Calorie Restriction Impairs Anti-Tumor Immune Responses in an Immunogenic Preclinical Cancer Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Animals and Diet
2.3. Antibody Treatment
2.4. Tumor-Infiltrating Lymphocyte (TIL) Isolation and Flow Cytometry
2.5. Real-Time CD8+ T Cell Metabolic Analysis
2.6. Statistical Analysis
3. Results
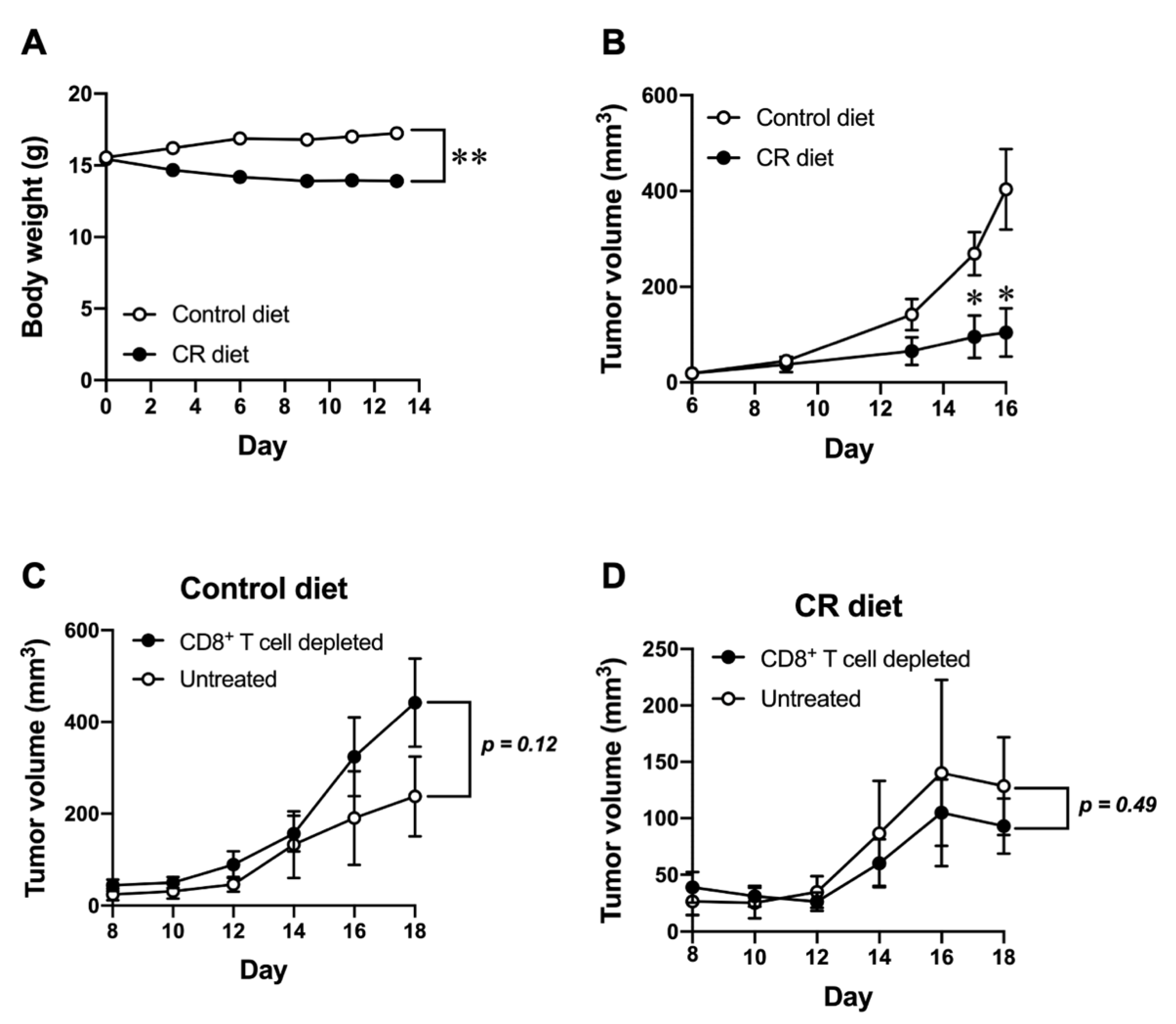
3.1. Effect of CR on Host Anti-Tumor Immunity
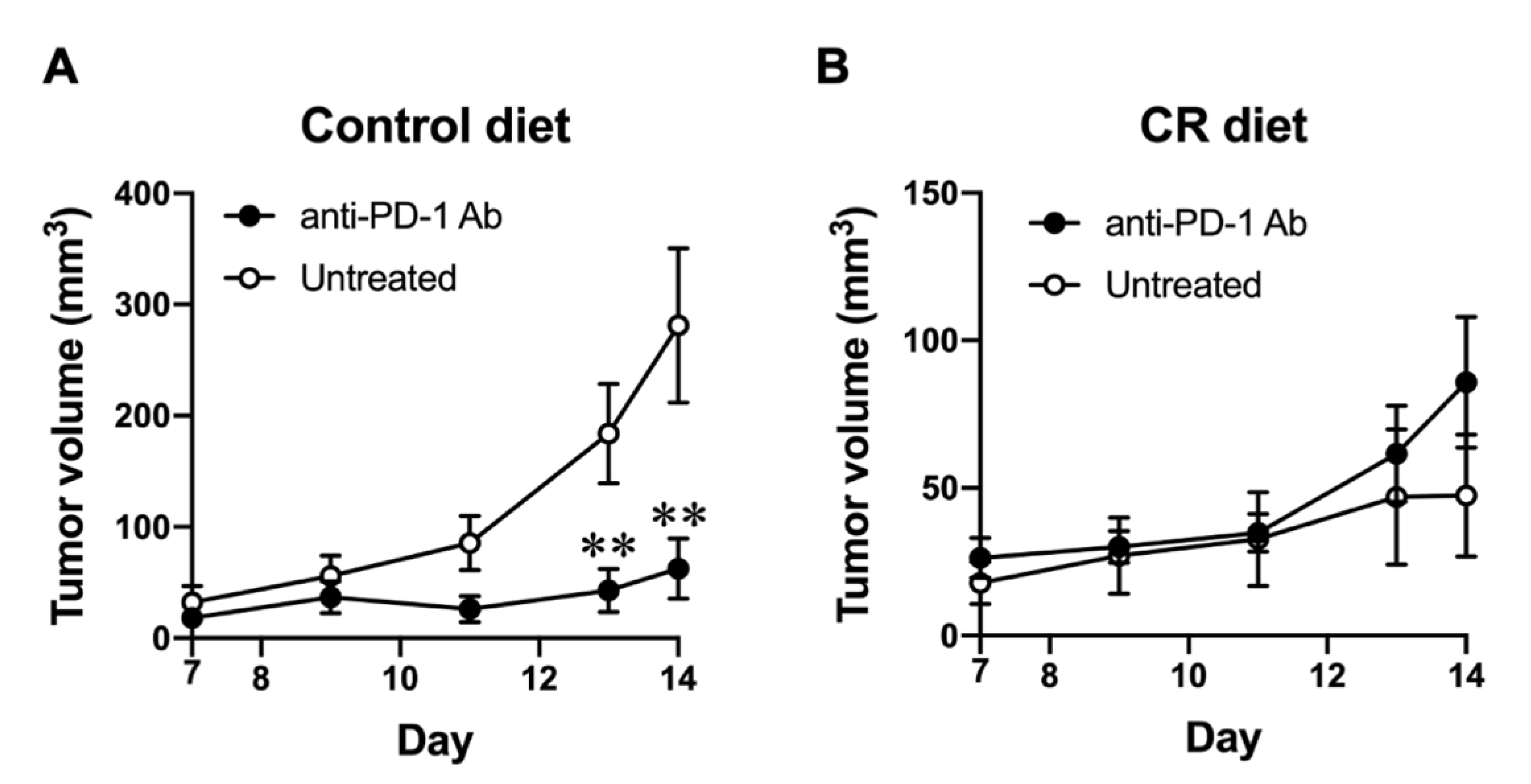
3.2. Effect of CR on the Responsiveness to Immune Checkpoint Blockade
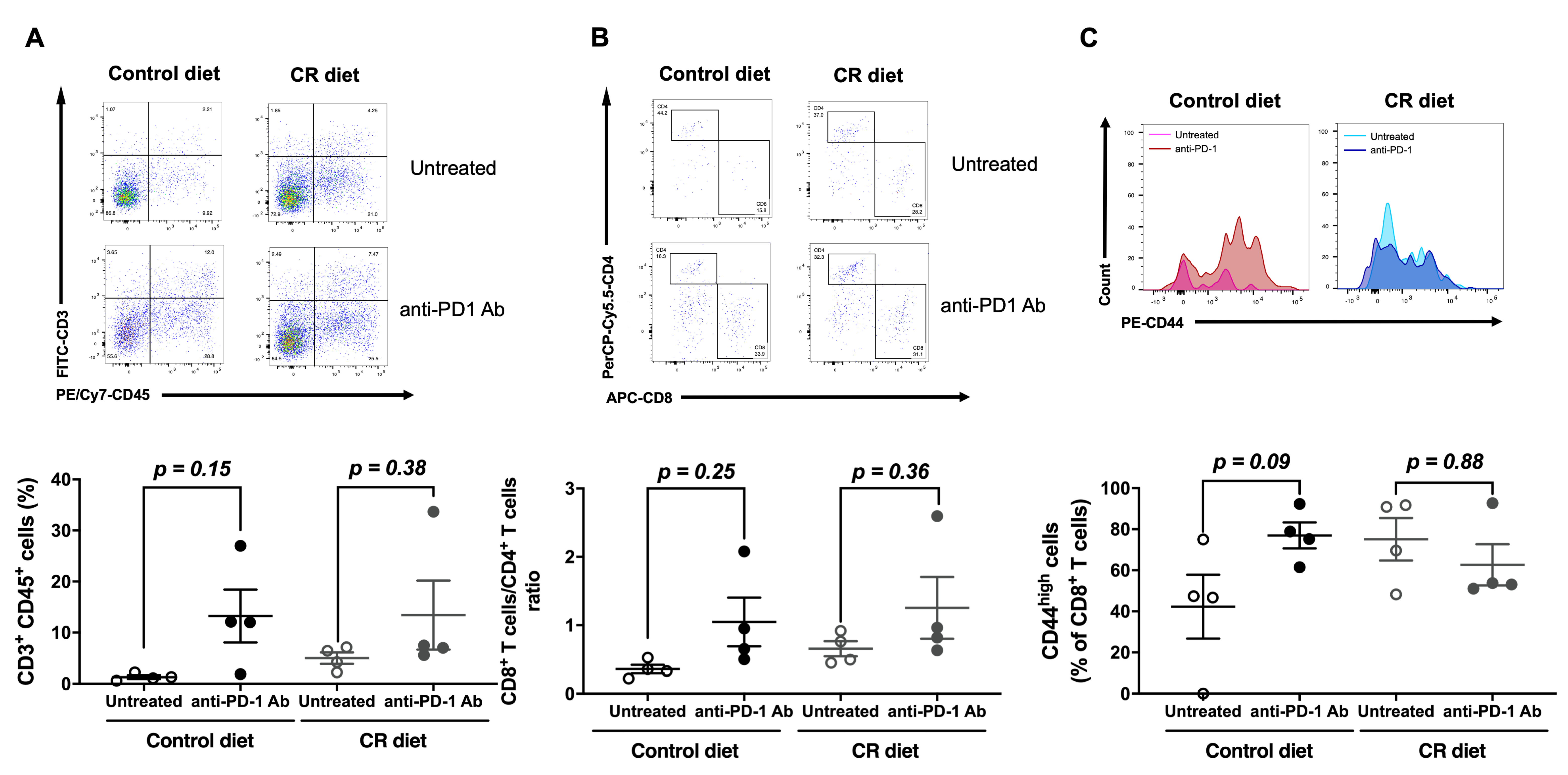
3.3. Effect of CR on the Population of Tumor-Infiltrating Lymphocytes (TILs)
3.4. Effect of CR on the Metabolic Status of CD8+ T Cell in B16-OVA Tumor-Bearing Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihaylova, M.M.; Chaix, A.; Delibegovic, M.; Ramsey, J.J.; Bass, J.; Melkani, G.; Singh, R.; Chen, Z.; William, W.J.; Shirasu-Hiza, M. When a calorie is not just a calorie: Diet quality and timing as mediators of metabolism and healthy aging. Cell Metab. 2023, 35, 1114–1131. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.A. Obesity and Cancer Pathogenesis. Ann. N. Y. Acad. Sci. 2014, 1311, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Cancer 2014, 135, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.A.; Riboli, E. Diet and Cancer Prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur. J. Cancer 2010, 46, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A. Mechanisms Linking Physical Activity with Cancer. Nat. Rev. Cancer 2008, 8, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Bhaskaram, P. Immunobiology of Mild Micronutrient Deficiencies. Br. J. Nutr. 2001, 85 (Suppl. 2), S75–S80. [Google Scholar] [CrossRef]
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, Nutrition and Immune Function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Beck, M.A. The Impact of Obesity on the Immune Response to Infection. Proc. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Ferraresi, A.; Esposito, A.; Maheshwari, C.; Dhanasekaran, D.N.; Mollace, V.; Isidoro, C. Calorie Restriction for Cancer Prevention and Therapy: Mechanisms, Expectations, and Efficacy. J. Cancer Prev. 2021, 26, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Longo, V.D. Fasting and Caloric Restriction in Cancer Prevention and Treatment. Metab. Cancer 2016, 207, 241–266. [Google Scholar] [CrossRef]
- Pistollato, F.; Forbes-Hernandez, T.Y.; Iglesias, R.C.; Ruiz, R.; Zabaleta, M.E.; Dominguez, I.; Cianciosi, D.; Quiles, J.L.; Giampieri, F.; Battino, M. Effects of caloric restriction on immunosurveillance, microbiota and cancer cell phenotype: Possible implications for cancer treatment. Semin. Cancer Biol. 2021, 73, 45–57. [Google Scholar] [CrossRef]
