Natural Phytochemicals as SIRT Activators—Focus on Potential Biochemical Mechanisms
Abstract
1. Introduction
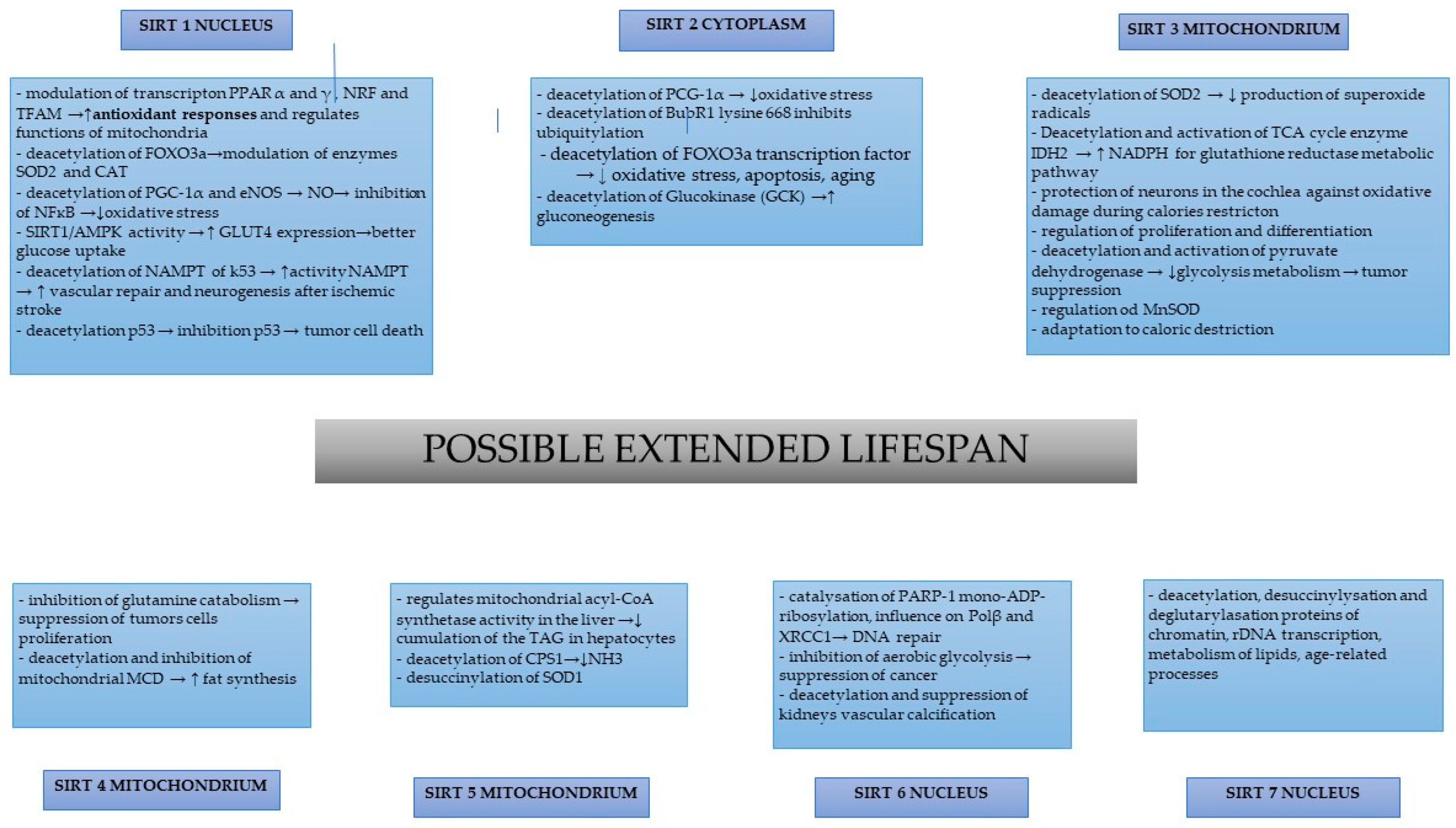
2. SIRT-1
3. SIRT-2
4. SIRT-3
5. SIRT-4
6. SIRT-5
7. SIRT-6
8. SIRT-7
9. Natural Phytochemicals as Sirtuin Activators
10. Resveratrol
11. Curcumin
12. Quercetin
13. Fisetin
14. Berberine
15. Kaempferol
16. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The Sirtuin Family in Health and Disease. Signal. Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as Anti-Inflammatory Agents in Animal Models of Prevalent Inflammatory Diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Shan, J.; Zhong, L.; Liang, B.; Zhang, D.; Li, M.; Tang, H. Dietary Phytochemicals That Can Extend Longevity by Regulation of Metabolism. Plant Foods Hum. Nutr. 2022, 77, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wierman, M.B.; Smith, J.S. Yeast Sirtuins and the Regulation of Aging. FEMS Yeast Res. 2014, 14, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The Role of Sirtuins in Cellular Homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, J.-H.; Lee, H.-Y.; Min, K.-J. Sirtuin Signaling in Cellular Senescence and Aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The Role of Mitochondrial Sirtuins in Health and Disease. Free. Radic. Biol. Med. 2016, 100, 164–174. [Google Scholar] [CrossRef]
- Katto, J.; Engel, N.; Abbas, W.; Herbein, G.; Mahlknecht, U. Transcription Factor NFκB Regulates the Expression of the Histone Deacetylase SIRT1. Clin. Epigenet. 2013, 5, 11. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Kahmini, F.R. A Comprehensive Review of Sirtuins: With a Major Focus on Redox Homeostasis and Metabolism. Life Sci. 2021, 282, 119803. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Khan, A.K.; Rashid, R.; Muneer, S.; Hasan, S.M.F.; Chen, J. FOXO Transcriptional Factors and Long-Term Living. Oxid. Med. Cell. Longev. 2017, 2017, 3494289. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Huang, Q.; Li, S.; Zhou, Y.; Li, Z. Cardioprotection of CAPE-ONO2 against Myocardial Ischemia/Reperfusion Induced ROS Generation via Regulating the SIRT1/ENOS/NF-ΚB Pathway in Vivo and in Vitro. Redox. Biol. 2018, 15, 62–73. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (ENOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar]
- Rogacka, D.; Piwkowska, A.; Audzeyenka, I.; Angielski, S.; Jankowski, M. SIRT1-AMPK Crosstalk Is Involved in High Glucose-Dependent Impairment of Insulin Responsiveness in Primary Rat Podocytes. Exp. Cell Res. 2016, 349, 328–338. [Google Scholar] [CrossRef]
- Rogacka, D.; Audzeyenka, I.; Rachubik, P.; Szrejder, M.; Typiak, M.; Angielski, S.; Piwkowska, A. Involvement of Nitric Oxide Synthase/Nitric Oxide Pathway in the Regulation of SIRT1-AMPK Crosstalk in Podocytes: Impact on Glucose Uptake. Arch. Biochem. Biophys. 2021, 709, 108985. [Google Scholar] [CrossRef]
- Rachubik, P.; Szrejder, M.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Angielski, S.; Piwkowska, A. The TRPC6-AMPK Pathway Is Involved in Insulin-Dependent Cytoskeleton Reorganization and Glucose Uptake in Cultured Rat Podocytes. Cell. Physiol. Biochem. 2018, 51, 393–410. [Google Scholar] [CrossRef]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic Syndrome and Insulin Resistance: Underlying Causes and Modification by Exercise Training. Compr. Physiol. 2013, 3, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Fleming Outeiro, T.; Cavadas, C. Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism. Trends Pharmacol. Sci. 2015, 36, 756–768. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, T.; Li, Y.; Kojima, H.; Nakajima, K.; Oshimura, M.; Inoue, T. Deacetylation of the Mitotic Checkpoint Protein BubR1 at Lysine 250 by SIRT2 and Subsequent Effects on BubR1 Degradation during the Prometaphase/Anaphase Transition. Biochem. Biophys. Res. Commun. 2014, 453, 588–594. [Google Scholar] [CrossRef]
- North, B.J.; Rosenberg, M.A.; Jeganathan, K.B.; Hafner, A.V.; Michan, S.; Dai, J.; Baker, D.J.; Cen, Y.; Wu, L.E.; Sauve, A.A.; et al. SIRT2 Induces the Checkpoint Kinase BubR1 to Increase Lifespan. EMBO J. 2014, 33, 1438–1453. [Google Scholar] [CrossRef]
- Keskin-Aktan, A.; Akbulut, K.G.; Abdi, S.; Akbulut, H. SIRT2 and FOXO3a Expressions in the Cerebral Cortex and Hippocampus of Young and Aged Male Rats: Antioxidant and Anti-Apoptotic Effects of Melatonin. Biol. Future 2022, 73, 71–85. [Google Scholar] [CrossRef]
- He, X.; Zeng, H.; Chen, J.-X. Emerging Role of SIRT3 in Endothelial Metabolism, Angiogenesis, and Cardiovascular Disease. J. Cell. Physiol. 2019, 234, 2252–2265. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Chen, Q.; Liu, N.; Yan, Y.; Liu, B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 7293861. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative Stress and Autophagy: The Clash between Damage and Metabolic Needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 Maintains Osteoblast Differentiation and Bone Formation by Regulating Mitochondrial Stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Betsinger, C.N.; Cristea, I.M. Mitochondrial Function, Metabolic Regulation, and Human Disease Viewed through the Prism of Sirtuin 4 (SIRT4) Functions. J. Proteome Res. 2019, 18, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Hwang, S.; Seong, R.H. SIRT4 Regulates Cancer Cell Survival and Growth after Stress. Biochem. Biophys. Res. Commun. 2016, 470, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, D.; Steegborn, C.; Mai, A.; Rotili, D. Sirt4: A Multifaceted Enzyme at the Crossroads of Mitochondrial Metabolism and Cancer. Front. Oncol. 2020, 10, 474. [Google Scholar] [CrossRef]
- Huang, G.; Zhu, G. Sirtuin-4 (SIRT4), a Therapeutic Target with Oncogenic and Tumor-Suppressive Activity in Cancer. Onco. Targets Ther. 2018, 11, 3395–3400. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Gao, J.; Yu, Y. The Roles of Mitochondrial SIRT4 in Cellular Metabolism. Front. Endocrinol. 2018, 9, 783. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, F.; Chen, X.; Wang, C.; Wang, J.; Liu, T.; Li, Y.; He, B. SIRT4 Is the Last Puzzle of Mitochondrial Sirtuins. Bioorg. Med. Chem. 2018, 26, 3861–3865. [Google Scholar] [CrossRef]
- Yang, L.; Ma, X.; He, Y.; Yuan, C.; Chen, Q.; Li, G.; Chen, X. Sirtuin 5: A Review of Structure, Known Inhibitors and Clues for Developing New Inhibitors. Sci. China Life Sci. 2017, 60, 249–256. [Google Scholar] [CrossRef]
