and 1320 mg, respectively, with an approximate 5% higher mean in the case group compared to the control group.

Table 1.
P mg intake in case and control groups.
Interestingly, nine cases initially reported unadjusted dietary P levels below 800 mg (substantially below the NHANES average of 1189 mg of P for U.S. women), which was reduced to two cases <800 mg of P after standardization. Among controls, 62 women initially reported unadjusted dietary P levels below 800 mg which was reduced to eight controls after standardization. Standardization appeared to reduce the proportion of initially reported dietary P levels <800 mg more so in cases (2 out of 9 or 22.2%) than in controls (8 out of 62 or 12.9%). Table 1 also shows that the maximum standardized dietary P intake levels in the cases and controls are 2180 mg and 2450 mg, respectively, which are well below the 4000 mg tolerable upper intake limit (UL) for P to prevent harmful effects according to the Institute of Medicine (IOM) [18]. The IOM notes that the UL was established to guide the use of dietary supplements, and P is not often consumed in supplements in the U.S.
The standardized mean P intakes for cases and controls in our study (1390 and 1320 mg, respectively) are below P levels in MyPlate recommendations (~1800). This suggests that MyPlate recommendations may not be attainable for many people. Additionally, Table 1 shows that minimum levels of standardized dietary P in cases is 770 mg, which meets the daily recommended dietary allowance (RDA) of 700 mg for adult men and women according to the IOM. The IOM also noted that RDAs provide additional P in women for lactation and pregnancy. By contrast, Table 1 shows that the standardized minimum level of dietary P is lower at 570 mg in the controls, yet this level is very close to the IOM’s estimated average requirement (EAR) of 580 mg of P for adult men and women.
Table 2 shows the division of standardized dietary P levels into six discrete dietary intake categories, each spanning 200 mg of P. Estimated relative risks (RR) of breast cancer are calculated by comparing risks from each of five categories of P intake to the reference P intake level of 800–1000 mg (the control level). Risk and RRs in Table 2 are shown rounded to two decimal places, and 95% confidence intervals (CIs) cross the null value of 1, indicating statistical non-significance. However, an increasing risk of breast cancer is associated with exposure to higher P intake levels. Furthermore, the highest level of >1800 mg of P is associated with the highest RR of breast cancer, 2.30, although the p-value is non-significant at 0.07.

Table 2.
Relative risks of breast cancer cases associated with dietary P levels compared to reference level of 800–1000 mg of P.
Figure 2 is a graph plotting the risks of breast cancer incidence associated with each category of dietary P mg. A curvilinear regression line fitted to the graph has an R2 of 0.9341, indicating a strong correlation between increasing dietary P levels and breast cancer risks.
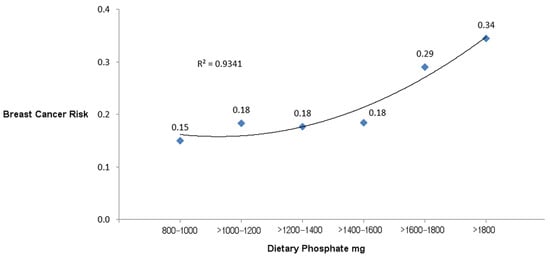
Figure 2.
Risks of breast cancer incidence associated with categories of dietary P.
4. Discussion
To the best of the authors’ knowledge, the present study is the first to report an increased risk of self-reported breast cancer incidence associated with a high dietary P intake. Compared to the lowest P intake level in this nested case–control study from the SWAN cohort of middle-aged females, exposure to the highest P intake of >1800 mg is associated with a 2.30 relative risk of breast cancer incidence, although this effect is not statistically significant (95% CI 0.94–5.61, p = 0.07). A curvilinear regression line of risks for breast cancer incidence shows a strong correlation with exposure to higher levels of dietary P; R2 equals 0.9341.