- Ibrahim, E.M.; Al-Foheidi, M.H.; Al-Mansour, M.M. Energy and caloric restriction, and fasting and cancer: A narrative review. Support. Care Cancer 2021, 29, 2299–2304. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular Mechanisms and Clinical Applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Lee, C.; Longo, V.D. Fasting vs Dietary Restriction in Cellular Protection and Cancer Treatment: From Model Organisms to Patients. Oncogene 2011, 30, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Raffaghello, L.; Brandhorst, S.; Safdie, F.M.; Bianchi, G.; Martin-Montalvo, A.; Pistoia, V.; Wei, M.; Hwang, S.; Merlino, A.; et al. Fasting Cycles Retard Growth of Tumors and Sensitize a Range of Cancer Cell Types to Chemotherapy. Sci. Transl. Med. 2012, 4, 124ra27. [Google Scholar] [CrossRef] [PubMed]
- Safdie, F.; Brandhorst, S.; Wei, M.; Wang, W.; Lee, C.; Hwang, S.; Conti, P.S.; Chen, T.C.; Longo, V.D. Fasting Enhances the Response of Glioma to Chemo- and Radiotherapy. PLoS ONE 2012, 7, e44603. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Summer, W.; Cutler, R.G.; Martin, B.; Hyun, D.-H.; Dixit, V.D.; Pearson, M.; Nassar, M.; Telljohann, R.; Maudsley, S.; et al. Alternate Day Calorie Restriction Improves Clinical Findings and Reduces Markers of Oxidative Stress and Inflammation in Overweight Adults with Moderate Asthma. Free Radic. Biol. Med. 2007, 42, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric Restriction and Intermittent Fasting: Two Potential