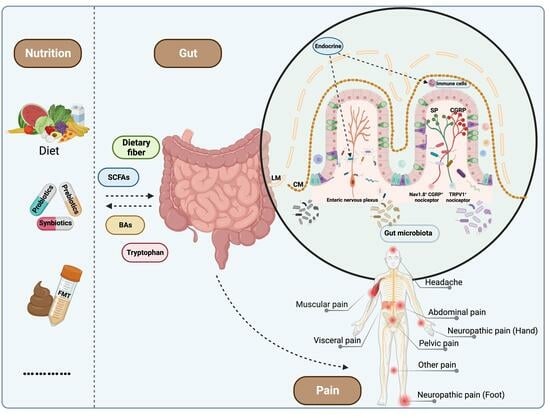
Global Trends in Research of Pain–Gut-Microbiota Relationship and How Nutrition Can Modulate This Link
Abstract
:1. Introduction
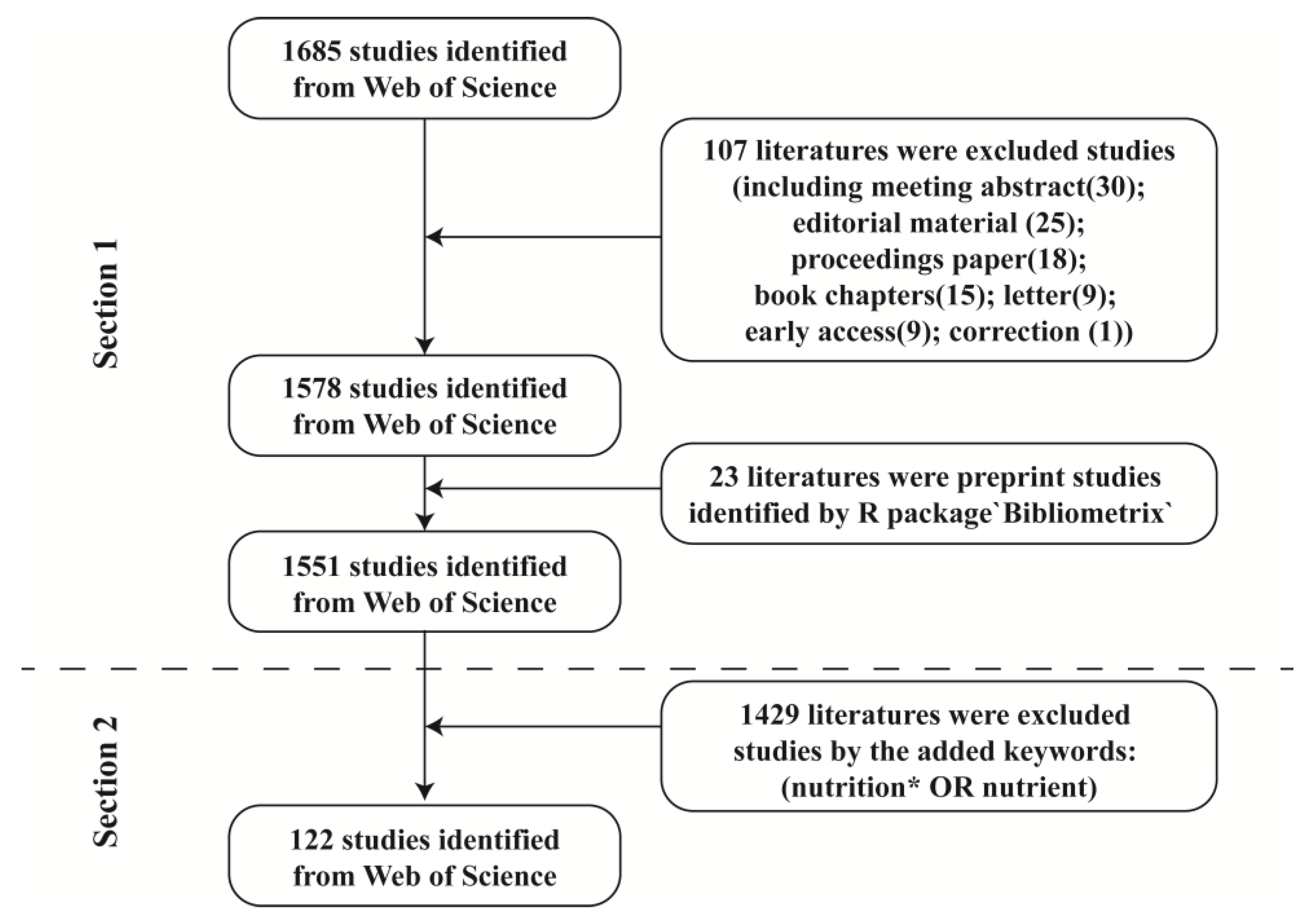
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Data Evaluation
2.3. Data Analysis and Visualization
3. Results
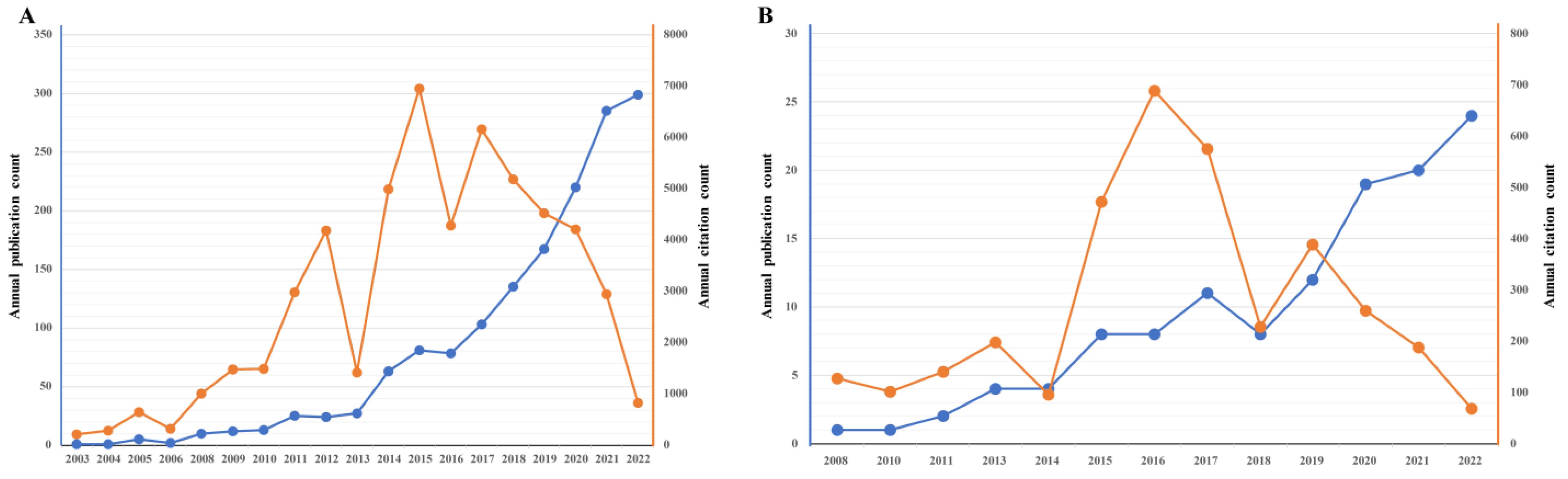
3.1. An Overview of Publications
3.2. Most Productive Entities in Recent Ten Years
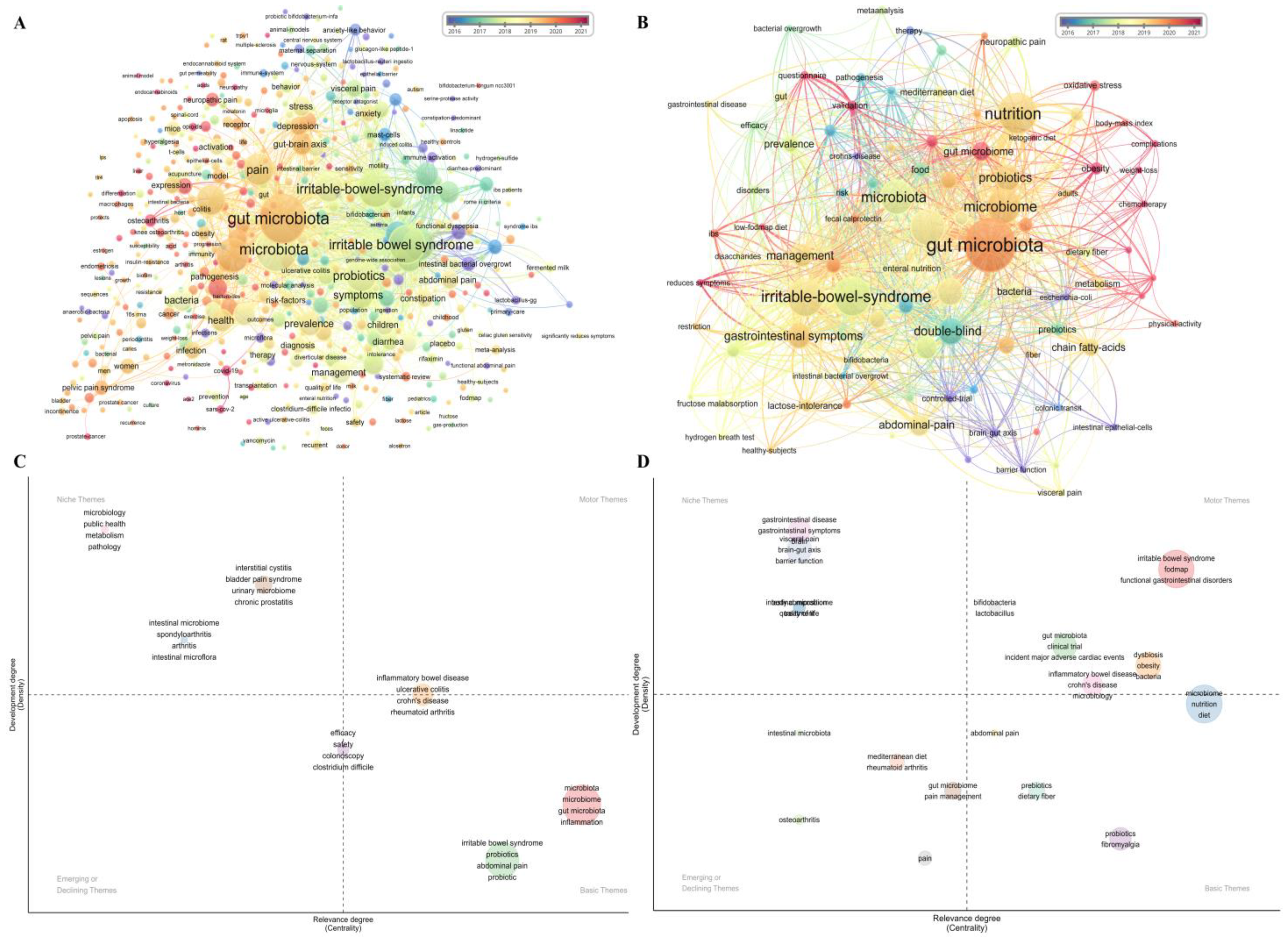
3.3. Popular Research Themes
3.4. Collaborative Network Analysis of Entities
3.5. Cited Literature Analysis
4. Discussion
4.1. Principal Findings
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| DM | Diabetes mellitus |
| DSPN | Distal symmetric polyneuropathy |
| FMT | Fecal microbiota transplantation |
| SCFAs | Short-chain fatty acids |
| BAs | Bile acids |
| WoSCC | Web of Science Core Collection |
| NP | Number of publications |
| NC | Number of citations |
| CPP | Citations per publication |
| LC | Local citations score |
| GC | Global citations score |
| SCP | Single-country publication |
| MCP | Multiple-country publication |
| APY | Average published year |
| FOS | Fructooligosaccharides |
| GOS | Galactooligosaccharides |
| IBS | Irritable bowel syndrome |
| IBD | Inflammatory bowel disease |
| FODMAP | Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols |
| WPF | Wheat peptides and fucoidan |
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. Can we conquer pain? Nat. Neurosci. 2002, 5 (Suppl. S11), 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J.; Ma, Q. Nociceptors--noxious stimulus detectors. Neuron 2007, 55, 353–364. [Google Scholar] [CrossRef]
- Aydede, M.; Shriver, A. Recently introduced definition of “nociplastic pain” by the International Association for the Study of Pain needs better formulation. Pain 2018, 159, 1176–1177. [Google Scholar] [CrossRef]
- Sinopoulou, V.; Gordon, M.; Akobeng, A.K.; Gasparetto, M.; Sammaan, M.; Vasiliou, J.; Dovey, T.M. Interventions for the management of abdominal pain in Crohn’s disease and inflammatory bowel disease. Cochrane Database Syst. Rev. 2021, 11, Cd013531. [Google Scholar]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diabetes Rep. 2019, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, X.; Wu, G.; Huang, F.; Shi, X.; Wei, W.; Zhang, Y.; Zhang, H.; Cheng, L.; Yu, L.; et al. Gut microbiota modulate distal symmetric polyneuropathy in patients with diabetes. Cell Metab. 2023; in press. [Google Scholar] [CrossRef]
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Bäckhed, F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef]
- O’ Mahony, S.M.; Dinan, T.G.; Cryan, J.F. The gut microbiota as a key regulator of visceral pain. Pain 2017, 158, S19–S28. [Google Scholar] [CrossRef]
- Grundy, L.; Erickson, A.; Brierley, S.M. Visceral Pain. Annu. Rev. Physiol. 2019, 81, 261–284. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Bonfiglio, F.; Belheouane, M.; Vallier, M.; Sauer, S.; Bang, C.; Bujanda, L.; Andreasson, A.; Agreus, L.; Engstrand, L.; et al. Faecal microbiota composition associates with abdominal pain in the general population. Gut 2018, 67, 778–779. [Google Scholar] [CrossRef]