Additionally, the risk ratios in the study did not reach statistical significance, but this may be due to the study’s limited statistical power and small sample size—breast cancer cases were reported in only 2.2% of the cohort. Nevertheless, the practical significance of the study’s large effect size is important, as “the effect size is the main finding of a quantitative study” [56].
Of particular concern is the 2.30 increased risk of breast cancer incidence associated with the highest level of >1800 mg of P compared to the reference level of 800–1000 mg of P. This high level of P is the approximate amount in menu plans promoted by the USDA. Powerful U.S. government agencies, like the USDA and the U.S. Department of Health and Human Services, currently write the Dietary Guidelines for Americans, “separating the science from the actual guidelines and making the process more political” [57]. Findings of the present study should alert the public to prioritize breast cancer prevention through promotion of dietary recommendations with lower P levels, which might also help reduce the global burden of 3 million new breast cancer cases predicted by 2040 [58].
Although RRs based on uncontrolled observational studies without randomization cannot prove causality, findings of the present study meet the criteria proposed by Bradford Hill, which infer causality in observational studies [59]. The criteria are detailed as follows:
- Strength of association: The magnitude of the relative risk of breast cancer incidence associated with high dietary P levels is up to 2.3 times greater than associations with low phosphorus levels. “As a measure of effect size, an RR value is generally considered clinically significant if it is less than 0.50 or more than 2.00; that is, if the risk is at least halved, or more than doubled” [60]. A recent review from the International Agency for Research on Cancer (IARC) found that most studies linking various cancers to occupational exposures known to be carcinogenic in humans reported relative risk values well below the 2.30 relative risk in the present study, and approximately one-third of the confidence intervals in the IARC review were not statistically significant [61];
- Consistency: the association of high dietary P with breast cancer [62] and with other cancers is similar across multiple studies [5];
- Specificity: the present study shows that P is a specific dietary factor in the association with breast cancer; notably, this does not preclude other risk factors that are associated with breast cancer;
- Temporality: exposure to high dietary P precedes breast cancer incidence, as revealed in the present nested case–control study’s longitudinal data;
- Biological gradient: compared to the lowest level of P intake, increasing levels of dietary P in the present study are associated with increasing risk of breast cancer;
- Plausibility: higher dietary P levels are associated with dysregulated phosphate metabolism and phosphate toxicity, which may lead to tumorigenesis [5,62,63,64,65];
- Coherence: dysregulated phosphate metabolism and phosphate toxicity fit the regulation-based model of cancer, which proposes that cancer is caused by dysregulated metabolic factors [8];
- Experimental evidence: Laboratory animal experiments confirm an association between high dietary P feeding and tumorigenesis [6,7]. Importantly, P from dietary sources in these animal experiments are not administered at the maximum tolerated dosages for chemical agents, which are often used in carcinogenic studies [66];
- Analogy: overgrowth of algae blooms in eutrophication, caused by excessive phosphate fertilizer agricultural runoff [67], is analogous to the ecosystem dynamics of cancer cell overgrowth [68] associated with high dietary P [2,10].
The study’s main strength is that it is the first report to show a large positive dose-dependent association between self-reported breast cancer incidence and increasing levels of dietary P intake in a cohort of middle-aged U.S. women. Limitations of the study include the small sample size of 3302 women in the SWAN cohort compared to nationwide studies of over 161,000 women in the Women’s Health Initiative [69] and 280,000 women in the Nurses’ Health Study [70]. However, the SWAN cohort provides the advantage of a broad ethnic cross-section of middle-aged women in the national population. Furthermore, Pink SWAN, supported by the National Cancer Institute, doubled the follow-up period of the SWAN cohort from 10 to 20 years, and Avis et al. identified 152 breast cancer cases [71], which is approximately twice the sample size of the present study.
Additionally, the nested case–control design of this study has certain limitations common to observational studies:
“The major disadvantage of nested case–control studies is that not all pertinent risk factors are likely to have been recorded. Furthermore, because many different healthcare professionals will be involved in patient care, risk factors and outcome(s) will probably not have been measured with the same accuracy and consistency throughout. It may also be problematic if the diagnosis of the disease or outcome changes with time”.[72]
Nevertheless, among epidemiological observational studies, the nested case–control design ranks high, providing the advantage of observing disease incidence within a cohort [73].