- Goetzman, E.S.; Bharathi, S.S.; Zhang, Y.; Zhao, X.-J.; Dobrowolski, S.F.; Peasley, K.; Sims-Lucas, S.; Monga, S.P. Impaired Mitochondrial Medium-Chain Fatty Acid Oxidation Drives Periportal Macrovesicular Steatosis in Sirtuin-5 Knockout Mice. Sci. Rep. 2020, 10, 18367. [Google Scholar] [CrossRef]
- Wang, D.Q.-H.; Portincasa, P.; Neuschwander-Tetri, B.A. Steatosis in the Liver. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1493–1532. ISBN 978-0-470-65071-4. [Google Scholar]
- Nishida, Y.; Rardin, M.J.; Carrico, C.; He, W.; Sahu, A.K.; Gut, P.; Najjar, R.; Fitch, M.; Hellerstein, M.; Gibson, B.W.; et al. SIRT5 Regulates Both Cytosolic and Mitochondrial Protein Malonylation with Glycolysis as a Major Target. Mol. Cell 2015, 59, 321–332. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 Regulation of Ammonia-Induced Autophagy and Mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin Functions and Modulation: From Chemistry to the Clinic. Clin. Epigenetics 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Peng, C.; Anderson, K.A.; Chhoy, P.; Xie, Z.; Dai, L.; Park, J.; Chen, Y.; Huang, H.; Zhang, Y.; et al. Lysine Glutarylation Is a Protein Posttranslational Modification Regulated by SIRT5. Cell Metab. 2014, 19, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Bunton-Stasyshyn, R.K.A.; Saccon, R.A.; Fratta, P.; Fisher, E.M.C. SOD1 Function and Its Implications for Amyotrophic Lateral Sclerosis Pathology: New and Renascent Themes. Neuroscientist 2015, 21, 519–529. [Google Scholar] [CrossRef]
- Lin, Z.-F.; Xu, H.-B.; Wang, J.-Y.; Lin, Q.; Ruan, Z.; Liu, F.-B.; Jin, W.; Huang, H.-H.; Chen, X. SIRT5 Desuccinylates and Activates SOD1 to Eliminate ROS. Biochem. Biophys. Res. Commun. 2013, 441, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Denu, J.M. Sirtuin Catalysis and Regulation. J. Biol. Chem. 2012, 287, 42419–42427. [Google Scholar] [CrossRef]
- Fouquerel, E.; Goellner, E.M.; Yu, Z.; Gagné, J.-P.; Barbi de Moura, M.; Feinstein, T.; Wheeler, D.; Redpath, P.; Li, J.; Romero, G.; et al. ARTD1/PARP1 Negatively Regulates Glycolysis by Inhibiting Hexokinase 1 Independent of NAD+ Depletion. Cell Rep. 2014, 8, 1819–1831. [Google Scholar] [CrossRef]
- Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Koczor, C.A.; Saville, K.M.; Andrews, J.F.; Clark, J.; Fang, Q.; Li, J.; Al-Rahahleh, R.Q.; Ibrahim, M.; McClellan, S.; Makarov, M.V.; et al. Temporal Dynamics of Base Excision/Single-Strand Break Repair Protein Complex Assembly/Disassembly Are Modulated by the PARP/NAD+/SIRT6 Axis. Cell Rep. 2021, 37, 109917. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, H.; Liu, H.; Zhang, W.; Zhou, J. Emerging Roles of SIRT6 in Human Diseases and Its Modulators. Med. Res. Rev. 2021, 41, 1089–1137. [Google Scholar] [CrossRef]
- Bae, J.S.; Noh, S.J.; Kim, K.M.; Park, S.-H.; Hussein, U.K.; Park, H.S.; Park, B.-H.; Ha, S.H.; Lee, H.; Chung, M.J.; et al. SIRT6 Is Involved in the Progression of Ovarian Carcinomas via β-Catenin-Mediated Epithelial to Mesenchymal Transition. Front. Oncol. 2018, 8, 538. [Google Scholar] [CrossRef]
- Li, W.; Feng, W.; Su, X.; Luo, D.; Li, Z.; Zhou, Y.; Zhu, Y.; Zhang, M.; Chen, J.; Liu, B.; et al. SIRT6 Protects Vascular Smooth Muscle Cells from Osteogenic Transdifferentiation via Runx2 in Chronic Kidney Disease. J. Clin. Investig. 2022, 132, e150051. [Google Scholar] [CrossRef] [PubMed]
- Lagunas-Rangel, F.A. SIRT7 in the Aging Process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Yoshizawa, T.; Karim, M.F.; Sobuz, S.U.; Korogi, W.; Kobayasi, D.; Okanishi, H.; Tasaki, M.; Ono, K.; Sawa, T.; et al. SIRT7 Has a Critical Role in Bone Formation by Regulating Lysine Acylation of SP7/Osterix. Nat. Commun. 2018, 9, 2833. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, X.; Zhang, S.; Jin, M.; Wang, M.; Deng, Z.; Liu, Z.; Qian, M.; Shi, W.; Wang, Z.; et al. SIRT7 Activates Quiescent Hair Follicle Stem Cells to Ensure Hair Growth in Mice. EMBO J. 2020, 39, e104365. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin Activators and Inhibitors: Promises, Achievements, and Challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef]
- Miceli, M.; Bontempo, P.; Nebbioso, A.; Altucci, L. Natural Compounds in Epigenetics: A Current View. Food Chem. Toxicol. 2014, 73, 71–83. [Google Scholar] [CrossRef]
- Karaman Mayack, B.; Sippl, W.; Ntie-Kang, F. Natural Products as Modulators of Sirtuins. Molecules 2020, 25, 3287. [Google Scholar] [CrossRef]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Ramesh, S.; Neel, L.; Fabbrini, M.; Buabeid, M.; Fujihashi, A.; Dwyer, D.; Lynd, T.; Shah, K.; Mohanakumar, K.P.; et al. Nutraceutical Based SIRT3 Activators as Therapeutic Targets in Alzheimer’s Disease. Neurochem. Int. 2021, 144, 104958. [Google Scholar] [CrossRef]
- Takaoka, M. Of the Phenolic Substrate of Hellebore (Veratrum Grandiflorum Loes. Fil.). J. Fac. Sci. Hokkaido Imper. Univ. 1940, 3, 1–16. [Google Scholar]
- Huang, Y.; Lu, J.; Zhan, L.; Wang, M.; Shi, R.; Yuan, X.; Gao, X.; Liu, X.; Zang, J.; Liu, W.; et al. Resveratrol-Induced Sirt1 Phosphorylation by LKB1 Mediates Mitochondrial Metabolism. J. Biol. Chem. 2021, 297, 100929. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [PubMed]
- Melk, A. Senescence of Renal Cells: Molecular Basis and Clinical Implications. Nephrol. Dial. Transplant. 2003, 18, 2474–2478. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.-A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 Activator, Ameliorates Aging-Related Progressive Renal Injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Siemann, E.H.; Creasy, L.L. Concentration of the Phytoalexin Resveratrol in Wine. Am. J. Enol. Vitic. 1992, 43, 49–52. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Yang, L.V.; Murata, R.M.; Rosalen, P.L.; Scalisi, A.; Neri, L.M.; Cocco, L.; et al. Effects of Resveratrol, Curcumin, Berberine and Other Nutraceuticals on Aging, Cancer Development, Cancer Stem Cells and MicroRNAs. Aging 2017, 9, 1477–1536. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The in Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Juhasz, B.; Varga, B.; Gesztelyi, R.; Kemeny-Beke, A.; Zsuga, J.; Tosaki, A. Resveratrol: A Multifunctional Cytoprotective Molecule. Curr. Pharm. Biotechnol. 2010, 11, 810–818. [Google Scholar] [CrossRef]
- Korsholm, A.S.; Kjær, T.N.; Ornstrup, M.J.; Pedersen, S.B. Comprehensive Metabolomic Analysis in Blood, Urine, Fat, and Muscle in Men with Metabolic Syndrome: A Randomized, Placebo-Controlled Clinical Trial on the Effects of Resveratrol after Four Months’ Treatment. Int. J. Mol. Sci. 2017, 18, 554. [Google Scholar] [CrossRef]