- Bonomo, R.R.; Cook, T.M.; Gavini, C.K.; White, C.R.; Jones, J.R.; Bovo, E.; Zima, A.V.; Brown, I.A.; Dugas, L.R.; Zakharian, E.; et al. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc. Natl. Acad. Sci. USA 2020, 117, 26482–26493. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.W.; Yang, X.; Zhao, B.C.; Deng, F.; Jiang, Y.M.; Pan, W.Y.; Chen, X.D.; Zhou, B.W.; Zhang, W.J.; Hu, J.J.; et al. Predictive and Preventive Potential of Preoperative Gut Microbiota in Chronic Postoperative Pain in Breast Cancer Survivors. Anesth. Analg. 2022, 134, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhan, G.; Chen, R.; Wang, D.; Guan, S.; Xu, H. Gut microbiota and its role in stress-induced hyperalgesia: Gender-specific responses linked to different changes in serum metabolites. Pharmacol. Res. 2022, 177, 106129. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The enteric nervous system. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef] [PubMed]
- de Clercq, N.C.; Groen, A.K.; Romijn, J.A.; Nieuwdorp, M. Gut Microbiota in Obesity and Undernutrition. Adv. Nutr. 2016, 7, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Georgieva, M.G.; Ognyanov, I.V.; Kordos, K.; Jóźwik, A.; Kühl, T.; Perry, G.; Petralia, M.C.; Mazzon, E.; et al. Reactive Oxygen Species and Their Impact in Neurodegenerative Diseases: Literature Landscape Analysis. Antioxid. Redox Signal. 2020, 34, 402–420. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.T.; Jóźwik, A.; Horbanczuk, O.K.; Polgar, T.; Pieczynska, M.D.; Sampino, S.; Nicoletti, F.; Berindan-Neagoe, I.; Battino, M.; et al. Food toxicology: Quantitative analysis of the research field literature. Int. J. Food Sci. Nutr. 2020, 71, 13–21. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, W.; Nie, Y.; Li, J.; He, X. From microbial technology to microbiota medicine as a clinical discipline: Sustainable development goal. Microb. Biotechnol. 2023; early view. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef]
- Chen, C. Science Mapping: A Systematic Review of the Literature. J. Data Inf. Sci. 2017, 2, 1–40. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, L.; Gaultier, E.; Del’Homme, C.; Cartier, C.; Delmas, E.; Dapoigny, M.; Fioramonti, J.; Bernalier-Donadille, A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol. Motil. 2013, 25, e272–e282. [Google Scholar] [CrossRef]
- Enck, P.; Aziz, Q.; Barbara, G.; Farmer, A.D.; Fukudo, S.; Mayer, E.A.; Niesler, B.; Quigley, E.M.M.; Rajilic-Stojanovic, M.; Schemann, M.; et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Croisier, E.; Brown, T.; Bauer, J. The Efficacy of Dietary Fiber in Managing Gastrointestinal Toxicity Symptoms in Patients with Gynecologic Cancers undergoing Pelvic Radiotherapy: A Systematic Review. J. Acad. Nutr. Diet. 2021, 121, 261–277.e2. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P.; et al. Rifaximin Therapy for Patients with Irritable Bowel Syndrome without Constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Verdú, E.F.; Bercik, P.; Verma-Gandhu, M.; Huang, X.X.; Blennerhassett, P.; Jackson, W.; Mao, Y.; Wang, L.; Rochat, F.; Collins, S.M. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006, 55, 182–190. [Google Scholar] [CrossRef]