Other study limitations include the reliance on cohort participants to self-report breast cancer incidence, which may be prone to inaccuracies and information bias, unless credible proof of diagnosis is presented to verify the diagnosis. Study limitations also include standardization of self-reported dietary intake from FFQ data. Although standardization is intended to provide a more realistic estimation of dietary intake to improve validity of the study, standardization cannot estimate actual dietary intake levels, and adjustments are based on averages rather than individual caloric needs of women. More accurate dietary information can be obtained using intervention studies with controlled feeding of participants—which can be very expensive. Furthermore, researchers have found a correlation between dietary phosphate intake and phosphate excreted in 24 h urine collection, which has potential use as a biomarker to estimate dietary phosphate intake in clinical studies [74]. However, compared to short-term measures such as a 24 h recall, FFQs are the most often used dietary tool for epidemiological studies with long follow-up periods [75].
Finally, potential confounding factors were not controlled in this observational study, such as exposures to environmental carcinogens, including alcohol and tobacco, and other risk factors like obesity, low physical activity, and family history of breast cancer [76]. Phosphorus needs may also decline during menopause compared to the reproductive years—which could explain findings of a study in which women at post menopause had increasing levels of serum P [77], which could be related to increasing breast cancer risk as women age [78]. Additionally, a participant’s individual renal function may modify the regulatory effect of dietary phosphate on breast cancer. Future studies should control for effect modification by stratifying the results according to the participant’s estimated glomerular filtration rate or other biomarkers of renal function. Furthermore, renal function declines with age [79], and so, findings of dietary phosphate and breast cancer in this middle-aged female SWAN cohort cannot be generalized to other segments of the population.
For future research on cancer therapies, Kuang et al. wrote, “our simulation results show that if an artificial mechanism (treatment) can cut the phosphorus uptake of tumor cells in half, then it may lead to a three-quarter reduction in ultimate tumor size, indicating an excellent potential of such a treatment” [63]. Furthermore, according to the National Institute of Cancer of the U.S. National Institutes of Health, “When evidence emerges from an epidemiologic study that a dietary component is associated with a reduced risk of cancer, a randomized trial may be done to test this possibility” [80]. Based on the epidemiologic evidence in the present study finding a clinically significant reduced risk of breast cancer incidence associated with low levels of dietary P compared to higher levels, further clinical studies are warranted to test a low-phosphate diet on tumor reduction in breast cancer patients.
5. Conclusions
Risk factors for breast cancer include the Western diet, which is high in the essential mineral P. Research has shown that higher amounts of dietary P are associated with disease and mortality. The present nested case–control study measured risk ratios of dietary P levels associated with self-reported breast cancer in middle-aged women from the SWAN cohort. Results in ten annual follow-up visits found that the highest dietary intake of P was associated with a clinically significant 2.30 relative risk of breast cancer incidence compared to the lowest intake level recommended by the U.S. NKF to treat chronic kidney disease. The highest level of P intake is within the approximate range promoted by the USDA. Evidence supports the criteria to infer breast cancer causation from high dietary P intake in observational studies, and further studies with larger cohorts are warranted. Additionally, clinical and preclinical studies with breast cancer patients should test the effect of a low-phosphate diet already in use for patients with chronic kidney disease.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu15173735/s1, Categorized Breast Cancer Cases.xlsx; Categorized Random Controls.xlsx; Multiple Imputed Breast Cancer Cases.rtf; Multiple Imputed Random Controls.rtf.