- Vang, O.; Ahmad, N.; Baile, C.A.; Baur, J.A.; Brown, K.; Csiszar, A.; Das, D.K.; Delmas, D.; Gottfried, C.; Lin, H.-Y.; et al. What Is New for an Old Molecule? Systematic Review and Recommendations on the Use of Resveratrol. PLoS ONE 2011, 6, e19881. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Dreses-Werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Therapeutic Potential of Resveratrol in Alzheimer’s Disease. BMC Neurosci. 2008, 9 (Suppl. 2), S6. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. A Review of the Sirtuin System, Its Clinical Implications, and the Potential Role of Dietary Activators like Resveratrol: Part 2. Altern. Med. Rev. 2010, 15, 313–328. [Google Scholar] [PubMed]
- Scrocchi, L.A.; Drucker, D.J. Effects of Aging and a High Fat Diet on Body Weight and Glucose Tolerance in Glucagon-like Peptide-1 Receptor −/− Mice. Endocrinology 1998, 139, 3127–3132. [Google Scholar] [CrossRef]
- Thomas, J.; Garg, M.L.; Smith, D.W. Dietary Resveratrol Supplementation Normalizes Gene Expression in the Hippocampus of Streptozotocin-Induced Diabetic C57Bl/6 Mice. J. Nutr. Biochem. 2014, 25, 313–318. [Google Scholar] [CrossRef]
- Anastácio, J.R.; Netto, C.A.; Castro, C.C.; Sanches, E.F.; Ferreira, D.C.; Noschang, C.; Krolow, R.; Dalmaz, C.; Pagnussat, A. Resveratrol Treatment Has Neuroprotective Effects and Prevents Cognitive Impairment after Chronic Cerebral Hypoperfusion. Neurol. Res. 2014, 36, 627–633. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Z.; Liu, Y.; Jia, Y.; Zhang, B.; Zhang, J. Resveratrol Improves Cognition and Reduces Oxidative Stress in Rats with Vascular Dementia. Neural. Regen. Res. 2013, 8, 2050–2059. [Google Scholar] [CrossRef]
- Zou, P.; Liu, X.; Li, G.; Wang, Y. Resveratrol Pretreatment Attenuates Traumatic Brain Injury in Rats by Suppressing NLRP3 Inflammasome Activation via SIRT1. Mol. Med. Rep. 2018, 17, 3212–3217. [Google Scholar] [CrossRef]
- Cosín-Tomàs, M.; Senserrich, J.; Arumí-Planas, M.; Alquézar, C.; Pallàs, M.; Martín-Requero, Á.; Suñol, C.; Kaliman, P.; Sanfeliu, C. Role of Resveratrol and Selenium on Oxidative Stress and Expression of Antioxidant and Anti-Aging Genes in Immortalized Lymphocytes from Alzheimer’s Disease Patients. Nutrients 2019, 11, 1764. [Google Scholar] [CrossRef]
- Le, K.; Chibaatar Daliv, E.; Wu, S.; Qian, F.; Ali, A.I.; Yu, D.; Guo, Y. SIRT1-Regulated HMGB1 Release Is Partially Involved in TLR4 Signal Transduction: A Possible Anti-Neuroinflammatory Mechanism of Resveratrol in Neonatal Hypoxic-Ischemic Brain Injury. Int. Immunopharmacol. 2019, 75, 105779. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol Prevents Cognitive Deficits Induced by Chronic Unpredictable Mild Stress: Sirt1/MiR-134 Signalling Pathway Regulates CREB/BDNF Expression in Hippocampus in Vivo and in Vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-L.; Zhang, H.; Ma, L.-N.; Dong, W.; Zhao, Z.-W.; Zhang, J.-S.; Wang, Y.-L.; Zhang, X.; Wang, R. Resveratrol Prevents High-Calorie Diet-Induced Learning and Memory Dysfunction in Juvenile C57BL/6J Mice. Neurol. Res. 2018, 40, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Vitti, M.; Di Carlo, M.; Santini, S.; D’Alessandro, A.M.; Falone, S.; Amicarelli, F. Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2015, 2015, 659687. [Google Scholar] [CrossRef]
- Morita, Y.; Wada-Hiraike, O.; Yano, T.; Shirane, A.; Hirano, M.; Hiraike, H.; Koyama, S.; Oishi, H.; Yoshino, O.; Miyamoto, Y.; et al. Resveratrol Promotes Expression of SIRT1 and StAR in Rat Ovarian Granulosa Cells: An Implicative Role of SIRT1 in the Ovary. Reprod. Biol. Endocrinol. 2012, 10, 14. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, W.; Ren, S.; Fu, Y.; Fang, X.; Wang, X.; Li, B. Roles of SIRT1 in Granulosa Cell Apoptosis during the Process of Follicular Atresia in Porcine Ovary. Anim. Reprod. Sci. 2014, 151, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V. The Role and Application of Sirtuins and MTOR Signaling in the Control of Ovarian Functions. Cells 2016, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Kim, S.W.; Lee, K.-L.; Song, S.-H.; Mesalam, A.; Chowdhury, M.M.R.; Uddin, Z.; Park, K.H.; Kong, I.-K. Polydatin Improves the Developmental Competence of Bovine Embryos in Vitro via Induction of Sirtuin 1 (Sirt1). Reprod. Fertil. Dev. 2017, 29, 2011–2020. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial Effect of Resveratrol on Bovine Oocyte Maturation and Subsequent Embryonic Development after in Vitro Fertilization. Fertil. Steril. 2014, 101, 577–586. [Google Scholar] [CrossRef]
- Cai, M.; Wang, J.; Sun, H.; Guo, Q.; Zhang, C.; Yao, H.; Zhao, C.; Jia, Y.; Zhu, H. Resveratrol Attenuates Hydrogen Peroxide-Induced Injury of Rat Ovarian Granulosa-Lutein Cells by Resisting Oxidative Stress via the SIRT1/Nrf2/ARE Signaling Pathway. Curr. Pharm. Des. 2023, 29, 947–956. [Google Scholar] [CrossRef]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sánchez, E.; Alinezhad, H.; Nabavi, S.M. Curcumin: A Natural Product for Diabetes and Its Complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef]
- Ren, B.-C.; Zhang, Y.-F.; Liu, S.-S.; Cheng, X.-J.; Yang, X.; Cui, X.-G.; Zhao, X.-R.; Zhao, H.; Hao, M.-F.; Li, M.-D.; et al. Curcumin Alleviates Oxidative Stress and Inhibits Apoptosis in Diabetic Cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt Signalling Pathways. J. Cell. Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T. Curcumin as a Functional Food-Derived Factor: Degradation Products, Metabolites, Bioactivity, and Future Perspectives. Food Funct. 2018, 9, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Suszek, M.; Wnuk, M.; Lewinska, A.; Wasiak, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin Elevates Sirtuin Level but Does Not Postpone in Vitro Senescence of Human Cells Building the Vasculature. Oncotarget 2016, 7, 19201–19213. [Google Scholar] [CrossRef] [PubMed]
- Demirovic, D.; Rattan, S.I.S. Curcumin Induces Stress Response and Hormetically Modulates Wound Healing Ability of Human Skin Fibroblasts Undergoing Ageing in Vitro. Biogerontology 2011, 12, 437–444. [Google Scholar] [CrossRef]
- Grabowska, W.; Kucharewicz, K.; Wnuk, M.; Lewinska, A.; Suszek, M.; Przybylska, D.; Mosieniak, G.; Sikora, E.; Bielak-Zmijewska, A. Curcumin Induces Senescence of Primary Human Cells Building the Vasculature in a DNA Damage and ATM-Independent Manner. Age Dordr. 2015, 37, 9744. [Google Scholar] [CrossRef]