- Saulnier, D.M.; Riehle, K.; Mistretta, T.A.; Diaz, M.A.; Mandal, D.; Raza, S.; Weidler, E.M.; Qin, X.; Coarfa, C.; Milosavljevic, A.; et al. Gastrointestinal Microbiome Signatures of Pediatric Patients With Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1782–1791. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.H.; Pothoulakis, C.; Mayer, E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 306–314. [Google Scholar] [CrossRef]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef]
- Ford, A.C.; Quigley, E.M.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.; Moayyedi, P. Efficacy of Prebiotics, Probiotics, and Synbiotics in Irritable Bowel Syndrome and Chronic Idiopathic Constipation: Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2014, 109, 1547–1561. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Felice, V.D.; Nally, K.; Savignac, H.M.; Claesson, M.J.; Scully, P.; Woznicki, J.; Hyland, N.P.; Shanahan, F.; Quigley, E.M.; et al. Disturbance of the gut microbiota in early-life selectively affects visceral pain in adulthood without impacting cognitive or anxiety-related behaviors in male rats. Neuroscience 2014, 277, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.A.; Sachs, D.; Costa, V.V.; Fagundes, C.T.; Cisalpino, D.; Cunha, T.M.; Ferreira, S.H.; Cunha, F.Q.; Silva, T.A.; Nicoli, J.R.; et al. Commensal microbiota is fundamental for the development of inflammatory pain. Proc. Natl. Acad. Sci. USA 2008, 105, 2193–2197. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Akkermans, L.M.; Soderholm, J.D. The role of microbiota and probiotics in stress-induced gastrointestinal damage. Curr. Mol. Med. 2008, 8, 282–298. [Google Scholar] [CrossRef]
- Waitzberg, D.L.; Logullo, L.C.; Bittencourt, A.F.; Torrinhas, R.S.; Shiroma, G.M.; Paulino, N.P.; Teixeira-da-Silva, M.L. Effect of synbiotic in constipated adult women—A randomized, double-blind, placebo-controlled study of clinical response. Clin. Nutr. 2013, 32, 27–33. [Google Scholar] [CrossRef]
- Le Nevé, B.; Posserud, I.; Böhn, L.; Guyonnet, D.; Rondeau, P.; Tillisch, K.; Naliboff, B.; Mayer, E.A.; Simrén, M.A. Combined Nutrient and Lactulose Challenge Test Allows Symptom-Based Clustering of Patients With Irritable Bowel Syndrome. Am. J. Gastroenterol. 2013, 108, 786–795. [Google Scholar] [CrossRef]
- Tick, H. Nutrition and Pain. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 309–320. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef]
- Kelly, D.L.; Lyon, D.E.; Yoon, S.L.; Horgas, A.L. The Microbiome and Cancer Implications for Oncology Nursing Science. Cancer. Nurs. 2016, 39, E56–E62. [Google Scholar] [CrossRef]
- Molina-Infante, J.; Serra, J.; Fernandez-Banares, F.; Mearin, F. The low-FODMAP diet for irritable bowel syndrome: Lights and shadows. Gastroenterol. Hepatol. 2016, 39, 55–65. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Schoterman, M.H.C.; Vaughan, E.E.; Belzer, C.; Benninga, M.A. The effect of fiber and prebiotics on children’s gastrointestinal disorders and microbiome. Expert. Rev. Gastroenterol. Hepatol. 2017, 11, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Ding, X.; Li, Q.; Wu, X.; Dai, M.; Long, C.; He, Z.; Cui, B.; Zhang, F. Efficacy of faecal microbiota transplantation in Crohn’s disease: A new target treatment? Microb. Biotechnol. 2020, 13, 760–769. [Google Scholar] [CrossRef]