Author Contributions
Conceptualization, R.B.B.; methodology, R.B.B.; investigation, R.B.B.; writing—original draft preparation, R.B.B.; writing—review and editing, P.B., J.A.D. and J.G.M.; supervision, P.B., J.A.D. and J.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
https://www.swanstudy.org/swan-research/data-access/ (accessed on 2 July 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Li, L.; Ke, Y.; Cheng, J.; Xie, Y.; Chu, W.; Cheung, P.; Kim, J.H.; Colditz, G.A.; et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Gallant, K.M.H. Plant-based diets in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Razzaque, M.S. Endocrine Regulation of Phosphate Homeostasis. In Textbook of Nephro-Endocrinology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 539–548. [Google Scholar]
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta (BBA) Rev. Cancer 2018, 1869, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.; Turley, E.A.; Baum, M. A new biological framework for cancer research. Lancet 1996, 348, 1149–1151. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Olanbiwonnu, T.; Holden, R.M. Inorganic phosphate as a potential risk factor for chronic disease. CMAJ 2018, 190, E784–E785. [Google Scholar] [CrossRef]
- Moshfegh, A.; Kovalchik, A.; Clemens, J. Phosphorus intake of Americans: What we eat in America, NHANES 2011-2012. Food Surveys Research Group: Dietary Data Brief. U.S. Department of Agriculture. 2016; Volume 15, pp. 1–5. Available online: https://www.ars.usda.gov/arsuserfiles/80400530/pdf/dbrief/15_phosphorus_intake_1112.pdf (accessed on 2 July 2023).
- Williams, C.; Ronco, C.; Kotanko, P. Whole Grains in the Renal Diet—Is It Time to Reevaluate Their Role? Blood Purif. 2013, 36, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Isaksen, I.M.; Dankel, S.N. Ultra-processed food consumption and cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Wang, G.-P.; Chen, G.-Q.; Chen, H.-N.; Zhang, G.-Y. Association between ultra-processed foods and risk of cancer: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1175994. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Gunter, M.J.; Rauber, F.; Levy, R.B.; Huybrechts, I.; Kliemann, N.; Millett, C.; Vamos, E.P. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. eClinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- IOM. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academy of Sciences: Washington, DC, USA, 1997.
- nih.gov. Phosphorus—Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/#en29 (accessed on 27 February 2022).
- Eknoyan, G.; Levin, A.; Levin, N. Bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [Google Scholar] [CrossRef]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- myplate.gov. MyPlate Plan. Available online: https://www.myplate.gov/myplate-plan (accessed on 9 August 2021).
- fdc.nal.usda.gov. Milk, Nonfat, Fluid, without Added Vitamin A (Fat Free or Skim). Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/746776/nutrients (accessed on 1 March 2022).
- Fraser, G.E.; Jaceldo-Siegl, K.; Orlich, M.; Mashchak, A.; Sirirat, R.; Knutsen, S. Dairy, soy, and risk of breast cancer: Those confounded milks. Int. J. Epidemiol. 2020, 49, 1526–1537. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Warensjö Lemming, E.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ Br. Med. J. 2014, 349, g6015. [Google Scholar] [CrossRef]
- Wang, R.; Wen, Z.Y.; Liu, F.H.; Wei, Y.F.; Xu, H.L.; Sun, M.L.; Zhao, Y.H.; Gong, T.T.; Wang, H.H.; Wu, Q.J. Association between dietary acid load and cancer risk and prognosis: An updated systematic review and meta-analysis of observational studies. Front. Nutr. 2022, 9, 891936. [Google Scholar] [CrossRef]