- Grabowska, W.; Mosieniak, G.; Achtabowska, N.; Czochara, R.; Litwinienko, G.; Bojko, A.; Sikora, E.; Bielak-Zmijewska, A. Curcumin Induces Multiple Signaling Pathways Leading to Vascular Smooth Muscle Cell Senescence. Biogerontology 2019, 20, 783–798. [Google Scholar] [CrossRef]
- Lewinska, A.; Wnuk, M.; Grabowska, W.; Zabek, T.; Semik, E.; Sikora, E.; Bielak-Zmijewska, A. Curcumin Induces Oxidation-Dependent Cell Cycle Arrest Mediated by SIRT7 Inhibition of RDNA Transcription in Human Aortic Smooth Muscle Cells. Toxicol. Lett. 2015, 233, 227–238. [Google Scholar] [CrossRef]
- Sandur, S.K.; Ichikawa, H.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Sethi, G.; Aggarwal, B.B. Role of Pro-Oxidants and Antioxidants in the Anti-Inflammatory and Apoptotic Effects of Curcumin (Diferuloylmethane). Free Radic. Biol. Med. 2007, 43, 568–580. [Google Scholar] [CrossRef]
- Liao, V.H.-C.; Yu, C.-W.; Chu, Y.-J.; Li, W.-H.; Hsieh, Y.-C.; Wang, T.-T. Curcumin-Mediated Lifespan Extension in Caenorhabditis Elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The Role of Different SIRT1-Mediated Signaling Pathways in Toxic Injury. Cell Mol. Biol. Lett. 2019, 24, 36. [Google Scholar] [CrossRef]
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M.; et al. Modulation of Keap1/Nrf2/ARE Signaling Pathway by Curcuma- and Garlic-Derived Hybrids. Front. Pharmacol. 2019, 10, 1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, J.; Ye, B.; Wang, Q.; Xie, X.; Shen, H. Protective Effect of Curcumin on TNBS-Induced Intestinal Inflammation Is Mediated through the JAK/STAT Pathway. BMC Complement. Altern. Med. 2016, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Cai, N.; Xu, T.; He, F. Anti-Inflammatory Effects of Curcumin in Acute Lung Injury: In Vivo and in Vitro Experimental Model Studies. Int. Immunopharmacol. 2021, 96, 107600. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wung Yeh, D.; Wang, J.-J. Curcumin Attenuates Hemorrhagic Shock and Blood Replenish Resuscitation-Induced Impairment of Pulmonary Barrier Function by Increasing SIRT1 and Reducing Malondialdehyde and TNF-α Contents and Neutrophil Infiltration in Lung in a Dose-Dependent Fashion. Transplant. Proc. 2020, 52, 1875–1879. [Google Scholar] [CrossRef]
- Tang, F.; Ling, C. Curcumin Ameliorates Chronic Obstructive Pulmonary Disease by Modulating Autophagy and Endoplasmic Reticulum Stress through Regulation of SIRT1 in a Rat Model. J. Int. Med. Res. 2019, 47, 4764–4774. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Li, Y.; Xie, Y.; Shan, H.; Chen, M.; Zhang, J.; Yang, X.; Zhang, Q.; Yang, X. Curcumin Attenuates Skeletal Muscle Mitochondrial Impairment in COPD Rats: PGC-1α/SIRT3 Pathway Involved. Chem. Biol. Interact. 2017, 277, 168–175. [Google Scholar] [CrossRef]
- Mahlooji, M.A.; Heshmati, A.; Kheiripour, N.; Ghasemi, H.; Asl, S.S.; Solgi, G.; Ranjbar, A.; Hosseini, A. Evaluation of Protective Effects of Curcumin and Nanocurcumin on Aluminium Phosphide-Induced Subacute Lung Injury in Rats: Modulation of Oxidative Stress through SIRT1/FOXO3 Signalling Pathway. Drug Res. 2022, 72, 100–108. [Google Scholar] [CrossRef]
- Hodge, G.; Tran, H.B.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lymphocyte Senescence in COPD Is Associated with Decreased Sirtuin 1 Expression in Steroid Resistant Pro-Inflammatory Lymphocytes. Ther. Adv. Respir. Dis. 2020, 14, 1753466620905280. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin Inhibits the PERK-EIF2α-CHOP Pathway through Promoting SIRT1 Expression in Oxidative Stress-Induced Rat Chondrocytes and Ameliorates Osteoarthritis Progression in a Rat Model. Oxid. Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, H.; Zhao, G.; Qiao, C.; Sun, X.; Pang, C.; Zhang, D. Curcumin and Resveratrol Suppress Dextran Sulfate Sodium-induced Colitis in Mice. Mol. Med. Rep. 2019, 19, 3053–3060. [Google Scholar] [CrossRef]
- Yin, Y.; Wu, X.; Peng, B.; Zou, H.; Li, S.; Wang, J.; Cao, J. Curcumin Improves Necrotising Microscopic Colitis and Cell Pyroptosis by Activating SIRT1/NRF2 and Inhibiting the TLR4 Signalling Pathway in Newborn Rats. Innate. Immun. 2020, 26, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ugur, S.; Ulu, R.; Dogukan, A.; Gurel, A.; Yigit, I.P.; Gozel, N.; Aygen, B.; Ilhan, N. The Renoprotective Effect of Curcumin in Cisplatin-Induced Nephrotoxicity. Ren. Fail. 2015, 37, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Domínguez, B.; Aparicio-Trejo, O.E.; García-Arroyo, F.E.; León-Contreras, J.C.; Tapia, E.; Molina-Jijón, E.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin Prevents Cisplatin-Induced Renal Alterations in Mitochondrial Bioenergetics and Dynamic. Food. Chem. Toxicol. 2017, 107, 373–385. [Google Scholar] [CrossRef]
- Ghazipour, A.M.; Shirpoor, A.; Ghiasi, R.; Pourheydar, B.; Khalaji, N.; Naderi, R. Cyclosporine A Induces Testicular Injury via Mitochondrial Apoptotic Pathway by Regulation of Mir-34a and Sirt-1 in Male Rats: The Rescue Effect of Curcumin. Chem. Biol. Interact. 2020, 327, 109180. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Li, S.; Wang, Q.; Wang, H.; Xu, M.; An, Y. Tetrahydrocurcumin Protects against Sepsis-Induced Acute Kidney Injury via the SIRT1 Pathway. Ren. Fail. 2021, 43, 1028–1040. [Google Scholar] [CrossRef]
- He, L.; Peng, X.; Zhu, J.; Liu, G.; Chen, X.; Tang, C.; Liu, H.; Liu, F.; Peng, Y. Protective Effects of Curcumin on Acute Gentamicin-Induced Nephrotoxicity in Rats. Can. J. Physiol. Pharmacol. 2015, 93, 275–282. [Google Scholar] [CrossRef]
- Zhou, S.; Sun, L.; Qian, S.; Ma, Y.; Ma, R.; Dong, Y.; Shi, Y.; Jiang, S.; Ye, H.; Shen, Z.; et al. Iron Overload Adversely Effects Bone Marrow Haematogenesis via SIRT-SOD2-MROS in a Process Ameliorated by Curcumin. Cell. Mol. Biol. Lett. 2021, 26, 2. [Google Scholar] [CrossRef]
- Jiménez-Flores, L.M.; López-Briones, S.; Macías-Cervantes, M.H.; Ramírez-Emiliano, J.; Pérez-Vázquez, V. A PPARγ, NF-ΚB and AMPK-Dependent Mechanism May Be Involved in the Beneficial Effects of Curcumin in the Diabetic Db/Db Mice Liver. Molecules 2014, 19, 8289–8302. [Google Scholar] [CrossRef]
- Zendedel, E.; Butler, A.E.; Atkin, S.L.; Sahebkar, A. Impact of Curcumin on Sirtuins: A Review. J. Cell. Biochem. 2018, 119, 10291–10300. [Google Scholar] [CrossRef]
- Fusi, J.; Bianchi, S.; Daniele, S.; Pellegrini, S.; Martini, C.; Galetta, F.; Giovannini, L.; Franzoni, F. An in Vitro Comparative Study of the Antioxidant Activity and SIRT1 Modulation of Natural Compounds. Biomed. Pharmacother. 2018, 101, 805–819. [Google Scholar] [CrossRef]