- Qi, J.; Buzas, K.; Fan, H.; Cohen, J.I.; Wang, K.; Mont, E.; Klinman, D.; Oppenheim, J.J.; Howard, O.M.Z. Painful Pathways Induced by TLR Stimulation of Dorsal Root Ganglion Neurons. J. Immunol. 2011, 186, 6417–6426. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Lemarroy, C.R.; Rodriguez-Gutierrez, R.; Monreal-Robles, R.; Marfil-Rivera, A. Gastrointestinal disorders associated with migraine: A comprehensive review. World J. Gastroenterol. 2016, 22, 8149–8160. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Ward, S.A.; Kalantar-Zadeh, K.; El-Omar, E.M. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano 2020, 14, 5179–5182. [Google Scholar] [CrossRef]
- Bian, X.; Wu, W.; Yang, L.; Lv, L.; Wang, Q.; Li, Y.; Ye, J.; Fang, D.; Wu, J.; Jiang, X.; et al. Administration of Akkermansia muciniphila Ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Front. Microbiol. 2019, 10, 2259. [Google Scholar] [CrossRef]
- You, H.J.; Si, J.; Kim, J.; Yoon, S.; Cha, K.H.; Yoon, H.S.; Lee, G.; Yu, J.; Choi, J.-S.; Jung, M.; et al. Bacteroides vulgatus SNUG 40005 Restores Akkermansia Depletion by Metabolite Modulation. Gastroenterology 2023, 164, 103–116. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, X.; Hu, D.; Huang, J.; Guo, E.; Xiao, R.; Li, W.; Sun, C.; Chen, G. Akkermansia supplementation reverses the tumor-promoting effect of the fecal microbiota transplantation in ovarian cancer. Cell Rep. 2022, 41, 111890. [Google Scholar] [CrossRef]
- Tang, Y.; Du, J.; Wu, H.; Wang, M.; Liu, S.; Tao, F. Potential Therapeutic Effects of Short-chain Fatty Acids on Chronic Pain. Curr. Neuropharmacol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Cruz-Aguliar, R.M.; Wantia, N.; Clavel, T.; Vehreschild, M.J.; Buch, T.; Bajbouj, M.; Haller, D.; Busch, D.; Schmid, R.M.; Stein-Thoeringer, C.K. An Open-Labeled Study on Fecal Microbiota Transfer in Irritable Bowel Syndrome Patients Reveals Improvement in Abdominal Pain Associated with the Relative Abundance of Akkermansia Muciniphila. Digestion 2019, 100, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ji, X.; Lu, G.; Zhang, F. The potential of Akkermansia muciniphila in inflammatory bowel disease. Appl. Microbiol. Biotechnol. 2021, 105, 5785–5794. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Gajtani, Z.; Baydoun, M.; Reimer, R.A.; Piedalue, K.A.; Carlson, L.E. The Use of Prebiotic and Probiotic Interventions for Treating Gastrointestinal and Psychosocial Health Symptoms in Cancer Patients and Survivors: A Systematic Review. Integr. Cancer Ther. 2021, 20, 15. [Google Scholar] [CrossRef]
- Vulevic, J.; Tzortzis, G.; Juric, A.; Gibson, G.R. Effect of a prebiotic galactooligosaccharide mixture (B-GOS (R)) on gastrointestinal symptoms in adults selected from a general population who suffer with bloating, abdominal pain, or flatulence. Neurogastroenterol. Motil. 2018, 30, 7. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Benninga, M.A.; Tabbers, M.M. Effectiveness of Probiotics in Children With Functional Abdominal Pain Disorders and Functional Constipation A Systematic Review. J. Clin. Gastroenterol. 2018, 52, S10–S26. [Google Scholar] [CrossRef]
- Ooi, S.L.; Correa, D.; Pak, S.C. Probiotics, prebiotics, and low FODMAP diet for irritable bowel syndrome—What is the current evidence? Complement. Ther. Med. 2019, 43, 73–80. [Google Scholar] [CrossRef]