- Müller, A.; Zimmermann-Klemd, A.M.; Lederer, A.K.; Hannibal, L.; Kowarschik, S.; Huber, R.; Storz, M.A. A Vegan Diet Is Associated with a Significant Reduction in Dietary Acid Load: Post Hoc Analysis of a Randomized Controlled Trial in Healthy Individuals. Int. J. Environ. Res. Public Health 2021, 18, 9998. [Google Scholar] [CrossRef]
- Lees, J.S.; Elyan, B.M.P.; Herrmann, S.M.; Lang, N.N.; Jones, R.J.; Mark, P.B. The ‘other’ big complication: How chronic kidney disease impacts on cancer risks and outcomes. Nephrol. Dial. Transplant. 2022, 38, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Staplin, N.; Emberson, J.; Baigent, C.; Turner, R.; Chalmers, J.; Zoungas, S.; Pollock, C.; Cooper, B.; Harris, D.; et al. Chronic kidney disease and the risk of cancer: An individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016, 16, 488. [Google Scholar] [CrossRef] [PubMed]
- Stengel, B. Chronic kidney disease and cancer: A troubling connection. J. Nephrol. 2010, 23, 253–262. [Google Scholar]
- Tendulkar, K.K.; Cope, B.; Dong, J.; Plumb, T.J.; Campbell, W.S.; Ganti, A.K. Risk of malignancy in patients with chronic kidney disease. PLoS ONE 2022, 17, e0272910. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; Reid, J.; Jeyakumar, N.; Dixon, S.N.; Munoz, A.M.; Silver, S.A.; Booth, C.M.; Chan, C.T.M.; Garg, A.X.; Amir, E.; et al. Cancer Risk and Mortality in Patients With Kidney Disease: A Population-Based Cohort Study. Am. J. Kidney Dis. 2022, 80, 436–448.e1. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, Q.; Liu, B.; Ma, Q.; Zhang, T.; Huang, T.; Lv, Z.; Wang, R. Chronic Kidney Disease and Cancer: Inter-Relationships and Mechanisms. Front. Cell Dev. Biol. 2022, 10, 868715. [Google Scholar] [CrossRef]
- Movahhed, S.M.M.; Mousavi, S.S.B.; Hayati, F.; Shayanpour, S.; Halili, S.; Sabetnia, L.; Khazaei, Z. The relationship between chronic kidney disease and cancer. J. Nephropathol. 2018, 7, 115–116. [Google Scholar] [CrossRef][Green Version]
- Wei, Y.-F.; Chen, J.-Y.; Lee, H.-S.; Wu, J.-T.; Hsu, C.-K.; Hsu, Y.-C. Association of chronic kidney disease with mortality risk in patients with lung cancer: A nationwide Taiwan population-based cohort study. BMJ Open 2018, 8, e019661. [Google Scholar] [CrossRef]
- Guo, K.; Wang, Z.; Luo, R.; Cheng, Y.; Ge, S.; Xu, G. Association between chronic kidney disease and cancer including the mortality of cancer patients: National health and nutrition examination survey 1999-2014. Am. J. Transl. Res. 2022, 14, 2356–2366. [Google Scholar]
- Na, S.Y.; Sung, J.Y.; Chang, J.H.; Kim, S.; Lee, H.H.; Park, Y.H.; Chung, W.; Oh, K.-H.; Jung, J.Y. Chronic Kidney Disease in Cancer Patients: An Independent Predictor of Cancer-Specific Mortality. Am. J. Nephrol. 2011, 33, 121–130. [Google Scholar] [CrossRef]
- Yu, T.M.; Chuang, Y.W.; Yu, M.C.; Chen, C.H.; Yang, C.K.; Huang, S.T.; Lin, C.L.; Shu, K.H.; Kao, C.H. Risk of cancer in patients with polycystic kidney disease: A propensity-score matched analysis of a nationwide, population-based cohort study. Lancet Oncol. 2016, 17, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Bernardor, J.; Garnier, C.; Naud, C.; Ranchin, B. Hyperphosphatemia and Chronic Kidney Disease: A Major Daily Concern Both in Adults and in Children. Calcif. Tissue Int. 2021, 108, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Aoyagi, H. Understudied Hyperphosphatemia (Chronic Kidney Disease) Treatment Targets and New Biological Approaches. Medicina 2023, 59, 959. [Google Scholar] [CrossRef]
- Sowers, M.F.R.; Crawford, S.L.; Sternfeld, B.; Morganstein, D.; Gold, E.B.; Greendale, G.A.; Evans, D.A.; Neer, R.; Matthews, K.A.; Sherman, S. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In Menopause: Biology and Pathobiology; Academic Press: San Diego, CA, USA, 2000; pp. 175–188. [Google Scholar]
- swanstudy.org. SWAN: Study of Women’s Health Across the Nation. Available online: https://www.swanstudy.org/ (accessed on 2 July 2023).