- Li, K.; Zhai, M.; Jiang, L.; Song, F.; Zhang, B.; Li, J.; Li, H.; Li, B.; Xia, L.; Xu, L.; et al. Tetrahydrocurcumin Ameliorates Diabetic Cardiomyopathy by Attenuating High Glucose-Induced Oxidative Stress and Fibrosis via Activating the SIRT1 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 6746907. [Google Scholar] [CrossRef]
- Xiao, J.; Sheng, X.; Zhang, X.; Guo, M.; Ji, X. Curcumin Protects against Myocardial Infarction-Induced Cardiac Fibrosis via SIRT1 Activation in Vivo and in Vitro. Drug Des. Devel. Ther. 2016, 10, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef]
- Tan, C.; Zhou, L.; Wen, W.; Xiao, N. Curcumin Promotes Cholesterol Efflux by Regulating ABCA1 Expression through MiR-125a-5p/SIRT6 Axis in THP-1 Macrophage to Prevent Atherosclerosis. J. Toxicol. Sci. 2021, 46, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, M.-H.; Hu, H.-J.; Feng, H.; Fan, X.-J.; Zou, W.; Pan, Y.; Hu, X.; Wang, Z. Curcumin Enhanced Cholesterol Efflux by Upregulating ABCA1 Expression through AMPK-SIRT1-LXRα Signaling in THP-1 Macrophage-Derived Foam Cells. DNA Cell Biol. 2015, 34, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Tatebe, J.; Washizawa, N.; Morita, T. Curcumin Inhibits Age-Related Vascular Changes in Aged Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1476. [Google Scholar] [CrossRef] [PubMed]
- Dolati, S.; Ahmadi, M.; Aghebti-Maleki, L.; Nikmaram, A.; Marofi, F.; Rikhtegar, R.; Ayromlou, H.; Yousefi, M. Nanocurcumin Is a Potential Novel Therapy for Multiple Sclerosis by Influencing Inflammatory Mediators. Pharmacol. Rep. 2018, 70, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, T.; Poljak, A.; Smythe, G.; Braidy, N.; Münch, G.; Sachdev, P. The Role of Polyphenols in the Modulation of Sirtuins and Other Pathways Involved in Alzheimer’s Disease. Ageing Res. Rev. 2013, 12, 867–883. [Google Scholar] [CrossRef]
- Keskin-Aktan, A.; Akbulut, K.G.; Yazici-Mutlu, Ç.; Sonugur, G.; Ocal, M.; Akbulut, H. The Effects of Melatonin and Curcumin on the Expression of SIRT2, Bcl-2 and Bax in the Hippocampus of Adult Rats. Brain Res. Bull. 2018, 137, 306–310. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wang, Y. Curcumin Alleviates Aβ42-Induced Neuronal Metabolic Dysfunction via the Thrb/SIRT3 Axis and Improves Cognition in APPTG Mice. Neurochem. Res. 2021, 46, 3166–3178. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, N.; Wang, W.; Jin, H.; Xu, J.; Hu, H. Activation of SIRT1 by Curcumin Blocks the Neurotoxicity of Amyloid-Β25-35 in Rat Cortical Neurons. Biochem. Biophys. Res. Commun. 2014, 448, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Sun, Q.; Su, Q.; Chen, G. SIRT1-Mediated Deacetylation of PGC1α Attributes to the Protection of Curcumin against Glutamate Excitotoxicity in Cortical Neurons. Biochem. Biophys. Res. Commun. 2016, 478, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Zhao, S.; Gao, Y.; Wang, R.; Wu, Q.; Wu, H.; Luo, T. Curcumin Pretreatment Attenuates Inflammation and Mitochondrial Dysfunction in Experimental Stroke: The Possible Role of Sirt1 Signaling. Brain Res. Bull. 2016, 121, 9–15. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, R.; He, D.; Zhou, G.; Wu, H.; Xu, C.; He, B.; Wu, L.; Wang, Y.; Chang, Y.; et al. Bisdemethoxycurcumin Inhibits Oxidative Stress and Antagonizes Alzheimer’s Disease by up-Regulating SIRT1. Brain Behav. 2020, 10, e01655. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Golab, F.; Morvaridzadeh, M.; Potter, E.; Akbari-Fakhrabadi, M.; Farsi, F.; Tanbakooei, S.; Shidfar, F. The Effects of Curcumin Supplementation on Oxidative Stress, Sirtuin-1 and Peroxisome Proliferator Activated Receptor γ Coactivator 1α Gene Expression in Polycystic Ovarian Syndrome (PCOS) Patients: A Randomized Placebo-Controlled Clinical Trial. Diabetes Metab. Syndr. 2020, 14, 77–82. [Google Scholar] [CrossRef]
- Azami, S.H.; Nazarian, H.; Abdollahifar, M.A.; Eini, F.; Farsani, M.A.; Novin, M.G. The Antioxidant Curcumin Postpones Ovarian Aging in Young and Middle-Aged Mice. Reprod. Fertil. Dev. 2020, 32, 292–303. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Song, N.-Y.; Suh, J.; Kim, D.-H.; Kim, W.; Ann, J.; Lee, J.; Baek, J.-H.; Na, H.-K.; Surh, Y.-J. Curcumin Suppresses Oncogenicity of Human Colon Cancer Cells by Covalently Modifying the Cysteine 67 Residue of SIRT1. Cancer Lett. 2018, 431, 219–229. [Google Scholar] [CrossRef]
- Gounden, S.; Chuturgoon, A. Curcumin Upregulates Antioxidant Defense, Lon Protease, and Heat-Shock Protein 70 Under Hyperglycemic Conditions in Human Hepatoma Cells. J. Med. Food. 2017, 20, 465–473. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, J.-Y.; Zhang, M.; Zhai, M.-G.; Di, S.-Y.; Han, Q.-H.; Jia, Y.-P.; Sun, M.; Liang, H.-L. Curcumin Attenuates IR-Induced Myocardial Injury by Activating SIRT3. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1150–1160. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Zhang, L.; Ying, Z.; Su, W.; Wang, T. Curcumin Attenuates D-Galactosamine/Lipopolysaccharide-Induced Liver Injury and Mitochondrial Dysfunction in Mice. J. Nutr. 2014, 144, 1211–1218. [Google Scholar] [CrossRef]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.-S.; Kwon, O.-S. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef]
- Du, S.; Zhu, X.; Zhou, N.; Zheng, W.; Zhou, W.; Li, X. Curcumin Alleviates Hepatic Steatosis by Improving Mitochondrial Function in Postnatal Overfed Rats and Fatty L02 Cells through the SIRT3 Pathway. Food Funct. 2022, 13, 2155–2171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Choi, M.-A.; Ro, S.W.; Yang, W.I.; Cho, A.E.H.; Ju, H.-L.; Baek, S.; Chung, S.I.; Kang, W.J.; Yun, M.; et al. Synergic Chemoprevention with Dietary Carbohydrate Restriction and Supplementation of AMPK-Activating Phytochemicals: The Role of SIRT1. Eur. J. Cancer Prev. 2016, 25, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic Preconditioning Combined with Curcumin Promotes Cell Survival and Mitochondrial Quality of Bone Marrow Mesenchymal Stem Cells, and Accelerates Cutaneous Wound Healing via PGC-1α/SIRT3/HIF-1α Signaling. Free Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef]
- Penedo-Vázquez, A.; Duran, X.; Mateu, J.; López-Postigo, A.; Barreiro, E. Curcumin and Resveratrol Improve Muscle Function and Structure through Attenuation of Proteolytic Markers in Experimental Cancer-Induced Cachexia. Molecules 2021, 26, 4904. [Google Scholar] [CrossRef]