- Barboi, O.B.; Chirila, I.; Ciortescu, I.; Anton, C.; Drug, V.L. Inulin, Choline and Silymarin in the Treatment of Irritable Bowel Syndrome with Constipation-Randomized Case-Control Study. J. Clin. Med. 2022, 11, 2248. [Google Scholar] [CrossRef]
- Anderson, J.L.; Hedin, C.R.; Benjamin, J.L.; Koutsoumpas, A.; Ng, S.C.; Hart, A.L.; Forbes, A.; Stagg, A.J.; Lindsay, J.O.; Whelan, K. Dietary intake of inulin-type fructans in active and inactive Crohn’s disease and healthy controls: A case-control study. J. Crohns Colitis 2015, 9, 1024–1031. [Google Scholar] [CrossRef]
- Yu, T.; Zheng, Y.P.; Tan, J.C.; Xiong, W.J.; Wang, Y.; Lin, L. Effects of Prebiotics and Synbiotics on Functional Constipation. Am. J. Med. Sci. 2017, 353, 282–292. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.; Turjeman, S.; Callens, K.; Koren, O. The intersection of undernutrition, microbiome, and child development in the first years of life. Nat. Commun. 2023, 14, 3554. [Google Scholar] [CrossRef] [PubMed]
- Kambale, R.M.; Ntagazibwa, J.N.; Kasengi, J.B.; Zigashane, A.B.; Francisca, I.N.; Mashukano, B.N.; Ngaboyeka, G.A.; Bahizire, E.; Zech, F.; Bindels, L.B.; et al. Probiotics for children with uncomplicated severe acute malnutrition (PruSAM study): A randomized controlled trial in the Democratic Republic of Congo. Am. J. Clin. Nutr. 2023, 117, 976–984. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Halkjær, S.I.; Christensen, A.H.; Lo, B.Z.S.; Browne, P.D.; Günther, S.; Hansen, L.H.; Petersen, A.M. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: Results from a randomised, double-blind placebo-controlled study. Gut 2018, 67, 2107–2115. [Google Scholar] [CrossRef]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Cui, B.; Feng, Q.; Wang, H.; Wang, M.; Peng, Z.; Li, P.; Huang, G.; Liu, Z.; Wu, P.; Fan, Z.; et al. Fecal microbiota transplantation through mid-gut for refractory Crohn’s disease: Safety, feasibility, and efficacy trial results. J. Gastroenterol. Hepatol. 2015, 30, 51–58. [Google Scholar] [CrossRef]
- Ding, X.; Li, Q.; Li, P.; Zhang, T.; Cui, B.; Ji, G.; Lu, X.; Zhang, F. Long-Term Safety and Efficacy of Fecal Microbiota Transplant in Active Ulcerative Colitis. Drug Saf. 2019, 42, 869–880. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: A pilot randomized controlled study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Field, R.; Pourkazemi, F.; Turton, J.; Rooney, K. Dietary Interventions Are Beneficial for Patients with Chronic Pain: A Systematic Review with Meta-Analysis. Pain. Med. 2021, 22, 694–714. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.; Philippou, E.; Rodomar, C.; Nikiphorou, E. The Mediterranean diet, fish oil supplements and Rheumatoid arthritis outcomes: Evidence from clinical trials. Autoimmun. Rev. 2018, 17, 1105–1114. [Google Scholar] [CrossRef]
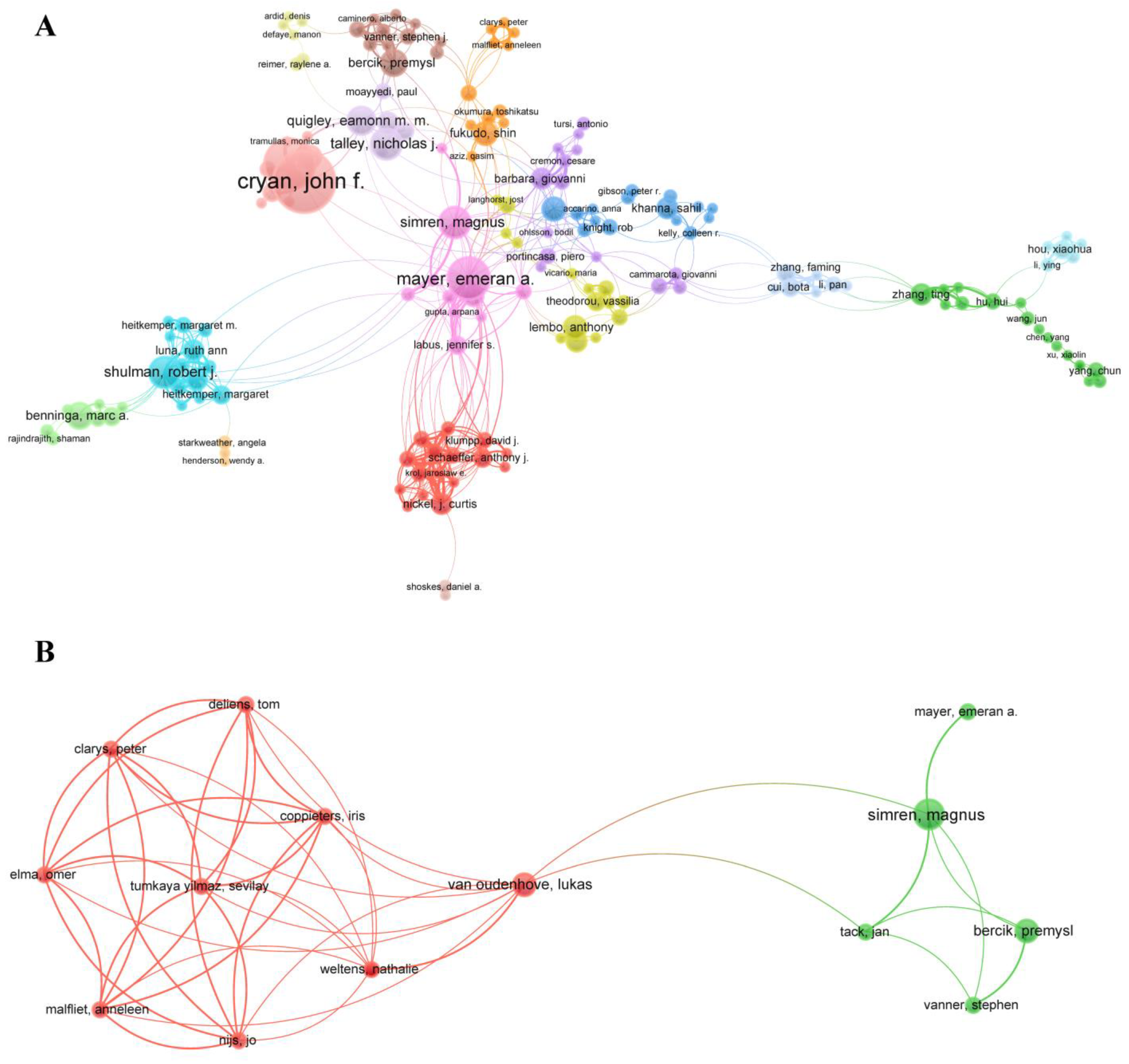
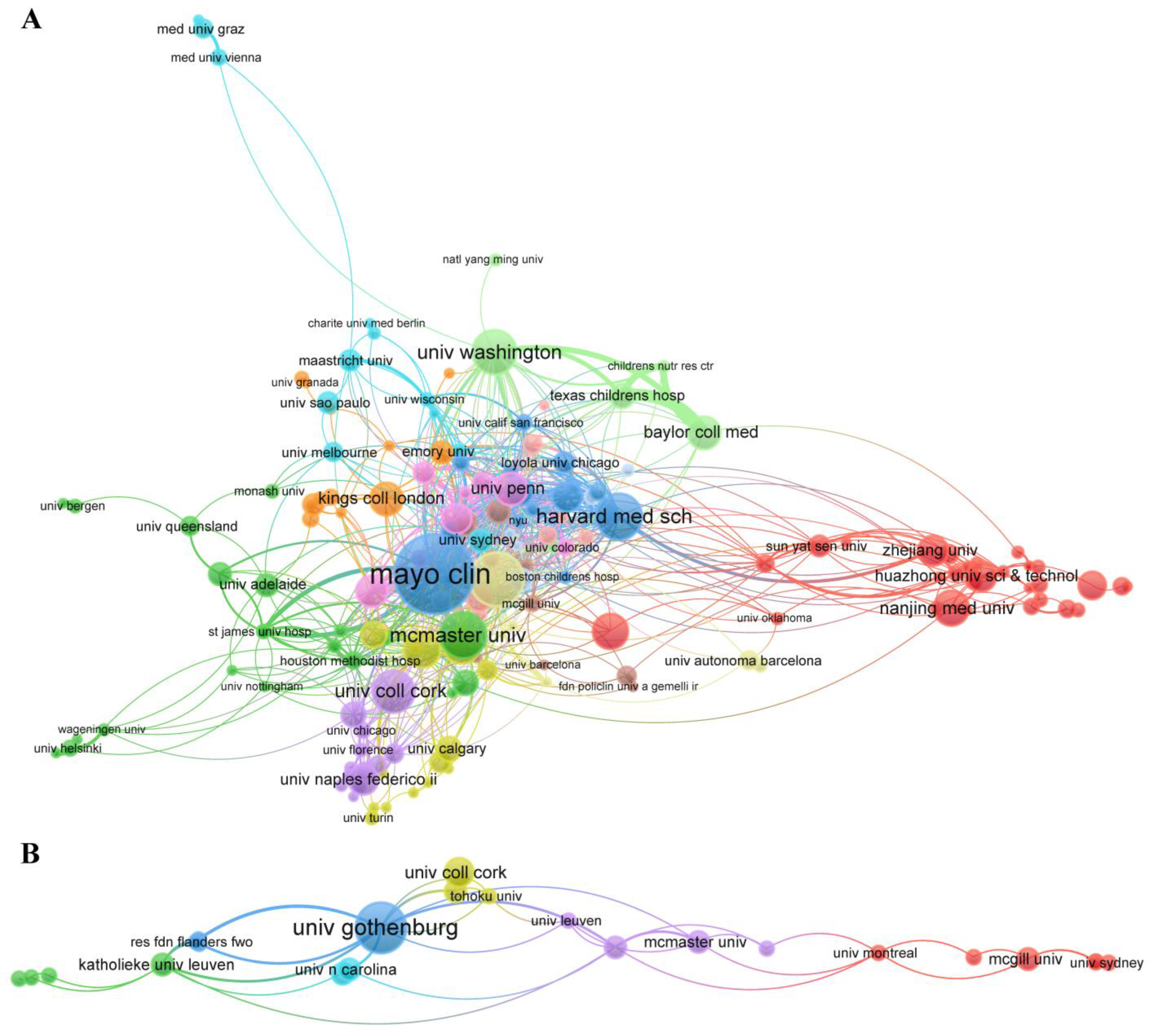
| Section 1. Researchers on Pain–Gut-Microbiota Relationship | ||||
|---|---|---|---|---|
| Author | Number of Publications, n (%) | Citations per Publication (CPP) | H-Index a | M-Index (First Year) a,b |
| Cryan JF | 28 (1.81) | 234.21 | 22 | 1.47 (2009) |
| Dinan TG | 23 (1.48) | 261.78 | 21 | 1.4 (2009) |
| Mayer EA | 16 (1.03) | 211.13 | 13 | 0.87 (2009) |