- Santoro, N.; Sutton-Tyrrell, K. The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet. Gynecol. Clin. N. Am. 2011, 38, 417–423. [Google Scholar] [CrossRef] [PubMed]
- swanstudy.org. About SWAN. Available online: https://www.swanstudy.org/about/about-swan/ (accessed on 2 July 2023).
- Grimes, D.A.; Schulz, K.F. Compared to what? Finding controls for case-control studies. Lancet 2005, 365, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, J.A.; Hunink, M.M.G.; Ikram, M.A.; Ikram, M.K. Do Case-Control Studies Always Estimate Odds Ratios? Am. J. Epidemiol. 2021, 190, 318–321. [Google Scholar] [CrossRef]
- cdc.gov. Principles of Epidemiology in Public Health Practice—Lesson 3: Measures of Risk. Available online: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/ (accessed on 6 July 2023).
- Knol, M.J.; Le Cessie, S.; Algra, A.; Vandenbroucke, J.P.; Groenwold, R.H. Overestimation of risk ratios by odds ratios in trials and cohort studies: Alternatives to logistic regression. CMAJ 2012, 184, 895–899. [Google Scholar] [CrossRef]
- medcalc.org. Relative Risk Calculator—Version 20.027. Available online: https://www.medcalc.org/calc/relative_risk.php (accessed on 17 March 2022).
- Wallace, T.C.; Jun, S.; Zou, P.; McCabe, G.P.; Craig, B.A.; Cauley, J.A.; Weaver, C.M.; Bailey, R.L. Dairy intake is not associated with improvements in bone mineral density or risk of fractures across the menopause transition: Data from the Study of Women’s Health Across the Nation. Menopause 2020, 27, 879–886. [Google Scholar] [CrossRef]
- Kong, J.S.; Kim, Y.M.; Woo, H.W.; Shin, M.H.; Koh, S.B.; Kim, H.C.; Shin, J.H.; Kim, M.K. Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS). Nutrients 2023, 15, 1186. [Google Scholar] [CrossRef]
- Blankers, M.; Koeter, M.W.J.; Schippers, G.M. Missing data approaches in eHealth research: Simulation study and a tutorial for nonmathematically inclined researchers. J. Med. Internet Res. 2010, 12, e54. [Google Scholar] [CrossRef]
- Lachin, J.M. Fallacies of last observation carried forward analyses. Clin. Trials 2016, 13, 161–168. [Google Scholar] [CrossRef] [PubMed]
- dietassessmentprimer.cancer.gov. Learn More about Energy Adjustment. Available online: https://dietassessmentprimer.cancer.gov/learn/adjustment.html (accessed on 13 August 2021).