Diets for Successful Brain Aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef] [PubMed]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-Driven Biomarkers to Guide Immune Checkpoint Blockade in Cancer Therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Irusta, P.M.; Navas, P.; de Cabo, R. Mitochondrial biogenesis and healthy aging. Exp. Gerontol. 2008, 43, 813–819. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Smith, S.R.; Ravussin, E. Diet, Energy Metabolism and Mitochondrial Biogenesis. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 679–687. [Google Scholar] [CrossRef]
- Nishioka, N.; Uchino, J.; Hirai, S.; Katayama, Y.; Yoshimura, A.; Okura, N.; Tanimura, K.; Harita, S.; Imabayashi, T.; Chihara, Y.; et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J. Clin. Med. 2019, 8, 450. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Tong, G.; Li, X.; You, D.; Cong, M. Prognostic Impact of Sarcopenia on Clinical Outcomes in Malignancies Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 726257. [Google Scholar] [CrossRef]
- Cao, J.; Liao, S.; Zeng, F.; Liao, Q.; Luo, G.; Zhou, Y. Effects of altered glycolysis levels on CD8+ T cell activation and function. Cell Death Dis. 2023, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Chamoto, K.; Chowdhury, P.S.; Kumar, A.; Sonomura, K.; Matsuda, F.; Fagarasan, S.; Honjo, T. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl. Acad. Sci. USA 2017, 114, E761–E770. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.S.; Chamoto, K.; Kumar, A.; Honjo, T. PPAR-Induced Fatty Acid Oxidation in T Cells Increases the Number of Tumor-Reactive CD8+ T Cells and Facilitates Anti–PD-1 Therapy. Cancer Immunol. Res. 2018, 6, 1375–1387. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dung, N.T.; Susukida, T.; Ucche, S.; He, K.; Sasaki, S.-i.; Hayashi, R.; Hayakawa, Y. Calorie Restriction Impairs Anti-Tumor Immune Responses in an Immunogenic Preclinical Cancer Model. Nutrients 2023, 15, 3638. https://doi.org/10.3390/nu15163638
Dung NT, Susukida T, Ucche S, He K, Sasaki S-i, Hayashi R, Hayakawa Y. Calorie Restriction Impairs Anti-Tumor Immune Responses in an Immunogenic Preclinical Cancer Model. Nutrients. 2023; 15(16):3638. https://doi.org/10.3390/nu15163638
Chicago/Turabian StyleDung, Nguyen Tien, Takeshi Susukida, Sisca Ucche, Ka He, So-ichiro Sasaki, Ryuji Hayashi, and Yoshihiro Hayakawa. 2023. "Calorie Restriction Impairs Anti-Tumor Immune Responses in an Immunogenic Preclinical Cancer Model" Nutrients 15, no. 16: 3638. https://doi.org/10.3390/nu15163638
APA StyleDung, N. T., Susukida, T., Ucche, S., He, K., Sasaki, S.-i., Hayashi, R., & Hayakawa, Y. (2023). Calorie Restriction Impairs Anti-Tumor Immune Responses in an Immunogenic Preclinical Cancer Model. Nutrients, 15(16), 3638. https://doi.org/10.3390/nu15163638