| Zhang L | 15 (0.97) | 35.93 | 10 | 1.25 (2016) |
| Zhang Y | 15 (0.97) | 11 | 8 | 1.33 (2018) |
| Talley NJ | 13 (0.84) | 69.38 | 13 | 0.93 (2010) |
| Simren M | 13 (0.84) | 74 | 9 | 0.75 (2012) |
| Shulman RJ | 12 (0.77) | 103.17 | 10 | 0.77 (2011) |
| Bercik P | 12 (0.77) | 44.25 | 9 | 0.5 (2006) |
| Clarke G | 11 (0.71) | 225.45 | 10 | 0.67 (2009) |
| Section 2. Researchers on Pain–Gut-Microbiota–Nutrition Relationship | ||||
| Author | Number of Publications, n (%) | Citations per Publication (CPP) | H-Index a | M-Index (First Year) a,b |
| Simren M | 5 (4.10) | 123.40 | 4 | 0.36 (2013) |
| Mayer EA | 3 (2.46) | 203.00 | 3 | 0.27 (2013) |
| Li L | 3 (2.46) | 131.67 | 3 | 0.43 (2017) |
| Van Oudenhove L | 3 (2.46) | 17.67 | 3 | 0.50 (2018) |
| Bercik P | 3 (2.46) | 3.33 | 2 | 0.50 (2020) |
| Chung YM | 2 (1.64) | 187.50 | 2 | 0.29 (2017) |
| Hazen Sl | 2 (1.64) | 187.50 | 2 | 0.29 (2017) |
| Luscher TF | 2 (1.64) | 187.50 | 2 | 0.29 (2017) |
| Mach F | 2 (1.64) | 187.50 | 2 | 0.29 (2017) |
| Matter CM | 2 (1.64) | 187.50 | 2 | 0.29 (2017) |
| Section 1. Organizations Regarding Pain–Gut-Microbiota Relationship | ||||
|---|---|---|---|---|
| Organization | Number of Publications, n (%) | Citations per Publication (CPP) | H-Index a | M-Index (First Year) a,b |
| Mayo Clinic | 31 (2.00) | 81.13 | 23 | 1.77 (2010) |
| University of California, Los Angeles | 29 (1.87) | 143.34 | 20 | 1.43 (2009) |
| Harvard Medical School | 25 (1.61) | 46.52 | 18 | 3.00 (2017) |
| McMaster University | 25 (1.61) | 81.60 | 16 | 0.94 (2006) |
| University of Washington | 24 (1.55) | 27.54 | 13 | 1.30 (2013) |
| University College Cork | 23 (1.48) | 51.26 | 11 | 0.73 (2008) |
| Baylor College of Medicine | 20 (1.29) | 83.25 | 12 | 1.00 (2011) |
| University of Gothenburg | 20 (1.29) | 50.35 | 10 | 0.91 (2012) |
| University of North Carolina | 20 (1.29) | 67.90 | 12 | 1.00 (2011) |
| Nanjing Medical University | 19 (1.23) | 33.00 | 12 | 1.50 (2015) |
| Section 2. Organizations Regarding Pain–Gut-Microbiota–Nutrition Relationship | ||||
| Organization | Number of Publications, n (%) | Citations per Publication (CPP) | H-Index a | M-Index (First year) a,b |
| University of Gothenburg | 7 (5.74) | 91.43 | 4 | 0.40 (2013) |
| King’s College London | 4 (3.28) | 39.75 | 4 | 0.33 (2011) |
| University College Cork | 4 (3.28) | 13.50 | 4 | 1.00 (2019) |
| Icahn School of Medicine at Mount Sinai | 3 (2.46) | 53.33 | 3 | 0.50 (2017) |
| Katholieke Universiteit Leuven | 3 (2.46) | 23.67 | 2 | 0.29 (2016) |
| Lerner Research Institute | 3 (2.46) | 126.33 | 3 | 0.50 (2017) |
| McGill University | 3 (2.46) | 8.33 | 2 | 0.29 (2016) |
| McMaster University | 3 (2.46) | 3.33 | 2 | 0.67 (2020) |
| Monash University | 3 (2.46) | 27.33 | 2 | 0.29 (2016) |
| Queen’s University | 3 (2.46) | 3.33 | 2 | 0.67 (2020) |
| Section 1. Countries Regarding Pain–Gut-Microbiota relationship | ||||
|---|---|---|---|---|
| Country | Number of Publications, n (%) | Citations per Publication (CPP) | SCP a | MCP b (MCP/(SCP+MCP) c, %) |
| United States | 356 (22.95) | 48.90 | 287 | 69 (19.38) |
| China | 283 (18.25) | 15.40 | 251 | 32 (11.31) |
| Italy | 119 (7.67) | 24.10 | 86 | 33 (27.73) |
| Australia | 58 (3.74) | 27.70 | 39 | 19 (32.76) |
| Canada | 55 (3.55) | 35.40 | 33 | 22 (40.00) |