- Fulgoni, K.; Fulgoni, V.L., 3rd. Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988-1994 to NHANES 2015–2016. Nutrients 2021, 13, 2249. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Nestle, M. Perspective: Challenges and Controversial Issues in the Dietary Guidelines for Americans, 1980–2015. Adv. Nutr. 2018, 9, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 14. [Google Scholar] [CrossRef]
- Andrade, C. Understanding relative risk, odds ratio, and related terms: As simple as it can get. J. Clin. Psychiatry 2015, 76, e857–e861. [Google Scholar] [CrossRef]
- Marant Micallef, C.; Shield, K.D.; Baldi, I.; Charbotel, B.; Fervers, B.; Gilg Soit Ilg, A.; Guénel, P.; Olsson, A.; Rushton, L.; Hutchings, S.J.; et al. Occupational exposures and cancer: A review of agents and relative risk estimates. Occup. Environ. Med. 2018, 75, 604–614. [Google Scholar] [CrossRef]
- Brown, R.B.; Bigelow, P.; Dubin, J.A.; Neiterman, E. Breast cancer, alcohol, and phosphate toxicity. J. Appl. Toxicol. 2023. [Google Scholar] [CrossRef]
- Kuang, Y.; Nagy, J.D.; Elser, J.J. Biological stoichiometry of tumor dynamics: Mathematical models and analysis. Discrete Contin. Dyn. Syst. Ser. B 2004, 4, 221–240. [Google Scholar]
- Brown, R.B. Vitamin D, cancer, and dysregulated phosphate metabolism. Endocrine 2019, 65, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Cancer Cachexia and Dysregulated Phosphate Metabolism: Insights from Mutant p53 and Mutant Klotho Mouse Models. Metabolites 2022, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- HASEMAN, J.K. Issues in Carcinogenicity Testing: Dose Selection. Toxicol. Sci. 1985, 5, 66–78. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pal, D.B. Chapter 11—Nutrients contamination and eutrophication in the river ecosystem. In Ecological Significance of River Ecosystems; Madhav, S., Kanhaiya, S., Srivastav, A., Singh, V., Singh, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–216. [Google Scholar] [CrossRef]
- Elser, J.J.; Nagy, J.D.; Kuang, Y. Biological Stoichiometry: An Ecological Perspective on Tumor Dynamics. BioScience 2003, 53, 1112–1120. [Google Scholar] [CrossRef]
- whi.org. Women’s Health Initiative. Available online: https://www.whi.org/ (accessed on 6 June 2023).
- nursesheatlhstudy.org. The Nurses’ Health Study and Nurses’ Health Study II Are among the Largest Investigations into the Risk Factors for Major Chronic Diseases in Women. Available online: https://nurseshealthstudy.org/ (accessed on 6 June 2023).
- Avis, N.E.; Levine, B.; Goyal, N.; Crawford, S.L.; Hess, R.; Colvin, A.; Bromberger, J.T.; Greendale, G.A. Health-related quality of life among breast cancer survivors and noncancer controls over 10 years: Pink SWAN. Cancer 2020, 126, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Nested case-control studies: Advantages and disadvantages. BMJ Br. Med. J. 2014, 348, g1532. [Google Scholar] [CrossRef]
- Partlett, C.; Hall, N.J.; Leaf, A.; Juszczak, E.; Linsell, L. Application of the matched nested case-control design to the secondary analysis of trial data. BMC Med. Res. Methodol. 2020, 20, 117. [Google Scholar] [CrossRef]
- Morimoto, Y.; Sakuma, M.; Ohta, H.; Suzuki, A.; Matsushita, A.; Umeda, M.; Ishikawa, M.; Taketani, Y.; Takeda, E.; Arai, H. Estimate of dietary phosphorus intake using 24-h urine collection. J. Clin. Biochem. Nutr. 2014, 55, 62–66. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef]
- cdc.gov. What Are the Risk Factors for Breast Cancer? Available online: https://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm (accessed on 1 June 2023).
- Zhang, D.; Maalouf, N.M.; Adams-Huet, B.; Moe, O.W.; Sakhaee, K. Effects of sex and postmenopausal estrogen use on serum phosphorus levels: A cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am. J. Kidney Dis. 2014, 63, 198–205. [Google Scholar] [CrossRef]
- Surakasula, A.; Nagarjunapu, G.C.; Raghavaiah, K.V. A comparative study of pre- and post-menopausal breast cancer: Risk factors, presentation, characteristics and management. J. Res. Pharm. Pract. 2014, 3, 12–18. [Google Scholar] [CrossRef]
- Toyama, T.; Kitagawa, K.; Oshima, M.; Kitajima, S.; Hara, A.; Iwata, Y.; Sakai, N.; Shimizu, M.; Hashiba, A.; Furuichi, K.; et al. Age differences in the relationships between risk factors and loss of kidney function: A general population cohort study. BMC Nephrol. 2020, 21, 477. [Google Scholar] [CrossRef]
- cancer.gov. Diet. National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/diet (accessed on 3 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).