- Mañas-García, L.; Guitart, M.; Duran, X.; Barreiro, E. Satellite Cells and Markers of Muscle Regeneration during Unloading and Reloading: Effects of Treatment with Resveratrol and Curcumin. Nutrients 2020, 12, 1870. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic Application of Quercetin in Aging-Related Diseases: SIRT1 as a Potential Mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin Induces Pro-Apoptotic Autophagy via SIRT1/AMPK Signaling Pathway in Human Lung Cancer Cell Lines A549 and H1299 in Vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Sang, A.; Wang, Y.; Wang, S.; Wang, Q.; Wang, X.; Li, X.; Song, X. Quercetin Attenuates Sepsis-Induced Acute Lung Injury via Suppressing Oxidative Stress-Mediated ER Stress through Activation of SIRT1/AMPK Pathways. Cell. Signal. 2022, 96, 110363. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Shi, J.-J.; Fang, J.; Wang, Q.; Chen, Y.-B.; Zhang, S.-J. Quercetin Ameliorates Diabetic Encephalopathy through SIRT1/ER Stress Pathway in Db/Db Mice. Aging 2020, 12, 7015–7029. [Google Scholar] [CrossRef]
- Iskender, H.; Dokumacioglu, E.; Sen, T.M.; Ince, I.; Kanbay, Y.; Saral, S. The Effect of Hesperidin and Quercetin on Oxidative Stress, NF-ΚB and SIRT1 Levels in a STZ-Induced Experimental Diabetes Model. Biomed. Pharmacother. 2017, 90, 500–508. [Google Scholar] [CrossRef]
- Ying, L.; Chaudhry, M.T.; Xiao, F.; Mao, Y.; Wang, M.; Wang, B.; Wang, S.; Li, Y. The Effects and Mechanism of Quercetin Dietary Supplementation in Streptozotocin-Induced Hyperglycemic Arbor Acre Broilers. Oxid. Med. Cell. Longev. 2020, 2020, 9585047. [Google Scholar] [CrossRef]
- Tang, J.; Lu, L.; Liu, Y.; Ma, J.; Yang, L.; Li, L.; Yang, J. Quercetin Improve Ischemia/Reperfusion-Induced Cardiomyocyte Apoptosis in Vitro and in Vivo Study via SIRT1/PGC-1α Signaling. J. Cell. Biochem. 2019, 120, 9747–9757. [Google Scholar] [CrossRef]
- Leyton, L.; Hott, M.; Acuña, F.; Caroca, J.; Nuñez, M.; Martin, C.; Otth, C. Nutraceutical Activators of AMPK/Sirt1 Axis Inhibit Viral Production and Protect Neurons from Neurodegenerative Events Triggered during HSV-1 Infection. Virus Res. 2015, 205, 63–72. [Google Scholar] [CrossRef]
- Yang, R.; Shen, Y.J.; Chen, M.; Zhao, J.Y.; Chen, S.H.; Zhang, W.; Du, G.H. Quercetin Attenuates Ischemia Reperfusion Injury by Protecting the Blood-Brain Barrier through Sirt1 in MCAO Rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin Attenuates Oxidative Stress-Induced Apoptosis via SIRT1/AMPK-Mediated Inhibition of ER Stress in Rat Chondrocytes and Prevents the Progression of Osteoarthritis in a Rat Model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Kim, S.C.; Kim, Y.H.; Son, S.W.; Moon, E.-Y.; Pyo, S.; Um, S.H. Fisetin Induces Sirt1 Expression While Inhibiting Early Adipogenesis in 3T3-L1 Cells. Biochem. Biophys. Res. Commun. 2015, 467, 638–644. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Ameliorative Effects of Fisetin in Letrozole-Induced Rat Model of Polycystic Ovary Syndrome. J. Steroid Biochem. Mol. Biol. 2021, 213, 105954. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Garg, G.; Singh, A.K.; Tripathi, S.S.; Rizvi, S.I. Fisetin, a Potential Caloric Restriction Mimetic, Modulates Ionic Homeostasis in Senescence Induced and Naturally Aged Rats. Arch. Physiol. Biochem. 2022, 128, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, A.K.; Garg, G.; Rizvi, S.I. Fisetin as a Caloric Restriction Mimetic Protects Rat Brain against Aging Induced Oxidative Stress, Apoptosis and Neurodegeneration. Life Sci. 2018, 193, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Feng, Z.; You, S.; Zhang, H.; Tao, Z.; Wang, Q.; Wu, Y. Fisetin Inhibits IL-1β-Induced Inflammatory Response in Human Osteoarthritis Chondrocytes through Activating SIRT1 and Attenuates the Progression of Osteoarthritis in Mice. Int. Immunopharmacol. 2017, 45, 135–147. [Google Scholar] [CrossRef]
- Rizk, F.H.; Soliman, N.A.; Abo-Elnasr, S.E.; Mahmoud, H.A.; Abdel Ghafar, M.T.; Elkholy, R.A.; ELshora, O.A.; Mariah, R.A.; Amin Mashal, S.S.; El Saadany, A.A. Fisetin Ameliorates Oxidative Glutamate Testicular Toxicity in Rats via Central and Peripheral Mechanisms Involving SIRT1 Activation. Redox. Rep. 2022, 27, 177–185. [Google Scholar] [CrossRef]
- Tabrizi, F.B.; Yarmohammadi, F.; Hayes, A.W.; Karimi, G. The Modulation of SIRT1 and SIRT3 by Natural Compounds as a Therapeutic Target in Doxorubicin-Induced Cardiotoxicity: A Review. J. Biochem. Mol. Toxicol. 2022, 36, e22946. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological Properties and Clinical Applications of Berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and Pharmacological Activities of Berberine in Diabetes Mellitus Treatment. Evid. Based Complement. Altern. Med. 2021, 2021, 9987097. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Liu, C.-S.; Huang, C.-L.; Chen, L.; Zheng, Y.-R.; Huang, S.-H.; Long, X.-Y. Nanoemulsion Improves Hypoglycemic Efficacy of Berberine by Overcoming Its Gastrointestinal Challenge. Colloids Surf. B Biointerfaces 2019, 181, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Yu, L.; Li, Q.; Yu, B.; Yang, Y.; Jin, Z.; Duan, W.; Zhao, G.; Zhai, M.; Liu, L.; Yi, D.; et al. Berberine Attenuates Myocardial Ischemia/Reperfusion Injury by Reducing Oxidative Stress and Inflammation Response: Role of Silent Information Regulator 1. Oxid. Med. Cell. Longev. 2016, 2016, 1689602. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Fu, W.; Liu, Y.; Yu, P.; Sun, M.; Li, X.; Yu, X.; Sui, D. Ginsenoside Rb2 Alleviates Myocardial Ischemia/Reperfusion Injury in Rats through SIRT1 Activation. J. Food Sci. 2020, 85, 4039–4049. [Google Scholar] [CrossRef] [PubMed]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and Barberry (Berberis Vulgaris): A Clinical Review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- El-Zeftawy, M.; Ghareeb, D.; ElBealy, E.R.; Saad, R.; Mahmoud, S.; Elguindy, N.; El-Kott, A.F.; El-Sayed, M. Berberine Chloride Ameliorated PI3K/Akt-p/SIRT-1/PTEN Signaling Pathway in Insulin Resistance Syndrome Induced in Rats. J. Food Biochem. 2019, 43, e13049. [Google Scholar] [CrossRef]
- Mi, D.-H.; Fang, H.-J.; Zheng, G.-H.; Liang, X.-H.; Ding, Y.-R.; Liu, X.; Liu, L.-P. DPP-4 Inhibitors Promote Proliferation and Migration of Rat Brain Microvascular Endothelial Cells under Hypoxic/High-Glucose Conditions, Potentially through the SIRT1/HIF-1/VEGF Pathway. CNS Neurosci. Ther. 2019, 25, 323–332. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Nutraceutical Activation of Sirt1: A Review. Open Heart 2022, 9, e002171. [Google Scholar] [CrossRef]