| United Kingdom | 55 (3.55) | 55.80 | 33 | 22 (40.00) |
| France | 48 (3.09) | 31.80 | 31 | 17 (35.42) |
| South Korea | 44 (2.84) | 20.10 | 40 | 4 (9.09) |
| Spain | 44 (2.84) | 35.10 | 31 | 13 (29.55) |
| Germany | 42 (2.71) | 39.10 | 32 | 10 (23.81) |
| Section 2. Countries Regarding Pain–Gut-Microbiota–Nutrition Relationship | ||||
| Country | Number of Publications, n (%) | Citations per Publication (CPP) | SCP a | MCP b (MCP/(SCP+MCP) c, %) |
| United States | 31 (25.41) | 44.2 | 22 | 9 (29.03) |
| Italy | 17 (13.93) | 12.4 | 10 | 7 (41.18) |
| China | 9 (7.38) | 20.0 | 5 | 4 (44.44) |
| Australia | 7 (5.74) | 6.7 | 6 | 1 (14.29) |
| Canada | 7 (5.74) | 4.7 | 5 | 2 (28.57) |
| Germany | 7 (5.74) | 87.7 | 4 | 3 (42.86) |
| Brazil | 6 (4.92) | 15.3 | 6 | 0 (0) |
| Belgium | 4 (3.28) | 8.5 | 0 | 4 (100) |
| Spain | 4 (3.28) | 13.0 | 4 | 0 (0) |
| United Kingdom | 4 (3.28) | 41.0 | 2 | 2 (50.00) |
| Section 1. Journals Regarding Pain–Gut-Microbiota Relationship | ||||||
|---|---|---|---|---|---|---|
| Journal | Number of Publications, n (%) | Citations per Publication (CPP) | H-Index a | M-Index (First Year) a,b | 2023 Impact Factor | Research Area (Domain) |
| Nutrients | 46 (2.97) | 17.52 | 16 | 1.60 (2014) | 5.9 | Medicine |
| World Journal of Gastroenterology | 38 (2.45) | 44.76 | 19 | 1.27 (2009) | 4.3 | Medicine |
| Neurogastroenterology and Motility | 31 (2.00) | 36.74 | 18 | 1.29 (2010) | 3.5 | Medicine |
| PLoS ONE | 26 (1.68) | 29.19 | 14 | 1.08 (2011) | 3.7 | Multidisciplinary Sciences |
| Scientific Reports | 25 (1.61) | 35.92 | 15 | 1.67 (2015) | 4.6 | Multidisciplinary Sciences |
| Frontiers in Cellular and Infection Microbiology | 22 (1.42) | 11.27 | 8 | 1.14 (2017) | 5.7 | Medicine |
| International Journal of Molecular Sciences | 21 (1.35) | 15.48 | 10 | 1.43 (2017) | 5.6 | Chemistry |
| Medicine | 20 (1.29) | 11.20 | 7 | 0.78 (2015) | 1.6 | Medicine |
| Gastroenterology | 19 (1.23) | 104.63 | 18 | 1.38 (2011) | 29.4 | Medicine |
| Frontiers in Microbiology | 16 (1.03) | 11.25 | 7 | 0.54 (2011) | 5.2 | Microbiology |
| Section 2. Journals Regarding Pain–Gut-Microbiota–Nutrition Relationship | ||||||
| Journal | Number of Publications, n (%) | Citations per Publication (CPP) | H-Index a | M-Index (First Year) a,b | 2023 Impact Factor | Research Area (Domain) |
| Nutrients | 11 (9.02) | 20.55 | 5 | 0.56 (2015) | 5.9 | Medicine |
| World Journal of Gastroenterology | 5 (4.1) | 19.20 | 4 | 0.36 (2013) | 4.3 | Medicine |
| Animals | 3 (2.46) | 10.33 | 3 | 1.00 (2021) | 3.0 | Medicine |
| Nutrition in Clinical Practice | 3 (2.46) | 26.67 | 2 | 0.18 (2013) | 3.1 | Medicine |
| American Journal of Gastroenterology | 2 (1.64) | 17.50 | 2 | 0.18 (2013) | 9.8 | Medicine |
| Autoimmunity Reviews | 2 (1.64) | 32.50 | 2 | 0.25 (2016) | 13.6 | Medicine |
| BMJ Open | 2 (1.64) | 2.00 | 2 | 0.50 (2020) | 2.9 | Medicine |
| Clinical Gastroenterology and Hepatology | 2 (1.64) | 36.50 | 2 | 0.40 (2019) | 12.6 | Medicine |
| Clinical Nutrition | 2 (1.64) | 37.00 | 2 | 0.18 (2013) | 6.3 | Medicine |
| European Heart Journal | 2 (1.64) | 187.50 | 2 | 0.29 (2017) | 39.3 | Medicine |
| Section 1. Literature Regarding Pain–Gut-Microbiota Relationship | ||||||
|---|---|---|---|---|---|---|
| DOI | Author | Year | Journal | LC | GC | LC/GC (%) |
| 10.1038/nrn3346 | Cryan JF [24] | 2012 | Nature Reviews Neuroscience | 102 | 2385 | 4.28 |
| 10.1056/NEJMoa1004409 | Pimentel M [28] | 2011 | New England Journal of Medicine | 85 | 665 | 12.78 |
| 10.1136/gut.2005.066100 | Verdu EF [29] | 2006 | Gut | 78 | 307 | 25.41 |
| 10.1053/j.gastro.2011.06.072 | Saulnier DM [30] | 2011 | Gastroenterology | 64 | 440 | 14.55 |
| 10.1038/nrgastro.2009.35 | Rhee SH [31] | 2009 | Nature Reviews Gastroenterology & Hepatology | 63 | 766 | 8.22 |
| 10.1111/nmo.12103 | Crouzet L [25] | 2013 | Neurogastroenterology & Motility | 58 | 176 | 32.95 |
| 10.1136/gut.2008.167270 | Moayyedi P [32] | 2010 | Gut | 57 | 461 | 12.36 |
| 10.1038/ajg.2014.202 | Ford AC [33] | 2014 | American Journal of Gastroenterology | 56 | 456 | 12.28 |
| 10.1016/j.neuroscience.2014.07.054 | O’mahony SM [34] | 2014 | Neuroscience | 55 | 179 | 30.73 |
| 10.1073/pnas.0711891105 | Amaral FA [35] | 2008 | Proceedings of the National Academy of Sciences of The United States of America | 54 | 173 | 31.21 |
| Section 2. Literature Regarding Pain–Gut-Microbiota–Nutrition Relationship | ||||||
| DOI | Author | Year | Journal | LC | GC | LC/GC (%) |
| 10.1038/nrdp.2016.14 | Enck P [26] | 2016 | Nature Reviews Disease Primers | 3 | 514 | 0.58 |
| 10.1016/j.jand.2020.08.077 | Croisier E [27] | 2021 | Journal of the Academy of Nutrition and Dietetics | 2 | 7 | 28.57 |
| 10.2174/156652408784533779 | Lutgendorff F [36] | 2008 | Current Molecular Medicine | 1 | 127 | 0.79 |
| 10.1016/j.clnu.2012.08.010 | Waitzberg Dl [37] | 2013 | Clinical Nutrition | 1 | 54 | 1.85 |
| 10.1038/ajg.2013.75 | Le Neve B [38] | 2013 | American Journal of Gastroenterology | 1 | 31 | 3.23 |
| 10.1016/j.pmr.2014.12.006 | Tick H [39] | 2015 | Physical Medicine and Rehabilitation Clinics of North America | 1 | 21 | 4.76 |
| 10.3390/nu7095380 | Deng Yy [40] | 2015 | Nutrients | 1 | 168 | 0.60 |
| 10.1097/NCC.0000000000000286 | Kelly Dl [41] | 2016 | Cancer Nursing | 1 | 10 | 10.00 |
| 10.1016/j.gastrohep.2015.07.009 | Molina-Infante J [42] | 2016 | Gastroenterologia Y Hepatologia | 1 | 22 | 4.55 |
| 10.1080/17474124.2017.1359539 | Wegh Cam [43] | 2017 | Expert Review of Gastroenterology & Hepatology | 1 | 32 | 3.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, G.; Zhang, S.; Wang, R.; Zhang, Z.; Wang, W.; Wen, Q.; Zhang, F.; Li, P. Global Trends in Research of Pain–Gut-Microbiota Relationship and How Nutrition Can Modulate This Link. Nutrients 2023, 15, 3704. https://doi.org/10.3390/nu15173704
Lu G, Zhang S, Wang R, Zhang Z, Wang W, Wen Q, Zhang F, Li P. Global Trends in Research of Pain–Gut-Microbiota Relationship and How Nutrition Can Modulate This Link. Nutrients. 2023; 15(17):3704. https://doi.org/10.3390/nu15173704
Chicago/Turabian StyleLu, Gaochen, Sheng Zhang, Rui Wang, Zulun Zhang, Weihong Wang, Quan Wen, Faming Zhang, and Pan Li. 2023. "Global Trends in Research of Pain–Gut-Microbiota Relationship and How Nutrition Can Modulate This Link" Nutrients 15, no. 17: 3704. https://doi.org/10.3390/nu15173704
APA StyleLu, G., Zhang, S., Wang, R., Zhang, Z., Wang, W., Wen, Q., Zhang, F., & Li, P. (2023). Global Trends in Research of Pain–Gut-Microbiota Relationship and How Nutrition Can Modulate This Link. Nutrients, 15(17), 3704. https://doi.org/10.3390/nu15173704