- Zheng, Y.; Kou, J.; Wang, P.; Ye, T.; Wang, Z.; Gao, Z.; Cong, L.; Li, M.; Dong, B.; Yang, W.; et al. Berberine-Induced TFEB Deacetylation by SIRT1 Promotes Autophagy in Peritoneal Macrophages. Aging 2021, 13, 7096–7119. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, T.; Ma, G.; Zheng, L.; Jiang, X.; Yang, F.; Wang, Z.; Li, N.; He, Z.; Song, X.; et al. Berberine Modulates Deacetylation of PPARγ to Promote Adipose Tissue Remodeling and Thermogenesis via AMPK/SIRT1 Pathway. Int. J. Biol. Sci. 2021, 17, 3173–3187. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-Pot Synthesis of Hyperoside by a Three-Enzyme Cascade Using a UDP-Galactose Regeneration System. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Dietary Flavonoids and Cancer Risk: Evidence from Human Population Studies. Nutr. Cancer 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini. Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- BinMowyna, M.N.; AlFaris, N.A. Kaempferol Suppresses Acetaminophen-Induced Liver Damage by Upregulation/Activation of SIRT1. Pharm. Biol. 2021, 59, 146–156. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Wang, Y.-H.; Lai, Y.-Y.; Tsou, H.-K.; Liou, G.-G.; Ko, J.-L.; Wang, S.-H. Kaempferol Protects against Propacetamol-Induced Acute Liver Injury through CYP2E1 Inactivation, UGT1A1 Activation, and Attenuation of Oxidative Stress, Inflammation and Apoptosis in Mice. Toxicol. Lett. 2018, 290, 97–109. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, S.; Keswani, H.; Shrivastava, N. Kaempferol and Apigenin Suppresses the Stemness Properties of TNBC Cells by Modulating Sirtuins. Mol. Divers. 2022, 26, 3225–3240. [Google Scholar] [CrossRef]
- Yang, C.; Yang, W.; He, Z.; Guo, J.; Yang, X.; Wang, R.; Li, H. Kaempferol Alleviates Oxidative Stress and Apoptosis Through Mitochondria-Dependent Pathway During Lung Ischemia-Reperfusion Injury. Front. Pharmacol. 2021, 12, 624402. [Google Scholar] [CrossRef]
- Yang, C.; Yang, W.; He, Z.; He, H.; Yang, X.; Lu, Y.; Li, H. Kaempferol Improves Lung Ischemia-Reperfusion Injury via Antiinflammation and Antioxidative Stress Regulated by SIRT1/HMGB1/NF-ΚB Axis. Front. Pharmacol. 2019, 10, 1635. [Google Scholar] [CrossRef]
- Sun, C.; Wang, T.; Wang, C.; Zhu, Z.; Wang, X.; Xu, J.; An, H. The Protective Effect of Kaempferol Against Ischemia/Reperfusion Injury Through Activating SIRT3 to Inhibit Oxidative Stress. Braz. J. Cardiovasc. Surg. 2022, 37, 335–342. [Google Scholar] [CrossRef]
- Guo, Z.; Liao, Z.; Huang, L.; Liu, D.; Yin, D.; He, M. Kaempferol Protects Cardiomyocytes against Anoxia/Reoxygenation Injury via Mitochondrial Pathway Mediated by SIRT1. Eur. J. Pharmacol. 2015, 761, 245–253. [Google Scholar] [CrossRef] [PubMed]
| Bioactive Molecules Modulating Sirtuins | Sources |
|---|---|
| Resveratrol (stilben) | Grapes, red wine, blueberries, cranberries, raisins, peanuts, Polygonum cuspidatum |
| Fisetin (flavonoid) | Apples, grapes, persimmons, strawberries, cucumbers, onion |
| Curcumin | herbal medicine, dietary spice (from root of the Curcuma longa) |
| Quercetin (flavonoid) | Onion, Shallots, broccoli, peppers, capers, apples, blueberries, grapes, herbs, tea, wine |
| Berberine | Coptidis rhizoma |
| Honokiol | Magnolia grandiflora |
| Dihydromyricetin (Ampelopsin, flavonool) | Ingredient of the Chinese medicinal herb Ampelopsis grossedentata |
| Trans ε-viniferin (polyphenol stiblenoid) | Vine stalks and most woody parts of the vine |
| Trilobatin | Lithocarpus polystahyus Rehd |
| Salidroside | Extracted from Rhodiola rosea |
| Silybin | Silybum marianum (L.) seeds |
| Polydatin | Polygonum cuspidatum |
| Kaempferol (flavonoid) | Spinach, kale, herbs, dills, chives, tarragon, wild leeks, ramps |
| Luteolin (flavonoid) | Carrots, peppers, celery, olive oil, peppermint, thyme, rosemary, lettuce, pomegranate, turnip, capers, cucumber, lemon, beets, brussels sprouts, cabbage, cauliflower, chives, fennel, harwort, horseradish, kohlrabi, parsley, spinach, and green tea |
| Cyanidin (anthocyanidin) | Berries, black currant, grapes |
| Delphinidin (anthocyanidin) | Fruits, vegetables, grains |
| Icariin (prenylated flavonoid glycoside) | Herba epimedii |
| Fucoidan (polysaccharide) | Seaweeds, brown algae |
| Oleic acid (fatty acid) | Olive oil, nuts vegetable |
| Linoleic acid (fatty acid) | Plant-based oil, nut, meat, animal products |
| Author | Type of Sirtuin | Potential Mechanism of Action |
|---|---|---|
| Reinisalo, M. et al., 2015 [63] | SIRT1 | deacetylation and activation of liver kinase B1 (LKB1) and ↑AMPK → ↑cellular NAD+ and ↑ catabolism inhibition of the NF-κB -light chain (enhancer of activated B cells) → anti-inflammatory and anti-cancer effects |
| Vingtdeux, V. et al., 2008 [72] | SIRT1 | the anti-amyloidogenic effect of PPARγ activation |
| Kelly, G.S et al., 2010 [73] | SIRT1 | modulation of PPAR γ co-activator 1 α pathway, enhancing mitochondrial function and proteostasis |
| Thomas, J. et al., 2014 [75] | SIRT1 | reduction of pro-inflammatory signaling through Jak-Stat pathway (IL-15, IL-22, Socs2, and Socs5) |
| Le, K. et al., 2019 [80] | SIRT1 | inhibition of HMGB1/TLR4/MyD88/NF-kB signaling and subsequent neuroinflammatory responses, providing neuroprotective effects in neonatal hypoxic-ischemic brain injury |
| Shen, J. et al., 2018 [81] | SIRT1 | activation of SIRT1/miR-134 pathway↑ expression of CREB and BDNF in the hippocampus → prevent impairment of the cognition induced by stress |
| Cai, M. et al., 2023 [89] | SIRT1 | Activation of SIRT1/Nrf2 pathway → ↓ oxidative stress and apoptosis |
| Author | Type of Sirtuin | Potential Mechanism of Action |
|---|---|---|
| Feng, K., et al., 2019 [110] | SIRT1 | Inhibition of endoplasmic reticulum stress → ↓ osteoarthritis development |
| Zhang, L., et al., 2019 [111] | SIRT1 | protective effects by stimulating mTOR phosphorylation and SIRT1 expression and ↓ the expression of autophagy-related 12, Beclin-1 and microtubule-associated protein light chain 3 II → reduced body weight loss and disease severity |
| Yin, Y., et al., 2020 [112] | SIRT1 | Activation of the SIRT1/Nrf2 pathway and reduced TLR4 expression |
| Ugur, S., et al., 2015 [113] and Ortega-Domínguez, B. et al., 2017 [114] | SIRT1, SIRT3, SIRT4 | cisplatin induced renal impairment → ↓ oxidative stress and ↑ protection of the kidneys from pathological changes |
| Li, L., et al., 2021 [116] | SIRT1 | sepsis induced acute kidney injury increased survival → improved kidney function, reduced inflammatory response and oxidative stress, and prevention of cell apoptosis |
| He, L., et al., 2015 [117] | SIRT1 | protective effects in gentamicin-induced acute kidney injury by ↓ tubular cell apoptosis, oxidative stress and ↑ SIRT1 and Nrf2/HO-1 expression |
| Jiménez-Flores, L.M., et al., 2014 [119] | SIRT1 | Modulation of SIRT1 through AMPK → ↑ glucose absorption and metabolism |
| Zendedel, E., et al., 2018 [120] | SIRT1 | Activation of AMPK, ↑ ATP and superoxide synthesis → ↓ oxidative stress-induced damage to mitochondria and ↓ infarction size |
| Fusi, J., et al., 2018 [121] | SIRT1 | ↓ apoptosis and oxidative stress |
| Li, K., et al., 2019 [122] | SIRT1 | streptozotocin-induced diabetes, ↑ cardiac function, ↓ myocardial fibrosis and cardiac hypertrophy, ↓ ROS generation |
| Takano, K., et al., 2018 [127] | SIRT1 | Long-term administration in atherosclerosis induced by a high-fat diet protected against ↓ SIRT1 expression, senescent cell accumulation, and vascular inflammation |
| Sun, Q., et al., 2014 [132] | SIRT1 | ↓ the expression of Bax a protein involved in apoptosis, in the presence of Aβ25-35 |
| Jia, N., et al., 2016 [133] | SIRT1 | ↓ glutamate excitotoxicity in cultured neurons and ↓ the level of acetylated PGC1α through deacetylation |
| Zhang, M. et al., 2017 [107] | SIRT3 | ↑ expression of SIRT3 in COPD → ↓ oxidative stress and inflammation markers |
| Liu, M., et al., 2021 [131] | SIRT3 | ↑ SIRT3 expression → ↓ superoxide dismutase activity, ↓ oxidative stress, and ↓ iron loading-induced autophagy in cell models of iron overload |
| Tan, C., et al., 2021 [125] and Lin, X., et al., 2015 [126] | SIRT6 | ↓ foam cell formation and intracellular lipid accumulation, ↑ cholesterol efflux in macrophages and activation of AMPK-SIRT1-LXRα signaling pathway → anti-atherosclerotic effects |
| Author | Type of Sirtuin | Potential Mechanism of Action |
|---|---|---|
| Cui Z et al., 2022 [149] | SIRT1 | regulation of pathways: SIRT1/AMPK/NFκB, SIRT1/Keap1/Nrf2/HO-1 and SIRT1/PI3K/Akt → ↑ the activity of antioxidant enzymes and anti-inflammatory cytokines |
| Guo H. et al., 2021 [153] | SIRT1 | inhibition of oxLDL-induced mitochondrial dysfunction and ROS production |
| Sang A. et al., 2022 [154] | SIRT1 | ↓ expression mRNA of CHOP, GRP78, activation of transcription factor 6 (ATF6), ↑ activation of AMPK/SIRT1 pathway → prevention of mitochondrial dysfunction and suppression of oxidative stress |
| Feng K. et al., 2019 [161] | SIRT1 | ↓ chondrocytes apoptosis by attenuation of ER stress and downregulation of the factors: CHOP, GRP78 and caspase 3 |
| Tang J. et al., 2019 [158] | SIRT1 | SIRT1/PGC-1α signaling pathway → up-regulation Bcl-2 and down-regulation Bax expression → anti-apoptotic impact in ischemia-reperfusion injury |
| Yang R. et al., 2022 [160] | SIRT1 | NRF2/HO-1 pathway → ↓ ROS production in the mitochondria in ischemia-reperfusion injured brain cells |
| Author | Type of Sirtuin | Potential Mechanism of Action |
|---|---|---|
| Kim S.C. et al., 2015 [162] | SIRT1 | ↓ the binding of PPARγ to the PPARγ promotor and simultaneously ↑ the binding of SIRT1 to the promoter → possibly changes deacetylation and activity of PPARγ → ↓ adipogenesis and accumulation of lipids control of adipogenic transcription factors such as CCAAT enhancer binding protein (C/EBP) family, peroxisome proliferator-activated receptor gamma (PPARg), Krüppel-like factors (KLFs) and sterol regulatory element-binding protein 1c (SREBP1c) → induction of adipocyte differentiation |
| Singh S., et al., 2020 [164] and Singh S., et al., 2018 [165] | SIRT1 | enhanced NF-kB deacetylation and suppression of pro-inflammatory genes expression in brain cells → prevention of neuro-inflammation and natural aging |
| Zheng W. et al., 2017 [166] | SIRT1 | inhibition of IL-1β-induced inflammation and degradation of Sox-9, aggrecan and collagen-II → ↓ articular cartilage damage, subchondral bone sclerosis and synovitis |
| Rizk F.H., et al., 2022 [167] | SIRT1 | degradation of the transcription factor FOXO3a → ↓ apoptosis |
| Author | Type of Sirtuin | Potential Mechanism of Action |
|---|---|---|
| Yu L. et al., 2016 [174] | SIRT1 | ↑ expression of the antiapoptotic factor Bcl-2 and ↓ expression in the proapoptotic factors Bax and caspase- |
| Xue Y. et al., 2020 [175] | SIRT1 | downregulation of gp91phox → antioxidant and anti-inflammatory effect ↓activity of IL-1β, IL-6, and TNFα → antioxidant and anti-inflammatory effect |
| El-Zeftawy, M. et al., 2019 [177] | SIRT1 | PI3K/Akt-p/SIRT-1/PTEN pathway → ↓ insulin resistance |
| Xu, Y. et al., 2021 [181] | SIRT1 | AMPK/SIRT1 pathway → activation of PPARγ → tissue remodeling and thermogenesis |
| Tabrizi, F.B. et al., 2022 [168] | SIRT1 | SIRT1/LKB1/AMPK, SIRT1/PGC-1α, SIRT1/NLRP3 and SIRT3/FoxO pathways → ↓ doxorubicin cardiotoxicity |
| Author | Type of Sirtuin | Potential Mechanism of Action |
|---|---|---|
| BinMowyna, M.N. et al., 2021 [186] | SIRT1 | exposure to acetaminophen → deacetylation of p53, NF-κB and FOXO-1 → hepatoprotective effect |
| Yang, C., et al., 2021 [189] | SIRT1 | activation of the mitochondrial SIRT1/PGC-1α pathway → ↓ oxidative stress and apoptosis |
| Yang, C., et al., 2019 [190] | SIRT1 | activation of the SIRT1/HMGB1/NF-κB pathway → anti-inflammatory and antioxidant effects |
| Guo, Z., et al., 2015 [192] | SIRT1 | ↓ LDH release in cardiomyoytes, ROS, caspase-3 and apoptosis and ↑ Bcl2 |
| Sun, C., et al., 2022 [191] | SIRT3 | ↓ ROS, NADPH oxidase activity, Bax and ↑ glutathione, Bcl2 → reduced oxidative stress |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Erdmann, J.; Nowacka, A.; Kuźmiński, O.; Michalak, K.; Janowski, K.; Ohla, J.; Biernaciak, A.; Szambelan, M.; Zabrzyński, J. Natural Phytochemicals as SIRT Activators—Focus on Potential Biochemical Mechanisms. Nutrients 2023, 15, 3578. https://doi.org/10.3390/nu15163578
Wiciński M, Erdmann J, Nowacka A, Kuźmiński O, Michalak K, Janowski K, Ohla J, Biernaciak A, Szambelan M, Zabrzyński J. Natural Phytochemicals as SIRT Activators—Focus on Potential Biochemical Mechanisms. Nutrients. 2023; 15(16):3578. https://doi.org/10.3390/nu15163578
Chicago/Turabian StyleWiciński, Michał, Jakub Erdmann, Agnieszka Nowacka, Oskar Kuźmiński, Klaudia Michalak, Kacper Janowski, Jakub Ohla, Adrian Biernaciak, Monika Szambelan, and Jan Zabrzyński. 2023. "Natural Phytochemicals as SIRT Activators—Focus on Potential Biochemical Mechanisms" Nutrients 15, no. 16: 3578. https://doi.org/10.3390/nu15163578
APA StyleWiciński, M., Erdmann, J., Nowacka, A., Kuźmiński, O., Michalak, K., Janowski, K., Ohla, J., Biernaciak, A., Szambelan, M., & Zabrzyński, J. (2023). Natural Phytochemicals as SIRT Activators—Focus on Potential Biochemical Mechanisms. Nutrients, 15(16), 3578. https://doi.org/10.3390/nu15163578







